Abstract
The pathogenesis of thrombocytopenia in chronic hepatitis C (CHC) conceivably involves autoimmunity; however, the dynamics of autoantibodies and other autoimmune mechanisms remain unclear. In this study, we examined the changes in the frequency of anti-glycoprotein (GP) IIb/IIIa antibody-producing B cells and the levels of plasma B-cell-activating factor (BAFF), a proliferation-inducing ligand (APRIL), and interleukin (IL)-21 following treatment of CHC with direct-acting antiviral agents (DAA). We recruited 28 patients with CHC who underwent treatment with DAA for 8–12 weeks and subsequently tested negative for serum hepatitis C virus RNA. Thirty healthy controls were recruited for comparison. Platelet counts increased significantly (p = .016), and the frequency of anti-GPIIb/IIIa antibody-producing B cells decreased significantly (p = .002) in CHC patients with thrombocytopenia at the end of treatment (EOT) than before DAA treatment (baseline). However, these changes were not observed in CHC patients without thrombocytopenia. Plasma BAFF levels in CHC patients with thrombocytopenia significantly decreased from baseline to EOT (p = .002). Anti-GPIIb/IIIa antibody-producing B cells were positively correlated with plasma BAFF levels in these patients (r = 0.669, p = .039). These results suggest that DAA treatment suppresses the autoimmune response against platelets and improves thrombocytopenia.
Plain Language Summary
What is the context?
Production of antiplatelet antibodies is one of the mechanisms underlying thrombocytopenia in patients with chronic hepatitis C.
Antiplatelet antibodies against platelet membrane glycoprotein (GP) IIb/IIIa are commonly detected in hepatitis C virus-associated immune thrombocytopenia.
Hepatitis C virus elimination by direct-acting antiviral agents (DAA) improves thrombocytopenia in patients with hepatitis C; however, the dynamics of autoantibodies and other autoimmune mechanisms remain unclear.
What is new?
In this study, we determined whether DAA treatment can alter the autoimmune response against platelets and improve platelet count.
The frequency of anti-GPIIb/IIIa antibody-producing B cells decreased significantly from the baseline following DAA treatment in chronic hepatitis C patients with thrombocytopenia.
DAA treatment reduced the levels of B-cell-activating factor, a cytokine associated with autoantibody production.
What is the impact?
The study provides evidence that DAA treatment diminishes the autoimmune response to GPIIb/IIIa and, therefore, improves platelet counts in chronic hepatitis C patients with thrombocytopenia.
Introduction
Thrombocytopenia in chronic hepatitis C (CHC) involves multiple mechanisms, including hypersplenism, diminished production of thrombopoietin (TPO), and the production of antiplatelet autoantibodies [Citation1,Citation2]. Immune thrombocytopenia (ITP) is an autoimmune disease in which antiplatelet antibodies against glycoprotein (GP)IIb/IIIa, GPIb, and TPO receptors are produced, which consequently leads to accelerated platelet destruction and impaired platelet production [Citation3,Citation4]. Hepatitis C virus (HCV)-infected individuals have been reported to be at higher risk of ITP than uninfected individuals [Citation5,Citation6]. Therefore, HCV infection is thought to induce an autoimmune reaction to platelets. Direct-acting antiviral agents (DAA), which strongly inhibit HCV replication, have yielded excellent therapeutic outcomes in antiviral pharmacotherapy for CHC [Citation7]. Studies have shown that the baseline platelet counts (<15 × 104/μL) in CHC patients with thrombocytopenia increased by 1.1- to 1.3-fold at the end of treatment (EOT) with DAA [Citation8–11].
Recent studies report that anti-GPIIb/IIIa antibodies are the most prevalent in HCV-associated ITP, and patients with HCV-associated ITP are more likely to be positive for antiplatelet antibodies than patients with primary ITP [Citation12]. Moreover, antiplatelet antibodies are strongly associated with severe thrombocytopenia [Citation12–14]. Molecular mimicry between HCV proteins and GPIIIa exists, and cross-reactivity of anti-HCV antibodies with platelets is reportedly involved in the pathogenesis of ITP with HCV infection [Citation14,Citation15]. Anti-GPIIb/IIIa antibody-producing B cells are also detected in liver cirrhosis, and their frequency negatively correlates with the platelet count [Citation16]. Our recent study also demonstrated the association between the frequency of anti-GPIIb/IIIa antibody-producing B cells and platelet count changes induced by treatment with the TPO receptor agonist, lusutrombopag, in patients with liver disease [Citation17]. However, no study has yet analyzed changes in the frequency of anti-GPIIb/IIIa antibody-producing B cells associated with DAA treatment for CHC.
To evaluate whether HCV elimination with DAA treatment changes the autoimmune response to platelets and the platelet count, we examined changes in anti-GPIIb/IIIa antibody-producing B cells and the level of B-cell-activating factor (BAFF), a proliferation-inducing ligand (APRIL), and interleukin (IL)-21, cytokines strongly associated with autoantibody production [Citation18–20], following treatment with DAA in patients with CHC.
Methods
Ethical approval
Samples and clinical information were collected from patients and healthy controls who provided written informed consent. All procedures conformed to the ethics protocol of the Kitasato University School of Medicine/Hospital Institutional Review Boards (approval Nos: C17–288 and B18–095) and the 1975 Declaration of Helsinki (revised in 2008). This study was registered at the UMIN Clinical Trials Registry (UMIN: 000030968).
Study participants
This prospective study was conducted at a single center in Japan. We enrolled patients who had plasma HCV RNA levels >2 log10 IU/mL at screening and for at least six months prior to enrollment. The eligibility criteria included treatment-naïve patients or patients previously treated with a DAA-based therapy. Patients with comorbid decompensated cirrhosis or liver cancer and those with superinfection with hepatitis B virus or human immunodeficiency virus were excluded. Based on the inclusion criteria, we recruited 28 patients with CHC and 30 healthy individuals (control group) to determine the frequency of anti-GPIIb/IIIa antibody-producing B cells and the level of BAFF, APRIL, and IL-21.
Peripheral blood mononuclear cells (PBMCs) and plasma preparation
PBMCs were separated from heparinized peripheral blood by density gradient centrifugation using Lymphoprep (Axis-Shield, Oslo, Norway). Platelet-poor plasma was harvested from subjects and stored at − 30°C until use.
Quantification of anti-GPIIb/IIIa antibody-producing B cells
Platelet-associated IgG (PAIgG) was measured by detecting IgG components bound to platelets, whereas the enzyme-linked immunospot (ELISPOT) assay was used for detecting B cells producing anti-GPIIb/IIIa antibodies instead of directly measuring antiplatelet antibodies [Citation21]. The ELISPOT method has greater sensitivity and specificity than other methods and is therefore considered an effective diagnostic tool for ITP [Citation21,Citation22]. B cells producing IgG anti-GPIIb/IIIa antibodies in the peripheral blood were quantified using an ELISPOT assay, as previously described [Citation17,Citation21,Citation22]. The number of anti-GPIIb/IIIa antibody-producing B cells was expressed as the number per 105 PBMCs.
Quantification of plasma TPO, BAFF, APRIL, and IL-21 levels
TPO, BAFF, APRIL, and IL-21 levels in platelet-poor plasma were quantified using Human Thrombopoietin ELISA Kit (CUSABIO, Wuhan, China), Human BAFF Quantikine ELISA Kit (R&D Systems, Minneapolis, MN, USA), APRIL ELISA kit (Thermo Fisher Scientific, Waltham, MA, USA), and IL-21 ELISA kit (R&D Systems), respectively, according to manufacturer instructions.
Endpoints
We evaluated improvements in the platelet count and frequency of anti-GPIIb/IIIa antibody-producing B cells in patients with CHC to determine the influence of DAA on the autoimmune response to platelets and the elimination of HCV. Additionally, we evaluated the association between the autoimmune response and the presence of thrombocytopenia (platelet count <10 × 104/μL) in patients with CHC. Patients were divided into two groups with or without thrombocytopenia at baseline to examine DAA-treatment-associated changes in platelet count and the frequency of anti-GPIIb/IIIa antibody-producing B cells.
Statistical analysis
All continuous variables were presented as median range and compared using the Mann–Whitney U test or the Wilcoxon test. Spearman’s correlation coefficient was determined for correlation analysis. Statistical analyses were performed using GraphPad Prism software v.9.3.1 for Mac (GraphPad Software, San Diego, CA, USA). Results were considered statistically significant at p values <0.05.
Results
Patient characteristics
The baseline clinical characteristics of the patients with CHC (n = 28) are summarized in . The median age of the patients was 68 years, 37–82 years, and 19 patients were female (67.9%). Four patients (14.8%) had previously been treated with a DAA-based therapy, and ten patients had liver cirrhosis. Thrombocytopenia (platelet count <10 × 104/μL) was present in ten patients.
Table I. Baseline clinical characteristics of patients with chronic hepatitis C (n = 28).
Virological response
Elbasvir plus grazoprevir, glecaprevir plus pibrentasvir, and sofosbuvir plus velpatasvir were administered orally to 7, 19, and 2 patients with HCV, respectively. HCV RNA level at baseline was 5.9 log10 IU/mL (4.4–7.3 log10 IU/mL). The median HCV RNA at baseline was 5.35 log10 IU/mL (4.5–6.4 log10 IU/mL) in CHC patients with thrombocytopenia and 5.95 log10 IU/mL (4.4–7.3 log10 IU/mL) in CHC patients without thrombocytopenia, with no significant difference between the two groups (p = .065). All patients received DAA as a scheduled treatment, and the median decrease in plasma HCV RNA levels between baseline and week four was 5.1 log10 IU/mL. HCV RNA was undetectable at the EOT in all patients.
Changes in laboratory findings and thrombopoietin levels at baseline and EOT
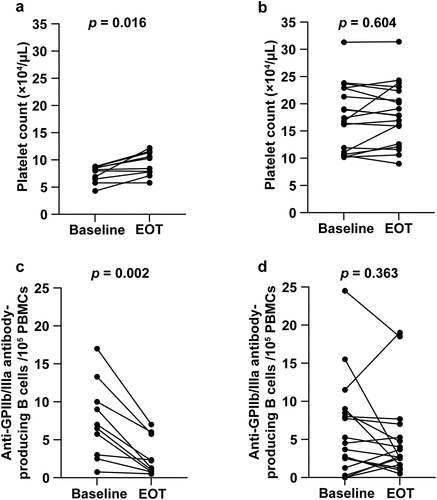
The changes in laboratory findings and thrombopoietin levels at the baseline and EOT are summarized in . A significant improvement in platelet count from the baseline to EOT was observed in CHC patients with thrombocytopenia [median (range), 8.45 (4.3–8.8) × 104/μL vs. 9.35 (5.8–12.2) × 104/μL; p = .016). In addition, the levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were significantly improved from the baseline to EOT in patients with CHC with or without thrombocytopenia. However, no correlation between AST or ALT and platelet count was observed at baseline (r = 0.249, p = .249 or r = 0.231, p = .519) and EOT (r = −0.488, p = .155 or r = −0.384, p = .272) in CHC patients with thrombocytopenia. In contrast, AST and platelet count were negatively correlated at the baseline (r = −0.778, p < .001) and EOT (r = −0.674, p = .002) in patients with CHC without thrombocytopenia; ALT was negatively correlated with platelet count only at the baseline in CHC patients without thrombocytopenia (r = −0.643, p = .004). Other laboratory data, including plasma TPO levels, showed no significant improvement.
Table II. Changes in laboratory findings and thrombopoietin levels at baseline and end of treatment (EOT) with direct-acting antiviral agents (DAA).
Frequency of anti-GPIIb/IIIa antibody-producing B cells in patients with CHC
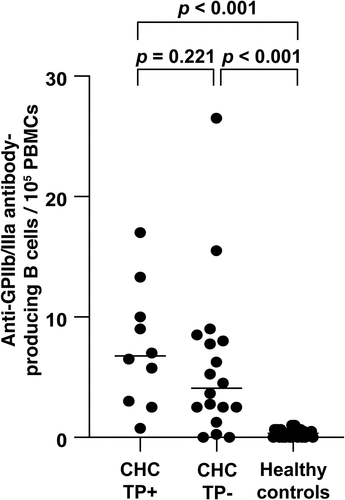
The frequency of anti-GPIIb/IIIa antibody-producing B cells was significantly higher in patients with CHC at baseline than that in healthy controls (5.5 (0–26.5)/105 PBMCs vs. 0.3 (0–0.67)/105 PBMCs, p < .001). Although the frequency of anti-GPIIb/IIIa antibody-producing B cells in CHC patients with thrombocytopenia [6.75 (0.75–17.0)/105 PBMCs] or without thrombocytopenia [4.09 (0–26.5)/105 PBMCs] at baseline was significantly higher than that in healthy controls [0.3 (0–0.67)/105 PBMCs] (p < .001), no significant difference was observed in the number of anti-GPIIb/IIIa antibody-producing B cells ().
DAA-treatment-associated changes in platelet count and the frequency of anti-GPIIb/IIIa antibody-producing B cells in patients with CHC
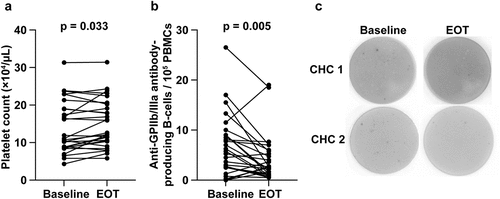
The platelet count increased significantly from baseline to EOT [10.9 (4.3–31.3) × 104/μL vs. 12.4 (5.8–31.4) × 104/μL, p = .033; ], and the frequency of anti-GPIIb/IIIa antibody-producing B cells decreased significantly from baseline to EOT [5.5 (0–26.5)/105 PBMCs vs. 2.6 (0.5–19.0)/105 PBMCs, p = .005; ]. shows a typical example of baseline and EOT assessments of anti-GPIIb/IIIa antibody-producing B cells, using ELISPOT.
Figure 2. Changes in the (a) platelet count and (b) number of anti-GPIIb/IIIa antibody-producing cells before and after DAA treatment. (c) Enzyme-linked immunospot data for anti-GPIIb/IIIa antibody-producing cells at baseline and after DAA therapy are shown.
The thrombocytopenia group demonstrated a significant improvement in platelet count from baseline to EOT [8.45 (4.3–8.8) × 104/μL vs. 9.35 (5.8–12.2) × 104/μL; p = .016; ]. In contrast, patients without thrombocytopenia showed no change in platelet count from baseline to EOT [17.1 (10.2–31.3) × 104/μL vs. 17.85 (9.0–31.4) × 104/μL; p = .604; ]. The frequency of anti-GPIIb/IIIa antibody-producing B cells decreased significantly from baseline to EOT in the thrombocytopenia group [6.75 (0.75–17.0)/105 PBMCs vs. 1.75 (0.5–7.0)/105 PBMCs; p = .002; ]. In contrast, patients without thrombocytopenia showed no change in anti-GPIIb/IIIa antibody-producing B cells from baseline to EOT [4.09 (0–26.5)/105 PBMCs vs. 2.71 (0.5–19.0)/105 PBMCs; p = .363; ].
DAA-treatment-associated changes in plasma BAFF, APRIL, and IL-21 levels in patients with CHC
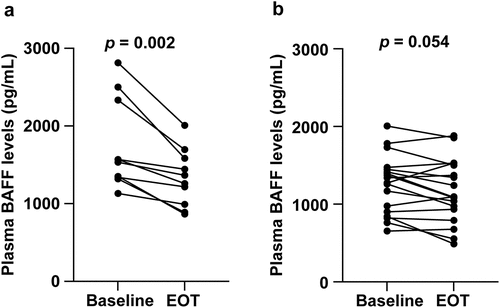
Because the frequency of anti-GPIIb/IIIa antibody-producing B cells was reduced after DAA treatment, we measured plasma levels of the cytokines BAFF, APRIL, and IL-21, which are involved in B cell proliferation, differentiation, and antibody production. Plasma BAFF and APRIL levels at baseline were significantly higher (p < .001) in patients with CHC [1361.4 (653.6–2813.7) pg/mL and 6.44 (2.18–50.0) ng/mL, respectively] than that in healthy controls [868.0 (431.2–1290.3) pg/mL and 2.39 (0.86–5.84) ng/mL, respectively]. Plasma IL-21 levels at baseline were not significantly different between patients with CHC and healthy controls [16.5 (15.6–929.8) pg/mL vs. 38.7 (15.6–808.3) pg/mL; p = .08]. Additionally, plasma BAFF levels of patients with CHC decreased significantly from baseline to EOT [1344.6 (653.6–2813.7) pg/mL vs. 1229.0 (489.3–2008.1) pg/mL; p < .001; ]. In contrast, DAA treatment did not significantly affect plasma APRIL [baseline vs. EOT: 6.44 (2.18–50.0) ng/mL vs. 6.34 (2.16–48.5) ng/mL; p = .061; ] and IL-21 [baseline vs. EOT: 16.5 (15.6–929.8) pg/mL vs. 15.6 (15.6–970.6) pg/mL; p = .188; ] levels. Furthermore, a significant positive correlation between anti-GPIIb/IIIa antibody-producing B cells and plasma BAFF levels was observed in patients with CHC at the baseline (r = 0.508, p = .006) and in patients with (r = 0.669, p = .039; ) and without thrombocytopenia (r = 0.482, p = .043; ).
Figure 4. Changes in (a) plasma BAFF, (b) APRIL, and (c) IL-21 levels after DAA treatment. (d, e) Correlation between the number of anti-GPIIb/IIIa antibody-producing cells and plasma BAFF levels before DAA treatment. Groups (d) with and (e) without thrombocytopenia at baseline.
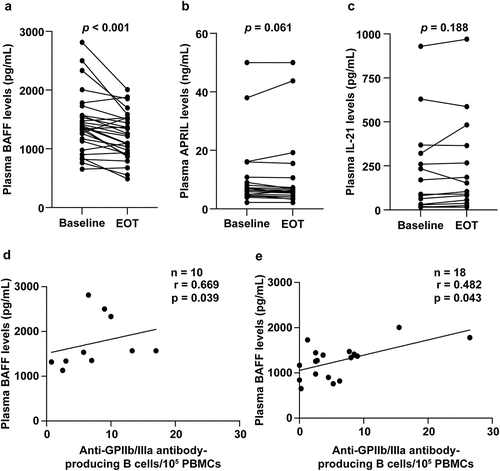
We then examined DAA-treatment-associated changes in plasma BAFF levels in patients with thrombocytopenia at baseline and those without thrombocytopenia. Plasma BAFF levels decreased significantly from baseline to EOT in the thrombocytopenia group [1551.3 (1130.8–2813.7) pg/mL vs. 1312.0 (867.0–2008.1) pg/mL; p = .002; ]. In contrast, although the plasma BAFF level was reduced from the baseline to EOT in the group of patients without thrombocytopenia, the reduction was not significant [1306.1 (653.6–2005.2) pg/mL vs. 1092.7 (489.3–1527.6) pg/mL; p = .054; ].
Discussion
This is the first study reporting that DAA treatment reduces the frequency of anti-GPIIb/IIIa antibody-producing B cells and reduces plasma BAFF levels in CHC patients with thrombocytopenia. In this study, we hypothesized that the elimination of HCV with DAA treatment would improve autoimmune response to platelets.
In previous reports, the platelet counts in CHC patients with thrombocytopenia (platelet count <15 × 104/μL) have been shown to increase by 1.1- to 1.3-fold following DAA treatment [Citation8–11]. DAA treatment has also been shown to increase the platelet count by 1.1-fold in patients with CHC with platelet counts <10 × 104/μL and >10 × 104/μL at baseline [Citation23]. In this study, thrombocytopenia was defined as a platelet count of <10 × 104/μL to examine changes in anti-GPIIb/IIIa antibody-producing cells. DAA treatment increased platelet counts in patients with CHC with thrombocytopenia but not in those without thrombocytopenia, suggesting that the underlying cause of thrombocytopenia reduced with HCV elimination.
In patients with CHC, DAA treatment improved AST and ALT but not plasma TPO levels. In addition, no correlation between AST or ALT and platelet counts was observed in patients with thrombocytopenia. These results suggest that thrombocytopenia is related to factors other than liver dysfunction. In this study, we considered the possibility that anti-GPIIb/IIIa antibody production due to HCV infection may induce thrombocytopenia.
In CHC patients with thrombocytopenia, DAA treatment reduced the frequency of anti-GPIIb/IIIa antibody-producing B cells. The level of anti-GPIIb/IIIa antibody-producing B cells in peripheral blood reflects autoimmune pathology in ITP; therefore, measuring this level is effective for assessing treatment responsiveness [Citation24]. The present study showed that measuring anti-GPIIb/IIIa antibody-producing B cells is also effective for assessing responsiveness to DAA treatment in patients with CHC. Previous reports implicated antiplatelet antibodies as a possible cause of thrombocytopenia in CHC [Citation12–14] and have shown that PAIgG diminished at EOT in patients with HCV-related liver disease [Citation25]. These studies suggest that anti-GPIIb/IIIa antibodies are one of the causes of thrombocytopenia in CHC. Furthermore, the elimination of HCV by DAA treatment potentially reduces the autoimmune response to platelets, resulting in an improved platelet count.
Although PAIgG includes autoantibodies against platelet membrane GPs, IgGs that bind nonspecifically to platelets are simultaneously detected; consequently, PAIgG levels are also sometimes elevated in nonimmune thrombocytopenia. Therefore, American diagnostic guidelines consider PAIgG “unnecessary and inappropriate” for diagnosing ITP [Citation26]. In the present study, the direct measurement of anti-GPIIb/IIIa antibody-producing B cells potentially compensates for the limitations of PAIgG testing. According to previous studies, anti-GPIIb/IIIa antibodies are the most commonly found antibodies in HCV-associated ITP [Citation12–14]. It has been shown that HCV infects not only liver cells but also B cells, resulting in the production of autoantibodies; this may represent the mechanism by which ITP is elicited [Citation27]. Therefore, given the significant involvement of B cells in the pathology of thrombocytopenia in CHC, the present finding of DAA-treatment-induced changes in anti-GPIIb/IIIa antibody-producing B cells in CHC patients with thrombocytopenia represents an important novel finding.
Anti-GPIIb/IIIa antibodies in patients with ITP have been reported to be primarily IgG1 antibodies [Citation28]. However, anti-GPIIb/IIIa antibodies in acquired Glanzmann’s thrombasthenia have been detected to be IgG2 or IgG4, and these autoantibodies have been reported to cause abnormal platelet function [Citation29]. IgG1 antibodies have a high affinity for the Fc receptor on macrophages. In contrast, IgG2 and IgG4 antibodies have low affinity for the Fc receptor on macrophages [Citation30], which may result in inadequate phagocytosis of antibody-bound platelets. Some CHC patients without thrombocytopenia showed a high frequency of anti-GPIIb/IIIa antibody-producing B cells before treatment, which was reduced after treatment. This result could be related to the immunoglobulin subclass of anti-GPIIb/IIIa antibodies. However, future studies should clarify the role of anti-GPIIb/IIIa antibodies by measuring the subclasses of anti-GPIIb/IIIa antibodies found in patients with CHC.
BAFF is a cytokine generated in various immune cells and plays an important role in B cell proliferation, differentiation, and antibody production [Citation31]. In this study, we identified elevated plasma BAFF levels at baseline that subsequently decreased at EOT in patients with CHC. A positive correlation was also observed between plasma BAFF levels and antibody-producing cell frequency at baseline. Additionally, plasma BAFF levels in patients with thrombocytopenia were considerably reduced at EOT compared to that at baseline. Consistent with the present study, previous studies have shown that BAFF levels are elevated in patients with CHC, suggesting that it has a crucial role in HCV-associated autoimmunity [Citation32]. Furthermore, BAFF levels are higher in patients with CHC with antinuclear antibodies than in those without antinuclear antibodies [Citation33]. In our recent study, we found that plasma BAFF levels were elevated in patients with liver cirrhosis with thrombocytopenia and were positively correlated with the number of anti-GPIIb/IIIa antibody-producing B cells [Citation34]. In contrast, in a previous report, BAFF mRNA expression measured by RT-PCR using PBMCs from patients with hepatitis C was increased after DAA treatment compared to that before treatment [Citation35]. The differences in the results of this study and our present study could be attributed to differences in the measured objects and measurement methods. The present study is the first to determine that plasma BAFF levels decline following DAA treatment, which may be important for understanding the autoimmune mechanisms in HCV-associated ITP. Therefore, it can be inferred that the activation of immunocompetent cells by HCV infection induces the production of BAFF.
In contrast, no changes in plasma APRIL levels were observed with DAA treatment, suggesting that the higher APRIL levels may not be directly related to HCV infection. Consistent with our study, a recent study in patients with ITP showed increased expression of APRIL in platelets, but its concentration in plasma did not differ after treatment, even in patients who had complete remission with treatment [Citation36]. However, in thrombocytopenia involving autoantibodies, examining the expression of APRIL in platelets may provide better insights into the association with APRIL. Therefore, analysis of DAA treatment-induced changes in APRIL expression in platelets needs to be considered in the future. In this study, plasma IL-21 levels did not differ significantly between healthy controls and patients with CHC. This suggests that IL-21 production is unlikely to be induced by HCV infection and that IL-21 levels are not altered by DAA treatment.
It has been reported that in CHC, interferon (IFN)-α/β and IFN-γ produced by dendritic cells in the liver are involved in the HCV-elimination mechanism [Citation37]. These inflammatory cytokines act on innate immune cells and induce BAFF production [Citation20,Citation38,Citation39], suggesting that in CHC, the increased BAFF levels associated with HCV infection promote the survival of autoreactive B cells and the induction of anti-GPIIb/IIIa antibody-producing B cells. Furthermore, elimination of HCV by DAA treatment inhibits the production of IFN-γ;[Citation40], therefore, the associated normalization of BAFF levels might have led to a decrease in the autoreactive anti-GPIIb/IIIa antibody-producing B cells.
This study has some limitations. First, the number of CHC patients with thrombocytopenia was low, and there were no patients with severe thrombocytopenia. Additionally, although samples were obtained immediately following the end of DAA treatment, subsequent follow-up samples were not obtained. A larger cohort of patients might enable suitable determination of an autoimmune response to platelets before and after DAA treatment. Future studies should consider a more detailed analysis of the cells involved in regulating autoantibody-producing B cells, such as changes in the percentage of memory B cells and plasmablasts in peripheral blood due to DAA treatment of CHC.
In conclusion, DAA treatment of patients with CHC reduced the frequency of anti-GPIIb/IIIa antibody-producing B cells. This reduction was seen not only in CHC patients with thrombocytopenia but also in other CHC patients whose platelet counts improved with treatment. These results suggest that anti-GPIIb/IIIa antibody-producing B cells may predict improved platelet counts following DAA therapy in CHC patients with thrombocytopenia. Additionally, the plasma level of BAFF, which is involved in B cell proliferation, survival, differentiation, and antibody production, was reduced by DAA treatment, which could be due to the elimination of HCV. Collectively, these findings contribute to our understanding of the mechanism of HCV-associated immune thrombocytopenia mediated by antiplatelet antibodies.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Peck-Radosavljevic M. Thrombocytopenia in chronic liver disease. Liver Int. 2017;37(6):778–9. doi:10.1111/liv.13317. PMID: 27860293.
- Rawi S, Wu GY. Pathogenesis of thrombocytopenia in chronic HCV infection: a review. J Clin Transl Hepatol. 2020;8(2):184–191. doi:10.14218/JCTH.2020.00007. PMID: 32832399.
- Kuwana M, Okazaki Y, Ikeda Y. Detection of circulating B cells producing anti-GPIb autoantibodies in patients with immune thrombocytopenia. PLoS One. 2014;9(1):e86943. doi:10.1371/journal.pone.0086943. PMID: 24466297.
- Nazy I, Kelton JG, Moore JC, Clare R, Horsewood P, Smith JW, Ivetic N, D’Souza V, Li N, Arnold DM. Autoantibodies to thrombopoietin and the thrombopoietin receptor in patients with immune thrombocytopenia. Br J Haematol. 2018;181(2):234–241. doi:10.1111/bjh.15165. PMID: 29532903.
- Liebman H. Other immune thrombocytopenias. Semin Hematol. 2007;44:S24–34. doi:10.1053/j.seminhematol.2007.11.004. PMID: 18096469.
- Chiao EY, Engels EA, Kramer JR, Pietz K, Henderson L, Giordano TP, Landgren O. Risk of immune thrombocytopenic purpura and autoimmune hemolytic anemia among 120 908 US veterans with hepatitis C virus infection. Arch Intern Med. 2009;169(4):357–363. doi:10.1001/archinternmed.2008.576. PMID: 19237719.
- Ioannou GN, Feld JJ. What are the benefits of a sustained virologic response to direct-acting antiviral therapy for hepatitis c virus infection? Gastroenterology. 2019;156(2):446–460. doi:10.1053/j.gastro.2018.10.033.
- Ishizu Y, Ishigami M, Hayashi K, Honda T, Kuzuya T, Ito T, Fujishiro M. Rapid increase of platelet counts during antiviral therapy in patients with hepatitis C virus infection. Hepatol Res. 2020;50(1):47–56. doi:10.1111/hepr.13426. PMID: 31496023.
- Chen YC, Tseng CW, Tseng KC. Rapid platelet count improvement in chronic hepatitis C patients with thrombocytopenia receiving direct-acting antiviral agents. Med (Baltimore). 2020;99(19):e20156. doi:10.1097/MD.0000000000020156. PMID: 32384505.
- Saif-Al-Islam M, Abdelaal UM, Younis MA, Alghany Algahlan HA, Khalaf S. Effect of direct-acting antiviral therapy on thrombocytopenic patients with hepatitis C virus-related chronic liver disease. Gastroenterol Res Pract. 2021;2021:8811203. doi:10.1155/2021/8811203. PMID: 34122539.
- Chen YC, Chang TS, Chen CH, Cheng PN, Lo CC, Mo LR, Chen CT, Huang CF, Kuo HT, Huang YH, et al. Factors associated with significant platelet count improvement in thrombocytopenic chronic hepatitis c patients receiving direct-acting antivirals. Viruses. 2022;14(2):333. doi:10.3390/v14020333. PMID: 35215926.
- Huang CE, Chen WM, Wu YY, Shen CH, Hsu CC, Li CP, Chen MC, Chang JJ, Chen YY, Lu CH, et al. Comparison of antiplatelet antibody profiles between hepatitis C virus-associated immune thrombocytopenia and primary immune thrombocytopenia. Platelets. 2021;32(8):1043–1050. doi:10.1080/09537104.2020.1820975. PMID: 32967492.
- Aref S, Sleem T, El Menshawy N, Ebrahiem L, Abdella D, Fouda M, Samara NA, Menessy A, Abdel-Ghaffar H, Bassam A, et al. Antiplatelet antibodies contribute to thrombocytopenia associated with chronic hepatitis C virus infection. Hematology. 2009;14(5):277–281. doi:10.1179/102453309X439818. PMID: 19843383.
- Satoh T, Kuwana M. Differential Diagnosis: Secondary ITP. In: Ishida Y Tomiyama Y, editors. Autoimmune thrombocytopenia. Singapore: Springer Nature; 2017; pp. 97–105.
- Zhang W, Nardi MA, Borkowsky W, Li Z, Karpatkin S. Role of molecular mimicry of hepatitis C virus protein with platelet GPIIIa in hepatitis C–related immunologic thrombocytopenia. Blood. 2009;113(17):4086–4093. doi:10.1182/blood-2008-09-181073. PMID: 19023115.
- Kajihara M, Kato S, Okazaki Y, Kawakami Y, Ishii H, Ikeda Y, Kuwana M. A role of autoantibody-mediated platelet destruction in thrombocytopenia in patients with cirrhosis. Hepatology. 2003;37(6):1267–1276. doi:10.1053/jhep.2003.50209. PMID: 12774004.
- Wada N, Uojima H, Satoh T, Okina S, Iwasaki S, Shao X, Takiguchi H, Arase Y, Itokawa N, Atsukawa M, et al. Impact of anti-GPIIb/IIIa antibody-producing B cells as a predictor of the response to lusutrombopag in thrombocytopenic patients with liver disease. Dig Dis. 2021;39(3):234–242. doi:10.1159/000510692. PMID: 32759604.
- Baert L, Manfroi B, Casez O, Sturm N, Huard B. The role of APRIL - a proliferation inducing ligand - in autoimmune diseases and expectations from its targeting. J Autoimmun. 2018;95:179–190. doi:10.1016/j.jaut.2018.10.016. PMID: 30385081.
- Long D, Chen Y, Wu H, Zhao M, Lu Q. Clinical significance and immunobiology of IL-21 in autoimmunity. J Autoimmun. 2019;99:1–14. doi:10.1016/j.jaut.2019.01.013. PMID: 30773373.
- Zhang Y, Tian J, Xiao F, Zheng L, Zhu X, Wu L, Zhao C, Wang S, Rui K, Zou H, et al. B cell-activating factor and its targeted therapy in autoimmune diseases. Cytokine Growth Factor Rev. 2022;64:57–70. doi:10.1016/j.cytogfr.2021.11.004. PMID: 34916133.
- Kuwana M, Okazaki Y, Satoh T, Asahi A, Kajihara M, Ikeda Y. Initial laboratory findings useful for predicting the diagnosis of idiopathic thrombocytopenic purpura. Am J Med. 2005;118(9):1026–1033. doi:10.1016/j.amjmed.2004.12.027. PMID: 16164890.
- Kuwana M, Kurata Y, Fujimura K, Fujisawa K, Wada H, Nagasawa T, Nomura S, Kojima T, Yagi H, Ikeda Y. Preliminary laboratory based diagnostic criteria for immune thrombocytopenic purpura: evaluation by multi-center prospective study. J Thromb Haemost. 2006;4(9):1936–1943. doi:10.1111/j.1538-7836.2006.02091.x. PMID: 16961601.
- Soliman Z, El Kassas M, Elsharkawy A, Elbadry M, Hamada Y, ElHusseiny R, El-Nahaas SM, Fouad R, Esmat G, Abdel Alem S. Improvement of platelet in thrombocytopenic HCV patients after treatment with direct-acting antiviral agents and its relation to outcome. Platelets. 2021;32(3):383–390. doi:10.1080/09537104.2020.1742313. PMID: 32250721.
- Kuwana M, Nomura S, Fujimura K, Nagasawa T, Muto Y, Kurata Y, Tanaka S, Ikeda Y. Effect of a single injection of humanized anti-CD154 monoclonal antibody on the platelet-specific autoimmune response in patients with immune thrombocytopenic purpura. Blood. 2004;103(4):1229–1236. doi:10.1182/blood-2003-06-2167. PMID: 14551140.
- Honma Y, Shibata M, Hayashi T, Kusanaga M, Ogino N, Minami S, Kumei S, Oe S, Miyagawa K, Senju M, et al. Effect of direct-acting antivirals on platelet-associated immunoglobulin G and thrombocytopenia in hepatitis C virus-related chronic liver disease. Liver Int. 2019;39(9):1641–1651. doi:10.1111/liv.14120. PMID: 31009141.
- George JN, Woolf SH, Raskob GE, Wasser JS, Aledort LM, Ballem PJ, Blanchette VS, Bussel JB, Cines DB, Kelton JG, et al. Idiopathic thrombocytopenic purpura: a practice guideline developed by explicit methods for the American society of hematology. Blood. 1996;88(1):3–40. doi:10.1182/blood.V88.1.3.3. PMID: 8704187.
- Pockros PJ, Duchini A, McMillan R, Nyberg LM, McHutchison J, Viernes E. Immune thrombocytopenic purpura in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 2002;97(8):2040–2045. doi:10.1111/j.1572-0241.2002.05845.x. PMID: 12190174.
- Chan H, Moore JC, Finch CN, Warkentin TE, Kelton JG. The IgG subclasses of platelet-associated autoantibodies directed against platelet glycoproteins IIb/IIIa in patients with idiopathic thrombocytopenic purpura. Br J Haematol. 2003;122(5):818–824. doi:10.1046/j.1365-2141.2003.04509.x. PMID: 12930395.
- Porcelijn L, Huiskes E, Maatman R, de Kreuk A, de Haas M. Acquired glanzmann’s thrombasthenia caused by glycoprotein IIb/IIIa autoantibodies of the immunoglobulin G1 (IgG1), IgG2 or IgG4 subclass: a study in six cases. Vox Sang. 2008;95(4):324–330. doi:10.1111/j.1423-0410.2008.01093.x. PMID: 19138263.
- Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520. doi:10.3389/fimmu.2014.00520. PMID: 25368619.
- Lake-Bakaar G, Jacobson I, Talal A. B cell activating factor (BAFF) in the natural history of chronic hepatitis C virus liver disease and mixed cryoglobulinaemia. Clin Exp Immunol. 2012;170(2):231–237. doi:10.1111/j.1365-2249.2012.04653.x. PMID: 23039894.
- Toubi E, Gordon S, Kessel A, Rosner I, Rozenbaum M, Shoenfeld Y, Zuckerman E. Elevated serum B-lymphocyte activating factor (BAFF) in chronic hepatitis C virus infection: association with autoimmunity. J Autoimmun. 2006;27(2):134–139. doi:10.1016/j.jaut.2006.07.005. PMID: 17029886.
- Himoto T, Fujita K, Nomura T, Tani J, Morishita A, Yoneyama H, Haba R, Masaki T. Verification of B-lymphocyte activating factor’s involvement in the exacerbation of insulin resistance as well as an autoimmune response in patients with nonalcoholic steatohepatitis and patients with HCV-related chronic liver disease. Diabetol Metab Syndr. 2017;9(1):45. doi:10.1186/s13098-017-0243-z. PMID: 28630652.
- Satoh T, Takiguchi H, Uojima H, Kubo M, Tanaka C, Yokoyama F, Wada N, Miyazaki K, Hidaka H, Kusano C, et al. B cell-activating factor is involved in thrombocytopenia in patients with liver cirrhosis. Ann Hematol. 2022;101(11):2433–2444. doi:10.1007/s00277-022-04973-x. PMID: 36098792.
- Hegazy MT, Allam WR, Hussein MA, Zoheir N, Quartuccio L, El-Khamisy SF, Ragab G. Mediterranean consortium for the study of cryoglobulinemic vasculitis. Increased genomic instability following treatment with direct acting anti-hepatitis C virus drugs. EBioMedicine.2018;35:106–113. doi:10.1016/j.ebiom.2018.08.007. PMID: 30139628.
- Hao YF, Bi H, Li HY, Yin LM, Yu JX, Tao W, Mu HL, Yang RC, Zhou ZP, Tai WL, et al. Aberrant expression of a proliferation-inducing ligand underlies autoimmune mechanisms in immune thrombocytopenia. J Immunology Res. 2021;2021:3676942. doi:10.1155/2021/3676942. PMID: 33564689.
- Yoshio S, Kanto T. Host–virus interactions in hepatitis B and hepatitis C infection. J Gastoenterol. 2016;51(5):409–420. doi:10.1007/s00535-016-1183-3. PMID: 26894594.
- Mackay F, Browning JL. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol. 2002;2(7):465–475. doi:10.1038/nri844. PMID: 12094221.
- Litinskiy MB, Nardelli B, Hillbert DM, He B, Scheffer A, Casali P, Cerutti A. Dcs induce CD40-independent immunoglobulin class switching through BLys and APRIL. Nat Immunol. 2002;3(9):822–829. doi:10.1038/ni829. PMID: 12154359.
- Nabeel MM, Darwish RK, Alakel W, Maher R, Mostafa H, Hashem A, Elbeshlawy M, Abul-Fotouh A, Shousha HI, Saeed Marie M. Changes in serum interferon gamma and interleukin-10 in relation to direct-acting antiviral therapy of chronic hepatitis C genotype 4: a pilot study. J Clin Exp Hepatol. 2022;12(2):428–434. doi:10.1016/j.jceh.2021.06.018. PMID: 35535108.