Abstract
Activated platelets possess procoagulant activity expressing on their surface phosphatidylserine (PS), a substrate for assembling coagulation complexes. We examined the effects of platelets activated by different agonists on fibrin formation and thrombin generation and compared these effects with each other and with PS expression. Modified plasma recalcification assay was developed to assess platelet effects on fibrin formation. Washed human platelets were left intact or activated by A23187 ionophore, collagen, arachidonic acid, ADP or TRAP (Thrombin Receptor Activating Peptide) and spun down in 96-well plates. Plasma was then added, recalcified, and fibrin formation was monitored by light absorbance. Platelets prepared in the same way were tested for their effect on thrombin generation. PS expression was evaluated by flow cytometry using annexin V staining. Platelets significantly accelerated fibrin formation and thrombin generation. They shortened lag phase and increased maximum rate of plasma clotting, and increased peak and maximum rate of thrombin generation. In both tests platelets were presumably activated by endogenous thrombin formed in plasma after triggering coagulation reactions. However, pretreatment with exogenous agonists additionally increased platelet procoagulant activity. It reached the maximum after incubation with A23187, being lower with collagen and arachidonic acid and minimum with ADP and TRAP (the latter might be ineffective due to competition with endogenous thrombin). The effects of platelets activated by different agonists on fibrin formation and thrombin generation correlate with each other and correspond to PS expression on their surface.
Plain Language Summary
Why was the study done?
Platelets and blood coagulation system interact with each other in hemostasis and intravascular thrombosis.
Direct platelet effects on fibrin formation (plasma clotting), the final stage of blood coagulation cascade, have been insufficiently studied.
The work is aimed at developing a method for studying platelet participation in fibrin formation in blood plasma and investigating the influence of platelet agonists on this reaction.
What is new?
Platelets significantly accelerate fibrin formation and their activation with various agonists (thrombin, collagen, arachidonic acid) enhances these effects.
Effects of platelets on fibrin formation correlated with their ability to stimulate thrombin generation in blood plasma
Effects of platelets on fibrin formation and thrombin generation correlated with the level of phosphatidylserine exposure on their surface
What is the impact?
This study provides further evidence that platelet procоagulant effects on fibrin formation should be considered in investigations of platelet involvement in hemostatic and thrombotic reactions and in the evaluation of the efficacy of antiplatelet drugs
Introduction
Activated platelets express on their surface negatively charged phosphatidylserine (PS) which serves as a substrate for assembling coagulation complexes. Active tenase and prothrombinase complexes form on activated platelets with participation of PS [Citation1–6]. In thrombin generation test (TGT) platelets markedly accelerate plasma thrombin generation. Different agonists potentiate platelet effects on thrombin generation, while antiplatelet agents reduce them [Citation7–12]. However, the effects of platelets on thrombin-catalyzed fibrin formation, the final step of the coagulation cascade, remain poorly investigated. Thromboelastography allows evaluation of platelet effect predominantly on fibrin clot strength (maximum amplitude parameter) but not on the time and rate of fibrin clot formation [Citation13,Citation14]. Recent studies in a sophisticated microfluidic flow chamber employing collagen- and tissue factor-coated surface for initiation of thrombus formation have demonstrated the role of collagen and its platelet receptor (glycoprotein VI) in platelet-dependent potentiation of blood clotting [Citation15–17].
Here we suggest a simple test based on plasma recalcification assay (PRA) for evaluation of platelet effects directly on fibrin formation. The effects of differently activated platelets on fibrin formation were compared with those on thrombin generation and both procoagulant activities were compared with PS expression.
Materials and methods
Platelets
Blood was collected from healthy volunteers. The donors sighed informed consent and the study was approved by Ethics Committee of National Medical Research Center of Cardiology (# 274 of 29.11.2021). Washed platelets were prepared as described [Citation18] and resuspended in Tyrode/HEPES solution (137 mM NaCl, 2.7 mM KCl, 0.36 mM NaH2PO4, 0.1% dextrose, 2 mM CaCl2, 1 mM MgCl2, 0.35% BSA, 5 mM HEPES, pH 7.35) at 0.5 × 108/ml. Platelets were left intact or activated by 10 µM A23187 ionophore (Thermo Fisher Scientific, Eugene, OR), 20 µM TRAP (sequence SFLLRN, provided by Dr. M.D. Ovchinnikov, National Medical Research Center for Cardiology), 0.2 mM arachidonic acid (Santa Cruz Biotechnology, Heidelberg, Germany), 10 µg/ml collagen (Helena Biosciences, UK) or 20 µM ADP (AppliChem GmbH, Darmstadt, Germany) without stirring for 5 min at room temperature.
Plasma recalcification assay (PRA)
Platelet ability to accelerate fibrin formation was tested in a modified PRA. Intact or activated platelets (100 µl, 0.5 x 107 per well) were added to 96-well cell culture flat bottom plates (Costar, Kennebunk, ME, Cat. # 3599) and sedimented in a baket rotor for 5 min at 1500 g. Under these conditions >90% platelets are immobilized on the well bottom (as assessed using radiolabeled platelets, Suppl. Table SI) and form a uniform layer covering plastic surface without forming aggregates (). After centrifugation the supernatant was removed by accurate aspiration with the multichannel pipette and 50 µl CaCl2 -free Tyrode/HEPES solution supplemented with 150 μg/ml corn trypsin inhibitor (provided by Dr. G.V. Shekhvatova, Institute of Protein, Pushchino, Russia) for partial inhibition of contact activation and 50 µl human citrated plasma (pooled plasma from 3 to 4 donors depleted of endogenous microparticles by centrifugation for 90 min at 20 000 g) were added to sedimented platelets. Control samples contained no platelets. Plasma was recalcified by adding 50 μl 25 mM CaCl2 (Diagnostica Stago, France) All procedures from platelet sedimentation to CaCl2 addition are usually performed within 3–5 minutes (depending on the number of analyzed samples). Fibrin formation (plasma clotting) was evaluated by changes in light absorbance at 450 nm (A450) for 60 min at 25°C in a Thermo Scientific Multiscan Go plate spectrophotometer (Thermo Fisher Scientific, Finland). The lag phase (time to reach 5% the maximum A450 increase, min) and the maximum rate of fibrin formation (Vmax, maximum increase in A450 per min as percentage of total increase at 60 min (%A450/min) were determined.
Figure 1. Platelets immobilized on plastic surface in 96-well plates. Washed platelets (0.5 x 108/ml) were not activated (a) or activated by 0.2 mM arachidonic acid (b) or 10 µm A23187 (C) without stirring for 5 min at room temperature and spun down on the bottom of 96-well plates (100 µl/well) at 1500 g, 5 min. (see “Materials and methods”). The supernatant was removed; platelets were fixed with 1% paraformaldehyde and analyzed in a phase-contrast microscope. Scale bar −10 µm. Close pictures were obtained after platelet activation by arachidonic acid (b) and ADP and collagen (not shown). After activation by A23187 most platelets acquire a rounded shape.
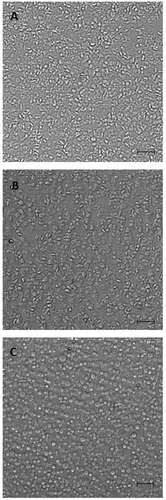
In preliminary experiments, we have shown that platelets attached to plastic provided more effective acceleration of plasma clotting than the same amount of platelets in suspension (Suppl. Figure S1). This difference might be explained by a lower availability of PS on platelets in suspension due to their ability to form aggregates under those conditions. The critical role of platelet PS was confirmed by complete inhibition of plasma clotting in the presence of PS blocker lactadherin (Suppl. Figure S2).
Thrombin generation test (TGT)
Platelets prepared in the same way as for PRA were tested in TGT. All reagents used in TGT were from Diagnostica Stago (France). A total of 80 µl citrated plasma and 20 µl trigger PRP reagent (tissue factor and minimum amount of phospholipids) were added to immobilized platelets. After incubation (10 min, 37 С) the reaction was started by the addition of 20 µl Fluo-Buffer (thrombin fluorogenic substrate and CaCl2). Final concentration of tissue factor was 0.5 pM. In control samples plasma thrombin generation was monitored with the use of “PPP reagent” that provided a final concentration of 5 pM for tissue factor and 4 µM for phospholipids. For calibration of fluorescent signal Thrombin Calibrator (750 nM Thrombin-α2-macroglobulin complex) was added instead of trigger reagent. Measurements were performed in a Fluoroscan Ascent plate fluorimeter (ThermoLab Systems, Finland). Thrombin generation curves were analyzed using Thrombinoscope software (Thrombinoscope BV, Netherlands).
Phosphatidylserine (PS) expression
PS expression on the surface of activated platelets was evaluated by flow cytometry using staining with Annexin V-FITC. Washed platelets (0.5 x 108/ml) prepared as described above were left intact or activated for 15-min at 37°C without stirring by 10 U/ml human thrombin (Haematologic Technologies, Inc., Essex Junction, VT), 10 µM A23187, 20 µM TRAP, 0.2 mM arachidonic acid, 10 µg/ml collagen or 20 µM ADP, or by thrombin in combination with arachidonic acid, collagen or ADP. Platelet suspension (50 µl) was then supplemented with 5 µl annexin V-FITC (BD Biosciences, San Jose, CA) and 3 µl CD42b-APC (BD Pharmingen, San Diego, CA) and incubated for 20 min at room temperature in the dark. Negative control contained no annexin V-FITC. Then Tyrode/HEPES (250 µl) was added and the samples were analyzed in a FACSCanto II flow cytometer using DivaTM Software (BD Biosciences, San Jose, CA). Platelets were gated according to their size and CD42b-positive staining. The percentage of annexin V-FITC (PS) positive platelets was calculated in comparison with negative control.
Statistics
Most of analyzed variables fit normal distribution (Shapiro-Wilk’s test). Data were expressed as means ± standard deviations (SD). The significance of differences between multiple groups was evaluated using one-way ANOVA with post-hoc analysis (Fisher test), and significance of differences between individual groups was evaluated using t-test (as indicated). Correlations were assessed using Pearson statistics.
Results
Platelets and fibrin formation
We used modified PRA for evaluation of direct platelet procoagulant effects on fibrin formation. Platelets were left intact or activated by different agonists and spun down in 96-well plates. After plasma addition clotting was initiated by the excess of CaCl2 and monitored by light absorbance. Typical clotting curves are presented in and statistical data in . Considerable acceleration of fibrin formation in recalcified plasma was observed in the presence of untreated and agonist-treated platelets. In the presence of untreated platelets the lag phase shortened almost 2-fold, and the maximum rate of plasma clotting increased almost 1.5-fold. In all samples platelets were presumably activated by endogenous thrombin forming in recalcified plasma. However, irrespective of thrombin effect other agonists further increased platelet effect on fibrin formation. A23187 ionophore shortened lag phase and increased maximum rate be approximately 2-fold in comparison with samples without exogenous agonists. A statistically significant decrease in lag phase was observed after incubation with collagen, arachidonic acid and ADP. An increase in the maximum rate was recorded after incubation with collagen. Among applied agonists, only TRAP was completely ineffective, which could be explained by employing for platelet activation the same receptor (PAR1, Protease Activated Receptor 1) as exogenous thrombin.
Figure 2. Effects of platelets on fibrin formation in modified PRA. Platelets were not activated by exogenous agonists (“No exo agonists”) or activated by 20 µM TRAP (“TRAP”), 20 µM ADP (“ADP”), 0.2 mM arachidonic acid (“Arach acid”), 10 µg/ml collagen (“Collagen”) or 10 µM A23187 (“A23187”) and spun down in 96-well plates. Platelets were not added to the control samples (“No platelets”). After plasma addition, PRA was performed as described in “Materials and methods.” Representative curves out of ≥ 8 experiments. Statistical data are given in .
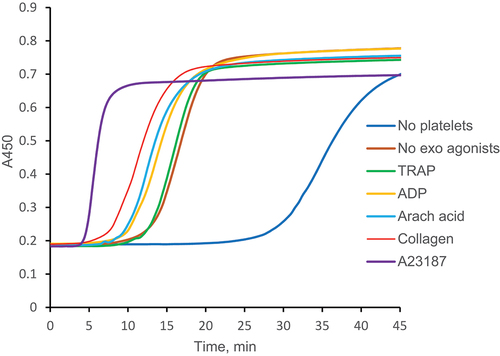
Table I. Platelet activation and fibrin formation in a PRA.
Platelets and thrombin generation
Platelets treated with the same agonists and prepared in the same way as in PRA (sedimentation in 96-well plates) were tested for their ability to accelerate thrombin generation. Tissue factor with minimum amount of phospholipids was used as a trigger reagent. As in PRA, in TGT platelets were exposed to endogenous thrombin formed after initiation of the coagulation cascade. Typical thrombin generation curves are given in and statistical data in . Very low concentrations of thrombin were detected when the reaction was performed without platelets. Platelets which were not treated with exogenous agonists induced a 3- to 4-fold increase in endogenous thrombin potential (ETP), thrombin peak and maximum rate of thrombin generation. The effect on lag phase was much less pronounced: it shortened by about 15%. Incubation with exogenous agonists had no effect on lag phase and ETP (lag phase slightly shortened only in the presence of A23187) but markedly increased peak and maximum rate of thrombin generation. Thrombin generation was accelerated almost in the same order as fibrin formation in PRA: A23187 >> collagen > arachidonic acid > TRAP > ADP ≈ no agonist. Weak effect of TRAP in TGT could be explained by its competition with exogenous thrombin.
Figure 3. Effects of platelets on thrombin generation in TGT. Platelets were not activated by exogenous agonists (“No exo agonists”), or activated by 20 µM TRAP (“TRAP”), 20 µM ADP (“ADP”), 0.2 mM arachidonic acid (“Arach acid”), 10 µg/ml collagen (“Collagen”) or 10 µM A23187 (“A23187”) and spun down in 96-well plates. Platelets were not added to the control samples (“No platelets”). TGT was performed as described in “Materials and methods.” Representative curves out of ≥ 7 experiments. Statistical data are given in .
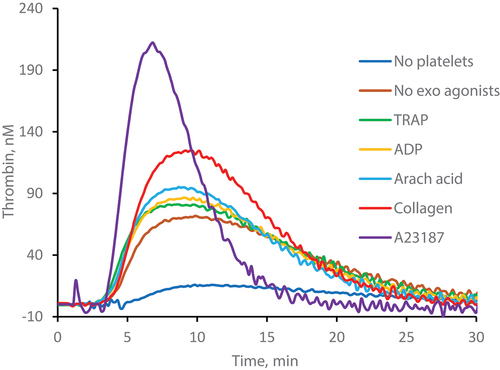
Table II. Platelet activation and thrombin generation.
By comparing the data obtained in PRA and TGT in the absence and presence of differently activated platelets () we have revealed a strong reverse correlation between the mean values of lag phase in PRA and thrombin peak (r = −0.906, p = .005) and Vmax (r = −0.814, p = .026) in TGT and a strong direct correlation between the mean values of V max in PRA and thrombin peak (r = 0.982, p < .001) and V max(r = 0.960, p = .001) in TGT (Correlation graphics – see Suppl. Figure S3).
Platelet activation and PS expression
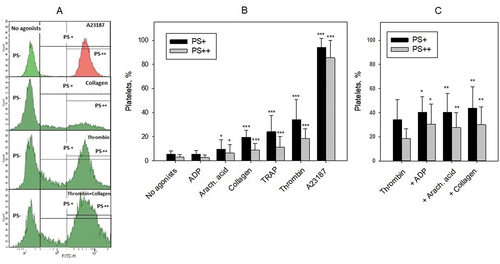
PS expression on the surface of activated platelets was evaluated by flow cytometry after staining with annexin V-FITC. In each sample we identified PS– platelets with FITC fluorescence as in the negative control (platelets incubated without annexin V-FITC), PS+ platelets with FITC fluorescence higher than in the control, and PS++ platelets with very high FITC fluorescence as after treatment with A23187 ionophore (). PS expression reached the maximum after activation with A23187: >90% PS++ platelets (). With other agonists the average numbers of PS+ and PS++ platelets did not exceed 40% and 20%, respectively, decreasing as follows: thrombin > TRAP ≈ collagen > arachidonic acid > ADP ≈ no agonists (). Collagen, arachidonic acid and ADP potentiated thrombin effect, increasing the number of PS+ and PS++ platelets by 20–30% and 50–60%, respectively (). The effect of exogenous thrombin on PS expression is presumably comparable to that of endogenous thrombin formed in PRA and TGT. Bearing this in mind we compared the mean levels of PS expression on platelets activated with thrombin alone, thrombin in combination with collagen, arachidonic acid and ADP as well as with A23187 (data presented in ) with the mean values of PRA and TGT indexes without (endogenous thrombin only) and with above mentioned agonists (corresponding data from ). Strong tendency to reverse correlation was revealed between the percent of PS+ platelets and lag phase in PRA (r = −0.835, p = .079) and significant direct correlations were revealed between the percent of PS+ platelets and Vmax in PRA (0.949, p = .014) and thrombin peak (r = 0.977, p = .04) and V max (r = 0.994, p = .001) in TGT (Correlation graphics – see Suppl. Figure S4).
Figure 4. Platelet activation and PS expression. Platelets were not activated (No agonists) or activated by indicated agonists and PS was detected by staining with annexin V-FITC using flow cytometry. Thrombin was used in a concentration of 10 U/ml, and other agonists (A23187, TRAP, collagen, arachidonic acid and ADP) in the same concentrations as in recalcification assay ( and ) and TGT ( and ). (A) Examples of flow cytometry analysis. Fluorescence regions for PS-, PS+, and PS++ platelets are shown. Vertical line separating PS- and PS+ regions corresponds to 95% of the fluorescent peak of the negative control (platelets without annexin V-FITC, not shown). Vertical line separating PS+ and PS++ regions corresponds to the 95% of the fluorescent peak of A23187 activated platelets (to the right). (B) Amounts of PS+ (black bars) and PS++ (gray bars) platelets after their activation with the agonists. Means ± SD (n ≥ 9) are shown; *p < 0.05, ***p < 0.001, significance of differences from “No agonists” group (t-test for means). (C) Amounts of PS+ (black bars) and PS++ (grey bars) platelets after their activation by thrombin alone and by thrombin in combination (+) with ADP, arachidonic acid and collagen. Means ± SD (n ≥ 11) are shown; *p < 0.05, **p < 0.001, significance of differences from “Thrombin” group (paired t-test).
Discussion
A new method was suggested for evaluation of platelet effects on fibrin formation and thrombin generation. Platelets were left intact or activated with different agonists, sedimented in 96-well plates, the supernatant was decanted and plasma was then added. Plasma clotting (fibrin formation) was initiated by adding CaCl2 (recalcification assay) and thrombin generation by adding CaCl2, low amount of tissue factor and minimum phospholipids. Fibrin formation was evaluated by light absorbance and thrombin generation was assessed with the use of fluorogenic substrate. Platelets markedly accelerated fibrin formation and thrombin generation. In PRA and TGT platelets were exposed to endogenous thrombin formed in plasma after triggering the coagulation cascade, and thus became strongly activated without preincubation with exogenous agonists. However, A23187 (served as a positive control), and to a less extent collagen increase platelet procoagulant activity in both tests. Arachidonic acid and ADP were significantly effective only in PRA (lag phase) and TRAP produced no significant effects. The results obtained in TGT are consistent with previous studies showing that platelet agonists could potentiate thrombin generation in platelet-rich plasma [Citation7–10]. The absence or very weak effect of TRAP, a strong platelet agonist, on fibrin formation and thrombin generation may result from its interaction with the same PAR-1 receptor as endogenous thrombin and indirectly confirm thrombin involvement in platelet activation in both assays. Concerning the effects of exogenous arachidonic acid and ADP, it should be taken into account that in control samples thromboxane A2 (a product of arachidonic acid metabolism) and ADP could be present due to their release from platelets activated by endogenous thrombin.
Sedimentation of platelets on plastic in both PRA and TGT allowed us to avoid their aggregation and to carry out both reactions in the absence of membrane microparticles which were removed with the supernatant. In PRA, platelet aggregation can potentially mask and reduce the availability of PS exposing platelet membranes which was indirectly confirmed by lower rate of plasma clotting in the presence of platelets in suspension in comparison with platelets attached to plastic surface (see Suppl. Figure S1). In TGT, aggregation was already shown to modify platelet-mediated effects on the rate and extent of thrombin generation [Citation10]. Stimulatory effects of platelet microparticles on fibrin formation and thrombin generation have been demonstrated in many studies [Citation18–23]. Moreover, it was suggested that microparticles express procoagulant PS on the membrane at a higher density than activated platelets [Citation24]. Thus, our approach allows one to assess procoagulant effects of non-aggregated platelets and to exclude the action of platelet-derived microparticles.
We have established correlations between the effects of platelets activated under different conditions on the parameters of fibrin formation and thrombin generation. Correlations were observed irrespective of different ways for triggering coagulation cascade: without TF in PRA and with TF in TGT. These findings indicate that potentiating effect of activated platelets on thrombin generation is directly retranslated into acceleration of fibrin clot formation, i.e., final reaction in the blood coagulation cascade.
The effects of various agonists on PS expression were evaluated after the addition of individual agonists or in combination with thrombin. The latter approach is similar to that when platelets are exposed to both endogenous thrombin and exogenous agonists in PRA and TGT. In general, our results describing effects of different agonists on PS exposure have no contradictions with previously published data [Citation6,Citation25–27]. As expected, the greatest number of PS-expressing platelets (>90%) was detected after incubation with A23187, which correlated with its powerful activating effects in PRA and TGT. Thrombin induced PS expression on about 20–30% platelets. TRAP was slightly less effective, probably because it interacts only with PAR-1, while thrombin binds to PAR-1 and PAR-4. Collagen was as active as TRAP, arachidonic acid produced a weaker effect, but still being able to induce PS expression exceeding the control level, while the weakest agonist ADP did not induce any significant increase in PS expression. Collagen, arachidonic acid and ADP potentiated thrombin effect on PS expression, which confirms that their potentiation of platelet effects on fibrin formation and thrombin generation in the presence of endogenous thrombin is associated with enhanced PS expression on platelet surface. The central role of PS expression in the implementation of platelet procoagulant activity on fibrin formation and thrombin generation was confirmed by revealing correlations between those reactions (level of PS expression versus plasma clotting and thrombin generation indexes).
Thus, our results indicate that the effects of activated platelets on fibrin formation and thrombin generation correlate with each other and are mediated by PS exposed on their surface.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Rosing J, van Rijn JL, Bevers EM, van Dieijen G, Comfurius P, Zwaal RF. The role of activated human platelets in prothrombin and factor X activation. Blood. 1985;65(2):1–8. doi:10.1182/blood.V65.2.319.319.
- Kempton CL, Hoffman M, Roberts HR, Monroe DM. Platelet Heterogeneity Variation in coagulation complexes on platelet subpopulations. Arterioscler Thromb Vasc Biol. 2005;25(4):861–866. doi:10.1161/01.ATV.0000155987.26583.9b.
- Panteleev MA, Ananyeva NM, Greco NJ, Ataullakhanov FI, Saenko EL. Two subpopulations of thrombin-activated platelets differ in their binding of the components of the intrinsic factor X-activating complex. J Thromb Haemost. 2005;3(11):2545–2553. doi:10.1111/j.1538-7836.2005.01616.x.
- Heemskerk JWM, Mattheij NJA, Cosemans JMEM. Platelet-based coagulation: different populations, different functions. J Thromb Haemost. 2013;11(1):2–16. doi:10.1111/jth.12045.
- Podoplelova NA, Sveshnikova AN, Kotova YN, Eckly A, Receveur N, Nechipurenko DY, Obydennyi SI, Kireev II, Gachet C, Ataullakhanov FI, et al. Coagulation factors bound to procoagulant platelets concentrate in cap structures to promote clotting. Blood. 2016;128(13):1745–1755. doi:10.1182/blood-2016-02-696898.
- Reddy EC, Rand ML. Procoagulant phosphatidylserine-exposing platelets in vitro and in vivo. Front Cardiovasc Med. 2020;7:15. doi:10.3389/fcvm.2020.00015.
- Altman R, Scazziota A, Rouvier J, Gonzalez C. Effect of sodium arachidonate on thrombin generation through platelet activation – inhibitory effect of aspirin. Thromb Haemost. 2000;84(12):1109–1112. doi:10.1055/s-0037-1614178.
- Altman R, Scazziota A, de Lourdes Herrera M, Gonzalez C. Recombinant factor VIIa reverses the inhibitory effect of aspirin or aspirin plus clopidogrel on in vitro thrombin generation. J Thromb Haemost. 2006;4(9):2022–2027. doi:10.1111/j.1538-7836.2006.02088.x.
- Altman R, Scazziota AS, de Lourdes Herrera M, Gonzalez C. Thrombin generation by activated factor VII on platelet activated by different agonists. Extending the cell-based model of hemostasis. Thromb J. 2006;4(1):5. doi:10.1186/1477-9560-4-5.
- Didelot M, Docq C, Wahl D, Lacolley P, Regnault V, Lagrange J. Platelet aggregation impacts thrombin generation assessed by calibrated automated thrombography. Platelets. 2018;29(2):156–161. doi:10.1080/09537104.2017.1356452.
- Panova-Noeva M, van der Meijden PEG, ten Cate H. Clinical applications, pitfalls, and uncertainties of thrombin generation in the presence of platelets. J Clin Med. 2020;9(1):92. doi:10.3390/jcm9010092.
- Wan J, Konings J, de Laat B, Hackeng TM, Roest M. Added value of blood cells in thrombin generation testing. Thromb Haemost. 2021;121(12):1574–1587. 10.1055/a-1450-8300.
- Reikvam H, Steien E, Hauge B, Knut Liseth K, Hagen KG, Størkson R, Hervig T. Thrombelastography. Transfus Apher Sci. 2009;40(2):119–123. doi:10.1016/j.transci.2009.01.019.
- Racine-Brzostek SE, Asmis LM. Assessment of platelet function utilizing viscoelastic testing. Transfusion. 2020;60(S6):S10–20. 10.1111/trf.16081.
- Swieringa F, Baaten CCFMJ, Verdoold R, Mastenbroek TG, Rijnveld N, van der Laan KO, Breel EJ, Collins PW, Lancé MD, Henskens YMC, et al. Platelet control of fibrin distribution and microelasticity in thrombus formation under flow. Arterioscler Thromb Vasc Biol. 2016;36(4):692–699. doi:10.1161/ATVBAHA.115.306537.
- Brouns SLN, van Geffen JP, Campello E, Swieringa F, Spiezia L, van Oerle R, Provenzale I, Verdoold R, Farndale RW, Clemetson KJ, et al. Platelet-primed interactions of coagulation and anticoagulation pathways in flow-dependent thrombus formation. Sci Rep. 2020;10(1):11910. doi:10.1038/s41598-020-68438-9.
- Navarro S, Stegner D, Nieswandt B, Heemskerk JWM, Kuijpers MJE. Temporal roles of platelet and coagulation pathways in collagen- and tissue factor-induced thrombus formation. Int J Mol Sci. 2021;23(1):358. doi:10.3390/ijms23010358.
- Khaspekova SG, Antonova OA, Shustova ON, Yakushkin VV, Golubeva NV, Titaeva EV, Dobrovolsky AB, Mazurov AV. Activity of tissue factor in microparticles produced in vitro by endothelial cells, monocytes, granulocytes, and platelets. Biochem Moscow. 2016;81(2):114–121. doi:10.1134/S000629791602005X.
- Aleman MM, Gardiner C, Harrison P, Wolberg AS. Differential contribution of monocyte- and platelet-derived microparticles towards thrombin generation and fibrin formation and stability. J Thromb Haemost. 2011;9(11):2251–2261. doi:10.1111/j.1538-7836.2011.04488.x.
- Van der Meijden PE, van Schilfgaarde M, van Oerle R, Renné T, ten Cate H, Spronk HM. Platelet- and erythrocyte-derived microparticles trigger thrombin generation via factor XIIa. J Thromb Haemost. 2012;10(7):1355–1362. doi:10.1111/j.1538-7836.2012.04758.x.
- Tripisciano C, Weiss R, Eichhorn T, Spittler A, Heuser T, Fischer MB, Weber V. Different potential of extracellular vesicles to support thrombin generation: contributions of phosphatidylserine, tissue factor, and cellular origin. Sci Rep. 2017;7(1):6522. doi:10.1038/s41598-017-03262-2.
- Shustova ON, Antonova OA, Golubeva NV, Khaspekova SG, Yakushkin VV, Aksuk SA, Alchinova IB, Karganov MY, Mazurov AV. Differential procoagulant activity of microparticles derived from monocytes, granulocytes, platelets and endothelial cells: impact of active tissue factor. Blood Coagul Fibrinolys. 2017;28(5):373–382. doi:10.1097/MBC.0000000000000609.
- Lipets EN, Antonova OA, Shustova ON, Losenkova KV, Mazurov AV, Ataullakhanov FI. Use of Thrombodynamics for revealing the participation of platelet, erythrocyte, endothelial, and monocyte microparticles in coagulation activation and propagation. PLoS ONE. 2020;15(5):e0227932. doi:10.1371/journal.pone.0227932.
- Sinauridze EI, Kireev DA, Popenko NY, Pichugin AV, Panteleev MA, Krymskaya OV, Ataullakhanov FI. Platelet microparticle membranes have 50- to 100-fold higher specific procoagulant activity than activated platelets. Thromb Haemost. 2007;97(03):425–434. doi:10.1160/TH06-06-0313.
- Thiagarajan P, Tait JF. Binding of annexin V/placental anticoagulant protein I to platelets. Evidence for phosphatidylserine exposure in the procoagulant response of activated platelets. J Biol Chem. 1990;265(29):17420–17423. doi:10.1016/S0021-9258(18)38177-8.
- Ramstrom S, Ranby M, Lindahl TL. Platelet phosphatidylserine exposure and procoagulant activity in clotting whole blood – different effects of collagen, TRAP and calcium ionophore A23187. Thromb Haemost. 2003;89(01):132–141. doi:10.1055/s-0037-1613552.
- Rukoyatkina N, Shpakova V, Panteleev M, Kharazova A, Gambaryan S, Geiger J. Multifaceted effects of arachidonic acid and interaction with cyclic nucleotides in human platelets. Thromb Res. 2018;171:22–30. doi:10.1016/j.thromres.2018.09.047.
