 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Primary immune thrombocytopenia (ITP) is an acquired autoimmune hemorrhagic disease. Loss of immune tolerance plays a crucial role in the pathogenesis of ITP. Monocytes and macrophages play an indispensable role in the pathophysiology of hematopoietic malignancies and have been implicated as key players in platelet destruction. Approximately 80% of adult patients with ITP exhibit corticosteroid treatment failure or become dependent, requiring novel therapy. Thrombopoietin (TPO) receptor agonists (TPO-RAs) have been used clinically to manage ITP effectively, however, little is known about the effect of TPO-RAs on monocyte and macrophage modulation in adult ITP. In this study, we investigated the phenotypic evolution and potential immunomodulatory roles of monocytes/macrophages in ITP patients receiving eltrombopag therapy. Results showed that the peripheral monocyte count positively correlated with IFN-γ/IL-4 ratio in ITP patients. Moreover, numerous phenotype-associated genes in ITP macrophages exhibited diverse responses, and ITP macrophages exhibited more M1-related characteristics. After eltrombopag therapy, the peripheral monocyte count and IFN-γ/IL-4 ratio significantly decreased in ITP patients. M1-related characteristics of ITP macrophages were partially reversed by eltrombopag. Therefore, this study revealed eltrombopag restored the monocyte dynamics and the associated Th1/Th2 imbalance, and partially reversed the M1-related characteristics of the ITP macrophages, which suggest the potential vital roles of TPO-RAs in regulating the monocyte/macrophage plasticity in ITP.
Plain Language Summary
What is the context?
Primary immune thrombocytopenia (ITP) is an acquired autoimmune hemorrhagic disease. Loss of immune tolerance plays a crucial role in the pathogenesis of ITP.
Monocytes and macrophages play an indispensable role in the pathophysiology of hematopoietic malignancies and have been implicated as key players in platelet destruction.
Approximately 80% of adult patients with ITP exhibit corticosteroid treatment failure or become dependent, requiring novel therapy. Thrombopoietin (TPO) receptor agonists (TPO-RAs) have been used clinically to manage ITP effectively, however, little is known about the effect of TPO-RAs on monocyte and macrophage modulation in ITP.
What is new?
In this study, we investigated the phenotypic evolution and potential immunomodula-tory roles of monocytes/macrophages in ITP patients receiving eltrombopag therapy.
The expansion of peripheral monocytes positively correlated with IFN-γ/IL-4 ratio in ITP patients.
ITP macrophages exhibited more M1-related characteristics.
After eltrombopag therapy, the peripheral monocyte count and IFN-γ/IL-4 ratio significantly decreased in ITP patients.
M1-related characteristics of ITP macrophages were partially reversed by eltrombopag.
What is the impact?
This study provides evidence that the potential vital roles of TPO-RAs in regulating the monocyte/macrophage plasticity in ITP.
Introduction
Primary immune thrombocytopenia (ITP) is an autoimmune hemorrhagic disorder with heterogeneous pathogenesis. It is primarily characterized by platelet destruction mainly due to anti-platelet autoantibodies and/or impaired platelet production. Accelerated platelet destruction remains the leading classical mechanism of ITP, predominantly in the reticuloendothelial system (RES), where immune cells are the major players involved in the process [Citation1].
Monocytes and macrophages are essential components of the immune system and participate in a diverse range of physiological and pathological processes [Citation2,Citation3]. Monocytes, as macrophage precursors, develop into macrophages in tissues upon recruitment [Citation4]. Macrophages exhibit considerable heterogeneity in response to various environmental signals. The classically activated M1 and alternatively activated M2 macrophages represent two extremes activation phenotypes and are polarized by LPS/IFN-γ and IL-4/IL-13 respectively [Citation5]. However, most macrophages undergo signal-dependent activation to exhibit a continuum of states ranging between the M1 and M2 extremes, although they are always considered M1/M2 or M1/M2-like macrophages. Due to the variable activation phenotypes, effector functions of macrophages show substantial heterogeneity within different or even the same tissue microenvironments. As vital components in the hematopoietic niche, monocytes and macrophages participated in regulating hematopoietic stem/progenitor cells (HSC/HPCs) and erythroid differentiation [Citation6,Citation7]. We previously demonstrated that monocytes and macrophages influenced the promotion of the pathologic processes of extramedullary distribution and intracerebral invasion of leukemia cells in hematopoietic neoplasms [Citation8,Citation9]. Monocytes/macrophages are thought to be involved in platelet destruction in the RES [Citation10–12], however, the phenotypic evolution and potential immunomodulatory roles of monocytes and macrophages have not been fully elucidated in ITP.
Less than 80% of ITP patients exhibit corticosteroid treatment failure or become dependent, requiring novel therapy [Citation13]. Hence, several new therapies have been introduced in the past decade, and a number of thrombopoietic agents have been developed [Citation14]. Thrombopoietin (TPO) receptor agonists (TPO-RAs), eltrombopag, avatrombopag and romiplostim, have been used clinically to manage ITP effectively [Citation15–17] Eltrombopag belongs to a class of small-molecule, non-peptide agonists and interacts with the transmembrane domain of the TPO receptor at residue H499 [Citation18]. Besides their role in stimulating platelet production from megakaryocytes via activating TPO receptors, TPO-RAs potentially affect immunomodulation. Improved regulatory T cell (Treg) activity with concomitant suppression of effector T helper functions on platelet autoantigen was observed in ITP patients responding to eltrombopag and other TPO-RAs [Citation19]. In addition, thrombopoietic treatment rectified defects occurring in functionally impaired regulatory B cells (Bregs) on monocyte activation in chronic ITP patients [Citation20]. Furthermore, eltrombopag influenced the monocyte FcγR balance, shifting it toward inhibitory FcγRIIb and attenuating monocyte/macrophage-mediated phagocytosis in vitro [Citation12]. Eltrombopag treatment also increased plasma levels of TGF-β1 in corticosteroid-resistant/relapsed chronic ITP patients [Citation12]. A recent work about the pediatric ITP found that eltrombopag induced macrophages to switch towards M2 phenotype [Citation21], but that no information was available about adult ITP patients.
In this study, we investigated the phenotypic evolution and potential immunomodulatory roles of monocytes/macrophages in adult ITP patients receiving eltrombopag therapy. The peripheral monocyte count positively correlated with IFN-γ/IL-4 ratio in ITP patients. Moreover, numerous phenotype-associated genes in ITP macrophages exhibited diverse responses, and ITP macrophages exhibited more M1-related characteristics. After eltrombopag therapy, the peripheral monocyte count and IFN-γ/IL-4 ratio significantly decreased in ITP patients. M1-related characteristics of ITP macrophages were partially reversed by eltrombopag. Therefore, this study revealed eltrombopag restored the monocyte dynamics and the associated Th1/Th2 imbalance, and partially reversed the M1-related characteristics of the ITP macrophages, which suggest the potential vital roles of TPO-RAs in regulating the monocyte/macrophage plasticity in ITP.
Materials and methods
Participants
Twenty primary ITP patients were enrolled in the study. Basic patient characteristics are described in Supplementary Table S1. Eleven patients of them received four-week eltrombopag (Novartis Pharma Schweiz AG) treatment at a dose of 50 mg once daily with no concomitant treatment. Patients received no other previously treatment but steroids and immunoglobulin. Only one patient presented ITP for over eight years had self-seeked herb treatment previously. Previous therapy was completed at least six weeks prior to enrollment in the eltrombopag treatment. More details concerning these patients are described in Supplementary Table S2. We measured variations in the monocyte distribution and IFN-γ and IL-4 levels before and after four weeks of eltrombopag treatment. The control group consisted of six age and gender-matched healthy individuals (two females and four males; age range: 33–65 years; median age: 48 years). Platelet counts ranged from 173 to 295 × 109/L, with a median count of 270 × 109/L. People participated in this research voluntarily and provided informed consent. The study was approved by the Ethics Committee of Nanjing First Hospital, Nanjing, China.
Isolation and culture of macrophages from ITP patients
Peripheral blood mononuclear cells (PBMCs) from healthy individuals and ITP patients before and after eltrombopag treatment (four-week eltrombopag (Novartis Pharma Schweiz AG) treatment at a dose of 50 mg once daily with no concomitant treatment) were isolated using Ficoll density-gradient centrifugation and resuspended in serum-free RPMI 1640 medium. Then the cells were seeded at a density of 2.5x106 cells/well in a six-well culture plate and held for three hours in an incubator at 37°C with a humidified atmosphere of 5% CO2. The wells were rinsed, and non-adherent cells were removed. The adherent cells were cultured for seven days in RPMI 1640 supplemented with 10% fetal bovine serum (Gibco), 1% penicillin-streptomycin (Sigma), and 100 ng/ml recombinant human (rh) M-CSF (R&D Systems). To generate control and ITP macrophages before and after eltrombopag treatment, PBMC-derived macrophages were incubated for eight hours in serum obtained from healthy individuals or ITP patients before and after eltrombopag treatment. PBMC-derived macrophages (PBMC-derived control macrophages, PBMC-derived ITP macrophages before eltrombopag treatment and PBMC-derived ITP macrophages after eltrombopag treatment) were incubated with serum from the same individual. Then the cells were collected for analysis.
Cytokine detection
Plasma from healthy individuals or ITP patients was harvested, and the concentrations of cytokines (IFN-γ and IL-4, Neobioscience Technology, Shenzhen, PR China) were measured by ELISA according to the manufacturer’s protocols.
Quantitative real-time polymerase chain reaction (qRT-PCR)
PBMC-derived macrophages after incubation with serum were lysed, and total RNA was extracted using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA). First-strand cDNA was synthesized with oligo-(dT) primers according to the manufacturer’s instructions. qPCR was performed on a 0.1-QuantStudio 5 (Thermo Fisher Scientific, USA). The target gene expression levels were analyzed using the relative quantity (RQ) value (2-ΔΔCt), which was calculated using the ΔΔCt method [ΔΔCt = (CtTARGET - CtGAPDH) sample - (CtTARGET - CtGAPDH) calibrator]. The primer sequences are listed in Supplementary Table S3.
Two-dimensional illustration of macrophage phenotypes
A two-dimensional illustration was established to describe the activation phenotypes of macrophages based on their expression of M1/M2 genes [Citation22]. Each macrophage gene value obtained from healthy individuals was designated as 0. The M1 and M2 values of a specific macrophage subset were calculated as follows, and the mean values of relative expression of genes were used for calculation:
Statistical analysis
GraphPad Prism version 6.0 software (San Diego, CA) and SPSS version 17.0 software (SPSS, Chicago, IL) were used for the statistical analysis. The unpaired Student’s t-test was used for comparisons between two groups, and one-way ANOVA was used for comparisons among multiple groups. The correlations among different variables were calculated with Pearson’s correlation. All results were expressed as means ± SEM. Statistical differences were considered significant when P was less than 0.05.
Results
Peripheral monocyte expansion in active ITP patients
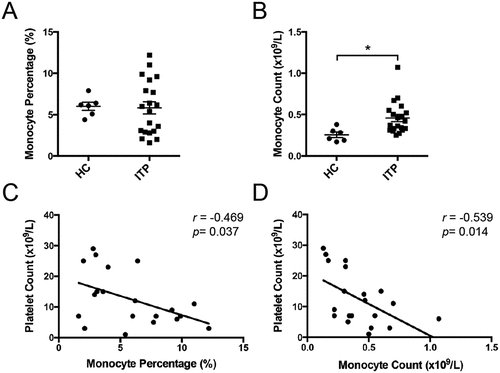
This study included twenty primary ITP patients (ten females and ten males). The median patient age was 61 and ranged between 29 and 79. Patient details are provided in Supplementary Table S1. The peripheral monocyte count was significantly elevated in the active ITP patients compared with HCs ((0.457 ± 0.044) × 109/L vs. (0.255 ± 0.033)× 109/L, p < .05, ), although no significant difference was observed in the monocyte proportion (5.830 ± 0.736% vs. 6.017 ± 0.490%, ). We evaluated the correlations between the peripheral monocyte proportion/count and the platelet count in ITP patients. Notably, both peripheral monocyte proportion (r=-0.469, p = .037, ) and monocyte count (r=-0.539, p = .014, ) exhibited negative correlations with platelet count. These results suggested that monocytes had a potentially vital role in ITP.
Figure 1. Peripheral monocytes in active ITP patients. Peripheral monocyte percentage (a) and count (b) from healthy controls (n = 6) and active ITP patients (n = 20). The correlations of peripheral monocyte percentage (c) and count (d) with platelet count in ITP patients. Bars represent mean ± SEM. *p < 0.05; unpaired Student’s t test and Pearson’s correlation test.
Peripheral monocytes are related to the Th1/Th2 imbalance in active ITP patients
Because the serum IFN-γ/IL-4 ratio reflects the immune imbalance for Th1/Th2 in ITP [Citation23], we assessed these two cytokines in active ITP patients. Elevated plasma IFN-γ () and decreased IL-4 levels () were found in ITP patients relative to healthy controls, which was in accordance with the previous study [Citation24]. Accordingly, the IFN-γ/IL-4 ratio in plasma was significantly increased in ITP patients (). We evaluated the relationship between these two cytokines and peripheral monocyte count in ITP patients. Interestingly, there was a significant positive correlation between peripheral monocyte count and IFN-γ levels (r = 0.500, p < .049, ) while no correlation was observed between peripheral monocyte count and IL-4 levels (r = −0.440, p = .08, ). However, the peripheral monocyte count positively correlated with the IFN-γ/IL-4 ratio (r = 0.764, p = .001, ). These results suggested that monocytes are positively related to the Th1/Th2 imbalance in ITP patients.
Figure 2. Plasma IFN-γ/IL-4 levels and the correlations with peripheral monocyte count in active ITP patients. Plasma IFN-γ (a) and IL-4 (b) levels from healthy controls (n = 6) and active ITP patients (n = 16). The correlations of plasma IFN-γ (c), IL-4 (d) levels and IFN-γ/IL-4 ratio (e) with peripheral monocyte count in ITP patients. Bars represent mean ± SEM. *p < 0.05; ** p < 0.01; unpaired Student’s t test and Pearson’s correlation test.
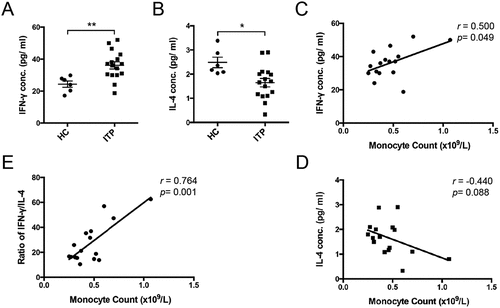
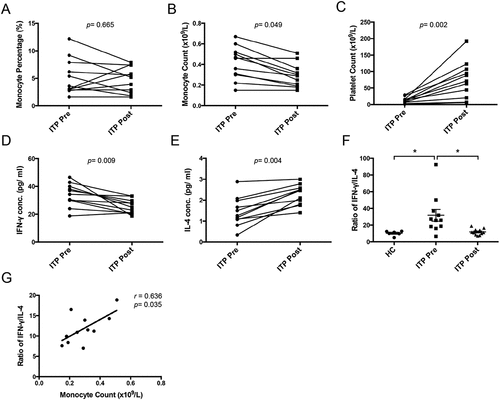
Figure 3. Changes of peripheral monocyte dynamics and plasma IFN-γ/IL-4 levels in ITP patients after eltrombopag treatment in vivo. Peripheral monocyte percentage (a), monocyte count (b), platelet count (c), plasma IFN-γ (d) and IL-4 (e) level in ITP patients (n = 11) before or after eltrombopag treatment in vivo. (f) Plasma IFN-γ/IL-4 ratio from healthy controls (n = 6) and ITP patients (n = 11) before or after eltrombopag treatment. (g) the correlations of plasma IFN-γ/IL-4 ratio (E) with peripheral monocyte count in ITP patients after eltrombopag treatment in vivo. Bars represent mean ± SEM. *p < 0.05; unpaired Student’s t test, one-way ANOVA tests and Pearson’s correlation test.
Monocyte count abnormal and Th1/Th2 imbalance rescue in ITP patients after effective therapy
The baseline platelet counts ranged from 1 to 29 × 109/L, with a median of 12 × 109/L. Platelet counts increased following four weeks of eltrombopag administration and ranged from 6 to 123 × 109/L, with a median of 71 × 109/L. Details are provided in Supplementary Table S2. There was no significant change in the monocyte proportion before or after eltrombopag treatment (). However, eltrombopag significant decreased the monocyte count () and increased platelet count () in ITP patients in vivo. Meanwhile, reduced plasma IFN-γ levels () and elevated IL-4 levels () were found in ITP patients after eltrombopag treatment. Eltrombopag also significant decreased the IFN-γ/IL-4 ratio in the plasma of ITP patients (). Moreover, peripheral monocyte count positively correlated with IFN-γ/IL-4 ratio in ITP patients after eltrombopag treatment (r = 0.636, p = .035, ). These results suggested that eltrombopag rescued the abnormal monocyte count and Th1/Th2 imbalance in ITP patients.
Macrophages from ITP patients before and after eltrombopag treatment present distinct activation phenotypes
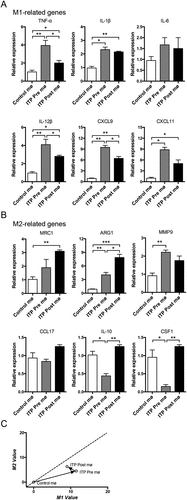
PBMCs were isolated from healthy individuals and ITP patients before or after eltrombopag treatment and were subsequently differentiated into control and ITP pre- or post-treatment macrophages. We analyzed the expression levels of a panel of phenotype-associated genes to investigate the activation phenotypes of the ITP pre- or post-treatment macrophages. Six genes for M1, including TNF-α, IL-1β, IL-6, IL-12β, CXCL9 and CXCL11, and six genes for M2, including MRC1, ARG1, MMP9, CCL17, IL-10 and CSF1 were investigated. Compared with the control group, the expression levels of most M1-related genes (TNF-α, IL-1β, IL-12β, CXCL9 and CXCL11) were elevated in ITP macrophages (). ITP macrophages also exhibited elevated expression levels of several M2-related genes (ARG1 and MMP9) but decreased IL-10 and CSF1 levels (). We generated a two-dimensional illustration of the macrophage to obtain an intuitive view of the phenotypic evolution of ITP macrophages. We found that the ITP macrophages were located in the M1 area (). Because extrinsic factors play critical roles in immune cell activation, we analyzed the effect of in vivo eltrombopag treatment on ITP macrophage activation. The ITP macrophages still exhibited elevated expression levels of several M1-related genes (TNF-α, IL-1β, IL-12β, CXCL9, and CXCL11) and M2-related genes (MRC1 and ARG1), compared with the control group. However, the expression of some M1-related genes (TNF-α, IL-12β, and CXCL9) decreased, and the expression of some M2-related genes (ARG1, IL-10, and CSF1) increased in ITP macrophages after eltrombopag treatment (). A two-dimensional illustration revealed that eltrombopag promoted ITP macrophages to have a reduced M1 value and increased M2 value, although they were still located in the M1 area (). These results suggested that the ITP macrophages exhibited more M1-related characteristics, and eltrombopag treatment reduced the M1-related characteristics of ITP macrophages.
Figure 4. The activation phenotypes of ITP macrophages before or after eltrombopag treatment in vivo. Expression of M1 (a) and M2 (b) phenotype-related genes in ITP macrophages before or after eltrombopag treatment was detected by real-time PCR. For each gene, the RQ value of control macrophages from healthy individuals was designated 1.000, respectively. (c) a two-dimensional illustration of the macrophage phenotype is shown. For each gene, the value of the control macrophages from healthy individuals was designated 0. The mean values of the relative expression of genes mentioned above were used for the calculation and the M1 and M2 values. The phenotypic evolution of ITP macrophages before or after eltrombopag treatment is indicated by arrows. The results are from three independent experiments. Bars represent mean ± SEM. *p < 0.05; ** p < 0.01; *** p < 0.001; one-way ANOVA tests.
Discussion
ITP is an acquired autoimmune hemorrhagic disease, and loss of immune tolerance plays a crucial role in the pathogenesis of ITP [Citation25]. Monocytes and macrophages are essential immune cells that participate in a broad range of physiological and pathological processes [Citation2,Citation3]. In our study, the expansion of peripheral monocytes was found and exhibited negative correlations with platelet count, suggesting potential vital roles of monocyte in ITP. As macrophage precursors, monocytes develop into macrophages in the tissues upon recruitment and exhibit diverse functions [Citation26]. Besides antigen presentation and phagocytosis, monocytes and macrophages regulate various functions of immune cells to support the adaptive immune response [Citation27]. Abnormal T cell subsets, such as elevated Th1 activation patterns and increased T helper reactivity against platelets, contributed to ITP pathogenesis [Citation28]. Since the serum ratio of IFN-γ/IL-4 reflects the immune imbalance of the Th1/Th2 in ITP [Citation23], we assessed these two cytokines. We discovered elevated IFN-γ levels and decreased plasma IL-4 levels in ITP patients, similar to the previous study [Citation24]. Importantly, there was a statistical positive relationship between peripheral monocyte count and IFN-γ levels while no statistically significant relationship between peripheral monocyte count and IL-4 levels. However, peripheral monocyte count positively correlated with IFN-γ/IL-4 ratio, which suggested that monocytes had a relationship with Th1/Th2 imbalance in ITP and might be a potential vital player in the immunopathogenesis of Th1 bias. Mechanisms of the immunoregulatory effects require further investigation.
Macrophages exhibit significant heterogeneity in response to various environmental signals [Citation29]. In our study, ITP macrophages expressed both M1 and M2-related genes. Although they expressed higher levels of ARG1 and MMP9, ITP macrophages expressed higher levels of proinflammatory cytokines, including TNF-α, IL-1β, IL-12β, as well as chemokines, including CXCL9 and CXCL11. Eventually, the ITP macrophages presented more M1-related characteristics. While seemingly contradictory, it is noted that M1 and M2 are two extremes, not stable differentiated states. Moreover, macrophages are activated in a spectrum of possible forms between the two extreme phenotypes [Citation30]. Generally, M1-like macrophages are considered to play anti-tumor roles, whereas M2-like macrophages play pro-tumor roles. In the previous research, phagocytic function was impaired in ITP macrophages due to the disturbed balance of the activating and inhibitory Fcγ receptor (FcγRs) [Citation12]. The impaired functional phenotypes seem tend to be an M2-like phenotype, in contrast to the M1-like phenotype of ITP macrophages observed in our study. Our previous research found that the monocyte-derived leukemia-associated macrophages (LAMs) subset with M1-like phenotype facilitated the extramedullary distribution of leukemia cells and promoted the pathologic process of hepatosplenomegaly in leukemia [Citation8]. Thus, the anti-tumor and pro-tumor roles of macrophages are not in exact one-to-one correspondence with the M1-like and M2-like phenotypes. Both CXCL9 and CXCL11 were considered as Th1-associated chemokines and promoting to polarize to Th1 [Citation31,Citation32]. In our study, besides high expression levels of pro-inflammatory cytokines from the ITP macrophages, ITP macrophages expressed high levels of CXCL9 and CXCL11, indicating the potential role of monocytes/macrophages in polarizing to Th1, which might also contribute to the pro-inflammatory microenvironment and ITP pathomechanism. However, CXCL9 and CXCL11 also recruited other immune cells, such as Tregs [Citation33–35], that might exert anti-inflammatory effects by secreting anti-inflammatory cytokines, including IL-10, IL-35 and TGF-β [Citation36]. Therefore, the traditional perception that M1 macrophages participate in a pro-inflammatory response while M2 macrophages participate in an anti-inflammatory response is somewhat imprecise. Hence, a comprehensive understanding of the multi-faceted pathologic roles of ITP macrophages requires further investigation.
Eltrombopag is in a class of small-molecule, non-peptide agonists that interacts with the transmembrane domain of the TPO receptor at residue H499. These agonists have been used clinically to manage ITP effectively [Citation18]. Remarkably, up to one-third ITP patients achieved a sustained remission off-treatment (SROT) after TPO-RAs had been discontinued for 24 weeks [Citation37]. Importantly, eltrombopag have been proposed to have immunomodulatory effects. Improved Treg activity with concurrent suppression of effector T helper functions through enhanced release of TGF-β1, which correlated with platelet counts, was found in ITP patients responding to eltrombopag [Citation19]. In addition, thrombopoietic treatment rectified the defects in causing functional impairment of Bregs on monocyte activation in chronic ITP patients [Citation20]. Moreover, eltrombopag modulated monocyte FcγR balance favoring the inhibitory FcγRIIb and attenuated monocyte/macrophage-mediated phagocytosis in corticosteroid-resistant/relapsed chronic ITP patients [Citation12]. Furthermore, TPO-RA might affect antigen processing and presentation by megakaryocytes (MKs) [Citation38]. In our study, we investigated the effects of eltrombopag on the monocyte dynamic and its potential correlation with the Th1/Th2 balance, as well as the expression levels of phenotype-associated genes and phenotypic evolution of macrophages in corticosteroid-resistant ITP patients. Significant increase of platelet counts mediated by eltrombopag was found. The abnormal monocyte count and Th1/Th2 imbalance rescued after eltrombopag therapy. TPO-RAs were generally considered as agents that may decrease platelet apoptosis, in addition, eltrombopag might bind to the BCL-2 family (i.e. BCL2, BAX, and BCL2L1) and inhibited pro-apoptotic member BAX-mediated apoptosis [Citation39,Citation40]. Nevertheless, the effect of eltrombopag on regulating immune cell apoptosis remains unclear. Eltrombopag involved in the rescue of monocyte count, which may partly due to monocyte apoptosis, however, the mechanisms require further study. Besides megacaryocytes and platelets, immune cells also expressed thrombopoietin receptor (MPL), such as monocytes, neutrophils, NK cells, B cells and T cells [Citation41,Citation42]. Eltrombopag has been shown to regulate immune cells through MPL. For example, according to ITP mathematical models, eltrombopag mediated MPL signaling through the activated JAK2-STAT3 pathway, induced the inhibition of IFN-γ signaling in T cells [Citation39]. Hence, MPL-mediated effect on T cells may contribute to the decrease of IFN-γ after eltrombopag treatment in ITP. However, beside the effect of eltrombopag through MPL on T cells, ectosomes derived from platelets interacted with CD4+ T cells diminished the release of cytokines IFN-γ [Citation43]. Multiple mechanisms may involve in the reestablishment of the immune equilibrium after eltrombopag treatment. The expression of M1-related genes (TNF-α, IL-12β, and CXCL9) decreased, whereas the expression of M2-related genes (ARG1, IL-10, and CSF1) increased in ITP macrophages after eltrombopag treatment. However, the ITP macrophages still exhibited elevated expression levels of several M1-related genes (TNF-α, IL-1β, IL-12β, CXCL9, and CXCL11) and M2-related genes (MRC1 and ARG1), compared with control macrophages. A two-dimensional illustration showed that eltrombopag polarized ITP macrophages toward more M2 value and less M1 value, although they were still located in the M1 area, which suggested that eltrombopag treatment reduced the M1-related characteristics of the ITP macrophages. A recent work about the pediatric ITP found eltrombopag mediated macrophage polarization from M1 to M2 phenotype [Citation21]. The mature status of immune system in microenvironment is diverse in pediatric and adult ITP, nevertheless, the effect of eltrombopag on polarizing ITP macrophages towards more M2 was consistent. Mechanisms of eltrombopag mediated the changes of immune cells in ITP may be complex. Eltrombopag was a powerful iron chelator that decreased cellular iron and enhanced iron mobilization [Citation44]. Evidence indicates that there is a close relationship between iron and monocytes/macrophages. Under different disease conditions and environments, iron induces macrophages to exhibit heterogeneous activation phenotypes via various mechanisms. For example, in diabetes, HO-1 expressed by macrophages mediated M1 polarization and promoting chronic metabolism-related inflammation [Citation45]. However, the accumulation of iron in healing wounds skewed macrophages towards M2-like [Citation46]. Hence eltrombopag mediated iron chelation may contribute to the phenotypic evolution of monocytes/macrophages in ITP. In addition, interestingly, platelets have been reported to regulated both innate and adaptive immune responses, and play immunomodulatory roles in monocytes/macrophages [Citation47]. For example, platelets released inflammatory mediators, such as HMGB1, which activated monocytes or potentially modulated monocyte immune phenotype and function [Citation48]. Platelet aggregation with monocytes polarized macrophages toward M1 phenotype in sepsis [Citation49]. Hence, besides the direct immunomodulatory effects of eltrombopag on monocytes/macrophages, the increased platelet mediated by eltrombopag may also play a crucial immunomodulatory role in monocytes/macrophages in ITP. The underlying mechanisms may reflect the recovery of immune tolerance after eltrombopag treatment and provide a possible explanation for why eltrombopag induced a prolonged response in the long-term EXTEND clinical trial [Citation50]. Nevertheless, given the complexity of tissue-specific components within the microenvironment at different stages, a comprehensive investigation of the multiple mechanisms of immune regulation is necessary. Furthermore, more types and larger numbers of ITP patients should be involved to evaluate the effect and study the further mechanism of eltrombopag therapy on immune system in future studies.
Collectively, our findings revealed the phenotypic evolution and potential immunomodulatory roles of monocytes/macrophages in ITP patients receiving eltrombopag therapy. Results showed that the expansion of peripheral monocytes positively correlated with IFN-γ/IL-4 ratio in ITP patients. Moreover, numerous phenotype-associated genes in ITP macrophages exhibited diverse responses, and ITP macrophages exhibited more M1-related characteristics. Eltrombopag therapy decreased the peripheral monocyte count and IFN-γ/IL-4 ratio in ITP patients. Furthermore, M1-related characteristics of ITP macrophages were partially reversed by eltrombopag. Therefore, this study revealed eltrombopag restored the monocyte dynamics and the associated Th1/Th2 imbalance, and partially reversed the M1-related characteristics of the ITP macrophages, which suggest the potential vital roles of TPO-RAs in regulating the monocyte/macrophage plasticity in ITP.
Supplemental Material
Download PDF (274.9 KB)Supplemental Material
Download PDF (202.2 KB)Supplemental Material
Download PDF (190.6 KB)Disclosure statement
The authors declare that there is no conflict of interest.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/09537104.2022.2135694.
Additional information
Funding
References
- Audia S, Mahévas M, Samson M, Godeau B, Bonnotte B. Pathogenesis of immune thrombocytopenia. Autoimmun Rev. 2017;16(6):1–9. doi: 10.1016/j.autrev.2017.04.012.
- DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19(6):369–382. doi: 10.1038/s41577-019-0127-6.
- Guilliams M, Mildner A, Yona S. Developmental and functional heterogeneity of monocytes. Immunity. 2018;49(4):595–613. doi: 10.1016/j.immuni.2018.10.005.
- Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11(11):762–774. doi: 10.1038/nri3070.
- Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. 2020;15:123–147. doi: 10.1146/annurev-pathmechdis-012418-012718.
- Ehninger A, Trumpp A. The bone marrow stem cell niche grows up: mesenchymal stem cells and macrophages move in. J Exp Med. 2011;208(3):421–428. doi: 10.1084/jem.20110132.
- Chow A, Lucas D, Hidalgo A, Méndez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208(2):261–271. doi: 10.1084/jem.20101688.
- Yang F, Feng W, Wang H, Wang L, Liu X, Wang R, Chen C, Yang X, Zhang D, Ren Q, et al. Monocyte-Derived leukemia-associated macrophages facilitate extramedullary distribution of T-cell acute lymphoblastic leukemia cells. Cancer Res. 2020;80(17):3677–3691. doi: 10.1158/0008-5472.CAN-20-0034.
- Wang H, Zhang D, Cui X, Dai Y, Wang C, Feng W, Lv X, Li Y, Wang L, Ru Y, et al. Loss of IRF7 accelerates acute myeloid leukemia progression and induces VCAM1-VLA-4 mediated intracerebral invasion. Oncogene. 2022;41(16):2303–2314. doi: 10.1038/s41388-022-02233-w.
- Kuwana M, Okazaki Y, Ikeda Y. Splenic macrophages maintain the anti-platelet autoimmune response via uptake of opsonized platelets in patients with immune thrombocytopenic purpura. J Thromb Haemost. 2009;7(2):322–329. doi: 10.1111/j.1538-7836.2008.03161.x.
- Saleh MN, Moore DL, Lee JY, LoBuglio AF. Monocyte-platelet interaction in immune and nonimmune thrombocytopenia. Blood. 1989;74(4):1328–1331. doi: 10.1182/blood.V74.4.1328.1328.
- Liu X-G, Liu S, Feng Q, Liu X-N, Li G-S, Sheng Z, Chen P, Liu Y, Wei Y, Dong X-Y, et al. Thrombopoietin receptor agonists shift the balance of Fcγ receptors toward inhibitory receptor IIb on monocytes in ITP. Blood. 2016;128(6):852–861. doi: 10.1182/blood-2016-01-690727.
- Ghanima W, Gernsheimer T, Kuter DJ. How I treat primary ITP in adult patients who are unresponsive to or dependent on corticosteroid treatment. Blood. 2021;137(20):2736–2744. doi: 10.1182/blood.2021010968.
- Cooper N, Ghanima W, Solomon CG. Immune Thrombocytopenia. N Engl J Med. 2019;381(10):945–955. doi: 10.1056/NEJMcp1810479.
- Stasi R, Evangelista ML, Amadori S. Novel thrombopoietic agents: a review of their use in idiopathic thrombocytopenic purpura. Drugs. 2008;68(7):901–912. doi: 10.2165/00003495-200868070-00002.
- Ghanima W, Cooper N, Rodeghiero F, Godeau B, Bussel JB. Thrombopoietin receptor agonists: undefined years later. Haematologica. 2019;104(6):1112–1123. doi: 10.3324/haematol.2018.212845.
- Markham A. Avatrombopag: a review in thrombocytopenia. Drugs. 2021;81(16):1905–1913. doi: 10.1007/s40265-021-01613-y.
- Cheng G. Eltrombopag, a thrombopoietin- receptor agonist in the treatment of adult chronic immune thrombocytopenia: a review of the efficacy and safety profile. Ther Adv Hematol. 2012;3(3):155–164. doi: 10.1177/2040620712442525.
- Bao W, Bussel JB, Heck S, He W, Karpoff M, Boulad N, Yazdanbakhsh K. Improved regulatory T-cell activity in patients with chronic immune thrombocytopenia treated with thrombopoietic agents. Blood. 2010;116(22):4639–4645. doi: 10.1182/blood-2010-04-281717.
- Li X, Zhong H, Bao W, Boulad N, Evangelista J, Haider MA, Bussel J, Yazdanbakhsh K. Defective regulatory B-cell compartment in patients with immune thrombocytopenia. Blood. 2012;120(16):3318–3325. doi: 10.1182/blood-2012-05-432575.
- Di Paola A, Palumbo G, Merli P, Argenziano M, Tortora C, Strocchio L, Roberti D, Santoro C, Perrotta S, Rossi F. Effects of eltrombopag on in vitro macrophage polarization in pediatric immune thrombocytopenia. Int J Mol Sci. 2020;22(1):97. doi: 10.3390/ijms22010097.
- Yang X, Feng W, Wang R, Yang F, Wang L, Chen S, Ru Y, Cheng T, Zheng G. Repolarizing heterogeneous leukemia-associated macrophages with more M1 characteristics eliminates their pro-leukemic effects. Oncoimmunology. 2018;7(4):e1412910. doi: 10.1080/2162402X.2017.1412910.
- Kostic M, Zivkovic N, Cvetanovic A, Marjanović G. CD4+ T cell phenotypes in the pathogenesis of immune thrombocytopenia. Cell Immunol. 2020;351:104096. doi: 10.1016/j.cellimm.2020.104096.
- Hao Y, Li Y, Li H, Lyu M, Zhang D, Fu R, Guan Y, Wang S, Sun B, Dou X, et al. Increased plasma sCXCL16 levels may have a relationship with Th1/Th2 imbalance in primary immune thrombocytopenia. Cytokine. 2017;99:124–131. doi: 10.1016/j.cyto.2017.08.024.
- Semple JW, Rebetz J, Maouia A, Kapur R. An update on the pathophysiology of immune thrombocytopenia. Curr Opin Hematol. 2020;27(6):423–429. doi: 10.1097/MOH.0000000000000612.
- Lavin Y, Mortha A, Rahman A, Merad M. Regulation of macrophage development and function in peripheral tissues. Nat Rev Immunol. 2015;15(12):731–744. doi: 10.1038/nri3920.
- Guilliams M, Thierry GR, Bonnardel J, Bajenoff M. Establishment and maintenance of the macrophage Niche. Immunity. 2020;52(3):434–451. doi: 10.1016/j.immuni.2020.02.015.
- Vrbensky JR, Nazy I, Clare R, Larché M, Arnold DM. T cell-mediated autoimmunity in immune thrombocytopenia. Eur J Haematol. 2022;108(1):18–27. doi: 10.1111/ejh.13705.
- Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity. 2014;41(1):21–35. doi: 10.1016/j.immuni.2014.06.013.
- Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, De Nardo D, Gohel TD, Emde M, Schmidleithner L, et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity. 2014;40(2):274–288. doi: 10.1016/j.immuni.2014.01.006.
- Ferrari SM, Fallahi P, Elia G, Ragusa F, Camastra S, Paparo SR, Giusti C, Gonnella D, Ruffilli I, Shoenfeld Y, et al. Novel therapies for thyroid autoimmune diseases: an update. Best Pract Res Clin Endocrinol Metab. 2020;34(1):101366. doi: 10.1016/j.beem.2019.101366.
- Karin N, Wildbaum G. The role of chemokines in shaping the balance between CD4(+) T cell subsets and its therapeutic implications in autoimmune and cancer diseases. Front Immunol. 2015;6:609. doi: 10.3389/fimmu.2015.00609.
- Wang Z, Zhang H, Liu R, Qian T, Liu J, Huang E, Lu Z, Zhao C, Wang L, Chu Y. Peyer’s patches-derived CD11b B cells recruit regulatory T cells through CXCL9 in dextran sulphate sodium-induced colitis. Immunology. 2018;155(3):356–366. doi: 10.1111/imm.12977.
- Tang Z, Gao J, Wu J, Zeng G, Liao Y, Song Z, Liang X, Hu J, Hu Y, Liu M, et al. Human umbilical cord mesenchymal stromal cells attenuate pulmonary fibrosis via regulatory T cell through interaction with macrophage. Stem Cell Res Ther. 2021;12(1):397. doi: 10.1186/s13287-021-02469-5.
- Zohar Y, Wildbaum G, Novak R, Salzman AL, Thelen M, Alon R, Barsheshet Y, Karp CL, Karin N. CXCL11-dependent induction of FOXP3-negative regulatory T cells suppresses autoimmune encephalomyelitis. J Clin Invest. 2018;128(3):1200–1201. doi: 10.1172/JCI120358.
- Wing JB, Tanaka A, Sakaguchi S. Human FOXP3+ regulatory T cell heterogeneity and function in autoimmunity and cancer. Immunity. 2019;50(2):302–316. doi: 10.1016/j.immuni.2019.01.020.
- Lucchini E, Palandri F, Volpetti S, Vianelli N, Auteri G, Rossi E, Patriarca A, Carli G, Barcellini W, Celli M, et al. Eltrombopag second-line therapy in adult patients with primary immune thrombocytopenia in an attempt to achieve sustained remission off-treatment: results of a phase II, multicentre, prospective study. Br J Haematol. 2021;193(2):386–396. doi: 10.1111/bjh.17334.
- Zufferey A, Kapur R, Semple JW. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP). J Clin Med. 2017;6(2):16. doi: 10.3390/jcm6020016.
- Lozano ML, Segú-Vergés C, Coma M, Álvarez-Roman MT, González-Porras JR, Gutiérrez L, Valcárcel D, Butta N. Elucidating the mechanism of action of the attributed immunomodulatory role of eltrombopag in primary immune thrombocytopenia: an in silico approach. Int J Mol Sci. 2021;22(13):6907. doi: 10.3390/ijms22136907.
- Spitz AZ, Zacharioudakis E, Reyna DE, Garner TP, Gavathiotis E. Eltrombopag directly inhibits BAX and prevents cell death. Nat Commun. 2021;12(1):1134. doi: 10.1038/s41467-021-21224-1.
- Schmiedel BJ, Singh D, Madrigal A, Valdovino-Gonzalez AG, White BM, Zapardiel-Gonzalo J, Ha B, Altay G, Greenbaum JA, McVicker G, et al. Impact of genetic polymorphisms on human immune cell gene expression. Cell. 2018;175(6):1701–1715. doi: 10.1016/j.cell.2018.10.022.
- Monaco G, Lee B, Xu W, Mustafah S, Hwang YY, Carré C, Burdin N, Visan L, Ceccarelli M, Poidinger M, et al. RNA-Seq signatures normalized by mRNA abundance allow absolute deconvolution of human immune cell types. Cell Rep. 2019;26(6):1627–1640. doi: 10.1016/j.celrep.2019.01.041.
- Sadallah S, Amicarella F, Eken C, Iezzi G, Schifferli JA. Ectosomes released by platelets induce differentiation of CD4+T cells into T regulatory cells. Thromb Haemost. 2014;112(12):1219–1229. doi: 10.1160/th14-03-0281.
- Vlachodimitropoulou E, Chen Y-L, Garbowski M, Koonyosying P, Psaila B, Sola-Visner M, Cooper N, Hider R, Porter J. Eltrombopag: a powerful chelator of cellular or extracellular iron(iii) alone or combined with a second chelator. Blood. 2017;130(17):1923–1933. doi: 10.1182/blood-2016-10-740241.
- Jais A, Einwallner E, Sharif O, Gossens K, T-H Lu T, Soyal SM, Medgyesi D, Neureiter D, Paier-Pourani J, Dalgaard K, et al. Heme oxygenase-1 drives metaflammation and insulin resistance in mouse and man. Cell. 2014;158(1):25–40. doi: 10.1016/j.cell.2014.04.043.
- Wilkinson HN, Roberts ER, Stafford AR, Banyard KL, Matteucci P, Mace KA, Hardman MJ. Tissue iron promotes wound repair via M2 macrophage polarization and the chemokine (C-C Motif) ligands 17 and 22. Am J Pathol. 2019;189(11):2196–2208. doi: 10.1016/j.ajpath.2019.07.015.
- Semple JW, Italiano JE, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11(4):264–274. doi: 10.1038/nri2956.
- Vogel S, Rath D, Borst O, Mack A, Loughran P, Lotze MT, Neal MD, Billiar TR, Gawaz M. Platelet-derived high-mobility group box 1 promotes recruitment and suppresses apoptosis of monocytes. Biochem Biophys Res Commun. 2016;478(1):143–148. doi: 10.1016/j.bbrc.2016.07.078.
- Carestia A, Mena HA, Olexen CM, Ortiz Wilczyñski JM, Negrotto S, Errasti AE, Gómez RM, Jenne CN, Carrera Silva EA, Schattner M. Platelets promote macrophage polarization toward pro-inflammatory phenotype and increase survival of septic mice. Cell Rep. 2019;28(4):896–908. doi: 10.1016/j.celrep.2019.06.062.
- Saleh MN, Bussel JB, Cheng G, Meyer O, Bailey CK, Arning M, Brainsky A. Safety and efficacy of eltrombopag for treatment of chronic immune thrombocytopenia: results of the long-term, open-label EXTEND study. Blood. 2013;121(3):537–545. doi: 10.1182/blood-2012-04-425512.
