Abstract
Multi-omics approaches are being used increasingly to study physiological and pathophysiologic processes. Proteomics specifically focuses on the study of proteins as functional elements and key contributors to, and markers of the phenotype, as well as targets for diagnostic and therapeutic approaches. Depending on the condition, the plasma proteome can mirror the platelet proteome, and hence play an important role in elucidating both physiologic and pathologic processes. In fact, both plasma and platelet protein signatures have been shown to be important in the setting of thrombosis-prone disease states such as atherosclerosis and cancer. Plasma and platelet proteomes are increasingly being studied as a part of a single entity, as is the case with patient-centric sample collection approaches such as capillary blood. Future studies should cut across the plasma and platelet proteome silos, taking advantage of the vast knowledge available when they are considered as part of the same studies, rather than studied as distinct entities.
Plain Language Summary
Platelets are key cellular elements of blood with plasma constituting the liquid component. Both platelets and proteins found in plasma rapidly work in unity to prevent/limit blood loss in response to blood vessel damage. Proteomics is the analysis of the entire protein complement of a cell, tissue, or organism under a specific, defined set of conditions. Of note, research to date has shown that platelet and plasma proteomes share many common proteins. In some disease scenarios, plasma proteomes can be used to identify platelet function or dysfunction, while in other scenarios, platelet-specific proteins are needed for physiological assessment. Thus, it may be beneficial to simultaneously study the plasma and platelet proteomes, thereby exploiting the considerable wealth of information provided under such circumstances.
Introduction
The days when features, elements, and entities of disease were explored in isolation are gradually waning. Improvements in technology (e.g. decreased sample volume, increase in throughput and automation, evolution of data analysis software – ability to generate and analyze big data, decrease in cost) have reduced the need for research laboratories to be highly specialized in order to make significant scientific and societal contributions. Such technological advances have led to increased use of multi-omics approaches, including proteomics, genomics, epigenomics, transcriptomics, lipidomics and metabolomics, as the means to obtain a global perspective of a particular scenario, whether disease, treatment, or outcome-based. Omics approaches facilitate the understanding of disease pathophysiology, identification of disease biomarkers, as well as markers associated with therapeutic response and/or with specific clinical outcomes.
This review focuses on proteomics, as the central omics approach, that studies proteins as functional elements ultimately responsible for a specific phenotype, and will specifically explore the similarities and differences between the plasma and platelet proteomes, and how these unique milieus/environments can be utilized to obtain a more granular scenario of the specific phenotype and interactions in blood.
Proteomics and the proteome
Proteomics is a methodological approach that allows for the simultaneous analysis of hundreds and sometimes thousands of proteins in an extremely small sample volume (e.g. 2 µL) and within an extremely rapid time-frame (e.g. 15 min sample run). Common proteomics techniques include untargeted and targeted mass spectroscopy, as well as targeted approaches such as the Olink® near proximity assay, among others. The main methods of choice for large-scale proteomic analyses are liquid chromatography followed by mass spectroscopy due to its unparalleled specificity and throughput, as well as the Olink® proximity extension assay due to its specificity and sensitivity. Approaches such as 2D gels and ELISA are no longer competitive, especially in the translational research and large-scale study landscape due to significant limitations such as extremely low throughput and inter-user dependence as is the case for 2D gels, as well as extremely low specificity as is the case for ELISA. The focus on proteins (rather than genes) as the key contributors to, and markers of, the phenotype and as key targets for therapeutic approaches is extremely important, as not all genetic changes lead to changes in protein concentration and function. In fact, the proteome is constantly changing with age, diseases, and therapies. In this context, the proteome describes proteins expressed in a certain biological fluid (e.g. blood, saliva), tissue (e.g. liver, kidney), or cell (e.g. platelets, neutrophils), with proteomics as a methodological approach that enables us to understand the phenotype of any given proteome. This provides comprehensive physiological and pathophysiological insights, especially in the context of translation of research findings into patient-specific benefits.
For the purpose of this review, we will focus on the plasma and the platelet proteomes as important components of the blood proteome.
Plasma vs serum: different sides of the same coin
Prior to focusing on the plasma proteome, it is important to distinguish the difference between plasma and serum, and the reasons for focusing on the plasma proteome when discussing platelets. The differential use of plasma versus serum in clinical diagnostics is generally respected, but the differences in specimen types used in proteomic studies can be significant and sometimes overlooked [Citation1,Citation2].
Plasma proteome focuses on the soluble proteins found in whole blood that are typically obtained upon centrifugation of the whole blood sample collected almost exclusively via venipuncture and in collection tubes containing anticoagulants to prevent spontaneous clot formation. Depending on the downstream analysis as well as specific centrifugation and collection techniques, plasma may or may not contain a large proportion of the platelets originally present in whole blood. Plasma that is enriched with platelets is termed platelet-rich plasma (PRP) and plasma that is devoid of platelets is termed platelet poor plasma (PPP) or platelet-free plasma (PFP). For the sake of simplicity, we will refer to plasma lacking platelets as PPP since there can still be a small but non-zero number of platelets present following standard PPP preparation techniques [Citation3]. This is in stark contrast to the serum proteome, which represents the liquid fraction of whole blood that remains following spontaneous or induced clot formation and removal. This process, however, is associated with activation of the coagulation and inflammatory pathways, which alters the protein content in the sample. Such alteration includes removal of the bulk of high abundance proteins such as fibrinogen, platelets that have been activated or formed into aggregates, and removal of other proteins that are either involved in clot formation or physically excluded within the clot [Citation2,Citation4]. As such, PRP and PPP represent an uncompromised, un-manipulated representation of the soluble whole blood protein fraction, and are more optimal when studying patient phenotype and/or biomarkers. The differences in the plasma and serum have been described from both the metabolomic and the exosome perspective [Citation5,Citation6].
Collection technique, pre-analytical handling, and sample-related issues can have significant downstream effects during proteomic analysis. Improper anticoagulant or mixing technique during sample collection can also lead to partial coagulation activation, dramatically affecting proteomic composition of what is expected to be plasma but in fact trends more toward serum protein composition [Citation7]. Anticoagulant type and time to processing post blood draw can have significant effects on the platelet function and platelet protein abundance and composition [Citation8]. In addition, contaminating erythrocyte or platelet proteins, most commonly clusterin, fibrinogen, prothrombin, kininogen, antithrombin-III, and platelet basic protein, can be misidentified as an outlier in analysis and therefore be misused as a biomarker for certain disease states [Citation7]. In one study, platelet, erythrocyte, PRP, and PPP proteomes included 5793, 2069, 1682, and 912 proteins, respectively, highlighting the significant effect that contaminating platelets or erythrocytes could have on what is expected to be a plasma proteome study [Citation7]. Plasma and serum represent extremely different proteomes and should be adequately distinguished during any study, with standardized and rigid collection techniques clearly stated and followed during the study.
Plasma vs platelet proteome: similarities and differences
The platelet proteome can provide information that the plasma proteome alone cannot. While there does exist a substantial overlap in proteins identified in PPP, PRP, and platelet fractions, reported at 20% [Citation9], 50% [Citation10], and up to 73% [Citation11], key platelet proteins may not be identified in PPP alone, such as secreted protein acidic and rich in cysteine (SPARC), calmodulin and calumenin, platelet glycoprotein V, platelet factor 4, type 1 collagen, talin, transforming growth factor beta-1, catalase, superoxide dismutase, platelet basic protein, pigment epithelium-derived factor, and multifunctional Heat shock protein Hsp 70 [Citation10]. Functionally significant proteins such as aminopeptidase N, hepatocyte growth factor-like protein, von Willebrand Factor, and selenoprotein P were found only in the PPP fraction () [Citation10]. A significant number of total proteins from PRP were found to be unique to that fraction (41.5%), whereas the PPP and plasma fractions included 4.8% and 2.9% unique proteins, respectively [Citation10]. Sample purity is vital in proteome analysis of blood components, with erythrocyte and leukocyte depletion necessary for an appropriate proteome analysis [Citation11]. A more systematic proteome characterization that identified approximately 4,000 platelet proteins as part of a platelet proteome protocol found that 12% of those proteins were exclusive to the platelet fraction [Citation11]. The proteins found exclusively in platelets could be vital to discovering aberrant characteristics of dysfunctional platelets through quantitative identification of protein–protein interactions, sub(cellular)-proteomes, post-translational modifications (PTMs), and in vitro signaling [Citation12].
Figure 1. Venn diagram comparing serum, PPP, and platelet fraction proteins that are either highly upregulated or found exclusively within that fraction.
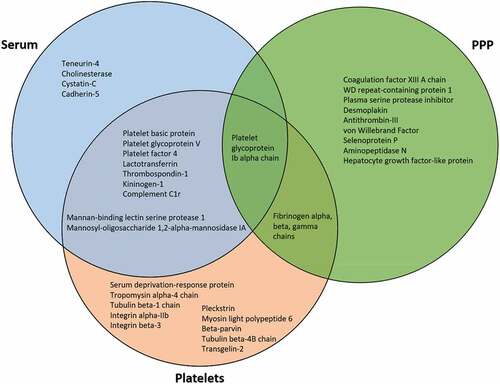
In addition, platelet membrane proteins, which provide the first line of contact for cell–matrix and cell–cell interactions in thrombosis and hemostasis, are often difficult to identify in global proteomics analyses performed in plasma due to biases toward soluble proteins [Citation13]. Further, platelet removal methods may also remove embedded platelet membrane proteins from the PPP sample to be analyzed. The process of platelet removal can lead to reduction or elimination of regulatory proteins including pigment epithelium-derived factor, platelet basic protein, platelet factor 4, calcium binding proteins such as caldesmon, calmodulin, calreticulin and calumenin, and Hsp70 [Citation10]. Significantly, up-regulation of platelet Hsp70 has been identified as a marker for β-thalassemia/HbE patients, highlighting vital information that can be missed when excluding platelet proteomics [Citation14,Citation15]. However, enrichment techniques can ensure that platelet membrane proteins are included in PRP proteomic analyses. A combination of three specific enrichment techniques has enabled significant enrichment of platelet membrane proteins, specifically more than 50% of the 1282 platelet proteins identified [Citation16].
Plasma as a mirror of platelet function
It has long been suggested that the anionic and cationic protein equilibrium in the plasma protein environment can influence platelet activity and function [Citation17], allowing for certain platelet functions and activities to be elucidated from plasma proteomics alone using an age-specific reference point. For instance, proteins of platelet activation such as thrombospondin 1, complement C5 beta chain, complement component C7, and angiotensin 2 were found to decrease in expression as age increased when analyzed using plasma [Citation18]. These and other hemostatic proteins clustered distinctly with age using principal component analysis, and could be used in an age-specific analysis to identify deviations from the norm in states of platelet dysfunction or dysregulation [Citation18]. PRP proteins can also be used as markers of platelet dysfunction following trauma, with 12 proteins either up- or down-regulated that significantly corresponded to platelet function in patients with and without severe trauma [Citation19].
An analysis of relative protein expression in samples of platelets isolated from PRP identified platelet proteins with functions in regulatory processes, cellular responses, developmental processes, platelet function, and immunity [Citation20]. The platelet proteins were mostly from intracellular origin at 46.5% of the proteins identified, while 20.9%, 20.9%, and 4.7% originated from membrane, endosomal, and extracellular regions, respectively [Citation20]. Platelet proteins were once again up-regulated in children compared to adults [Citation20].
However, the specific protein biomarker and disease state must be taken into consideration when choosing if PPP proteomics alone is sufficient for platelet analysis, as certain soluble proteins can be used as surrogate biomarkers for platelet activation and function while others cannot be found in the PPP fraction. For instance, Platelet Factor 4, a biomarker of tumor growth, is significantly increased in the presence of a tumor, but this increase is only seen in platelet proteomics analysis, while being absent in PPP proteomics [Citation21]. On the other hand, platelet glycoprotein VI (GPVI) is a platelet transmembrane protein that upon activation by ligands collagen or fibrin, in situations of thermal injury or thrombotic conditions, can release a soluble portion to be detected in plasma as a marker for platelet activation without requiring platelet enrichment for detection [Citation22–24]. P-selectin, an adhesion molecule that is found in the α granules of platelets and mediates cellular adhesion as a marker of platelet activation in inflammation and thrombogenic conditions, can be found as a soluble portion within PPP [Citation25–27].
An alternate source of platelet proteins found in PPP could originate from platelet-derived extracellular vesicles (EVs). Platelet-derived EVs including exosomes and microparticles/microvesicles are the largest contributor to EVs in circulation [Citation28,Citation29]. Platelet-derived EVs could indeed be a source of platelet proteins within the PPP fraction as a means to assess disease conditions such as tumor-associated conditions [Citation30], excessive thrombosis, autoimmunity, allergies, and infectious diseases [Citation31].
Clinical applicability of plasma proteome as a surrogate for platelet function
Platelets play an essential role in mediating hemostasis by initiating a rapid set of responses at the vascular endothelium level that limit bleeding [Citation32]. Once platelets are activated, they can release over 300 proteins in plasma that are implicated in the regulation of hemostatic, inflammatory, and angiogenic responses [Citation33–35]. The releasate contains a core group of proteins that are present with low variability in healthy adults and which can be used as a fingerprinting method to detect variations from the healthy state [Citation33]. In addition, the platelet releasate (regulatory proteins and biomolecules released upon activation), secretome (chemokines secreted upon activation), and sheddome (soluble protein fragments that are shed upon activation) can offer clues to platelet activation patterns or dysfunction in disease states such as coronary artery disease, peripheral artery disease, acute coronary syndrome and myocardial infarction, ischemic stroke, atrial fibrillation, and venous thrombosis, without the need for platelet-enriched proteomics [Citation34].
However, platelets can also contribute to the development of inflammatory responses and the progression of chronic diseases through specific maladaptive platelet subpopulations or phenotypes [Citation36]. These heterogenous platelet subpopulations have been implicated in the pathogenesis of multiple diseases including vascular diseases, metabolic syndrome, and cancer [Citation37–39]. While there exists a good understanding of the molecular mechanisms that lead to platelet activation states during hemostasis and thrombosis, there is still a significant knowledge gap regarding how these mechanisms may contribute to alterations in platelet phenotypes that lead to chronic disease.
The study of the plasma proteome may provide some answers by permitting the characterization of different platelet phenotypes and the identification of platelet biomarkers associated with disease states [Citation40,Citation41]. Prior studies have demonstrated conflicting correlations between the platelet transcriptome and the platelet proteome, with a recent analysis identifying up to 58% of platelet mRNAs with no corresponding protein in the proteomic analysis, suggesting a high presence of untranslated RNAs and low or controlled mRNA degradation in platelets [Citation42]. Despite this, 83.4% of the platelet proteins had a corresponding reference transcript in all study participants [Citation42], showing the validity of studying the plasma proteome as a way of obtaining novel insights on the pathogenesis of platelet disorders and the role of platelets in the development of chronic diseases [Citation43–45]. Among patients with acute venous thromboembolism, the quantitation of soluble platelet activation biomarkers within plasma has shown increased plasma levels of soluble P-selectin which have been associated with a high risk of recurrence [Citation46].
Similarly, specific platelet protein signatures, associated with non-canonical pathways primarily involved in the development of atherosclerosis and arterial thrombosis, have been identified in the plasma of patients with isolated pulmonary embolism (PE) [Citation47,Citation48]. This finding correlates with the phenotype observed in these individuals who demonstrate a higher prevalence of arterial hypertension, and chronic inflammatory, atherosclerotic and cardiovascular diseases compared to patients with isolated deep venous thrombosis (DVT) [Citation47,Citation48]. Further, these specific platelet protein signatures in plasma permit the differentiation between isolated PE versus DVT-associated PE [Citation48]. High levels of platelet microparticles in plasma have also been implicated in the pathogenesis of cancer-associated thrombosis, and may help predict the hypercoagulable state and risk of VTE in this population of patients [Citation49].
Innovations: moving from plasma to capillary samples that include platelets?
The scientific community has advocated for the use of multi-omics approaches focusing on a single cellular element (e.g. platelet) or component (e.g. plasma) of a complex biological fluid (e.g. whole blood). However, what could be more insightful is to focus on the different elements of a single biological fluid (e.g. platelets, plasma, red blood cells, neutrophils) with one methodological approach such as proteomics. This whole-blood-omics (WBO) approach would integrate proteomics data from the specific cellular elements and soluble proteins for increased insights into the setting that is being studied, whether a specific disease processes and/or markers associated with clinical outcomes.
Microsampling provides an innovative approach to capture diagnostic information from low volumes (<50 µL) of biological fluids such as whole blood, overcoming some of the limitations and challenges with traditional venipuncture methods and allowing for further adoption of point of care diagnostic techniques [Citation50]. This approach could be especially beneficial in pediatric populations, where typical volumes of whole blood collected from an adult can be difficult to obtain. In terms of platelet function, microsampling has been successfully implemented in mouse models of thromboembolism [Citation51] and shown to be comparable to standard phlebotomy when assessing certain quantitative protein biomarkers in humans [Citation52]. Capillary blood sampling from the retro-orbital venous plexus to assess platelet proteomes has been successfully implemented in murine models [Citation53]. That said, the feasibility of platelet analyses using microsampling in humans still needs to be investigated. Further development could lead to remote monitoring or at-home diagnostic tests becoming increasingly available in the clinical application of platelet and plasma analyses.
Conclusion
Platelets play key roles in numerous physiological and pathophysiological processes, and these important cellular elements require rigorous laboratory protocols to ensure appropriate isolation, to minimize activation and reduce contamination from other blood components, particularly when considering the platelet proteome. Proteomic studies need to have clear and transparent methods while recognizing the potential limitations of proteomic analyses of platelets using different sample types such as PPP, PRP, or enriched platelets. The plasma proteome can in some cases serve as a mirror of platelet function, and hence plays an important role, in addition to the platelet proteome, for elucidation of both physiological and disease processes, as both the plasma and platelet proteomes can provide unique but complementary information regarding platelet function and activity. Rapid innovations in sample collection, especially from the patient centric perspective are facilitating the inclusion of platelets in scenarios where capillary blood samples are quickly becoming the norm, rather than a rarity. We recommend that future studies cut across the plasma and platelet proteome silos, and take advantage of the vast knowledge available when these entities are considered as part of the same studies, rather than studied as distinct entities.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Lan J, Núñez Galindo A, Doecke J, Fowler C, Martins RN, Rainey-Smith SR, Cominetti O, Dayon L. Systematic evaluation of the use of human plasma and serum for mass-spectrometry-based shotgun proteomics. J Proteome Res. 2018;17(4):1426–6. doi: 10.1021/acs.jproteome.7b00788.
- Ignjatovic V, Geyer PE, Palaniappan KK, Chaaban JE, Omenn GS, Baker MS, Deutsch EW, Schwenk JM. Mass spectrometry-based plasma proteomics: considerations from sample collection to achieving translational data. J Proteome Res. 2019;18(12):4085–4097. doi: 10.1021/acs.jproteome.9b00503.
- Rikkert LG, Coumans FAW, Hau CM, Lwmm T, Nieuwland R. Platelet removal by single-step centrifugation. Platelets. 2021;32(4):440–443. doi: 10.1080/09537104.2020.1779924.
- Lundblad R. Considerations for the use of blood plasma and serum for proteomic analysis. Int J Genomics Proteomics. 2003;1(2).
- Liu X, Hoene M, Wang X, Yin P, Häring H-U, Xu G, Lehmann R. Serum or plasma, what is the difference? Investigations to facilitate the sample material selection decision making process for metabolomics studies and beyond. Anal Metabolomics. 2018;1037:293–300. doi: 10.1016/j.aca.2018.03.009.
- Cao F, Gao Y, Chu Q, Wu Q, Zhao L, Lan T, Zhao L. Proteomics comparison of exosomes from serum and plasma between ultracentrifugation and polymer-based precipitation kit methods. Electrophoresis. 2019 2022 Dec 16;40(23–24):3092–3098. doi: 10.1002/elps.201900295.
- Geyer PE, Voytik E, Treit PV, Doll S, Kleinhempel A, Niu L, Müller JB, Buchholtz ML, Bader JM, Teupser d, et al. plasma Proteome Profiling to detect and avoid sample-related biases in biomarker studies. EMBO Mol Med. 2019 2022 Nov 23;11(11):e10427. doi: 10.15252/emmm.201910427.
- Tassi Yunga S, Gower AJ, Melrose AR, Fitzgerald MK, Rajendran A, Lusardi TA, Armstrong RJ, Minnier J, Jordan KR, McCarty OJT, et al. Effects of ex vivo blood anticoagulation and preanalytical processing time on the proteome content of platelets. J Thromb Haemostasis. 2022 2023 Feb 2;20(6):1437–1450. doi: 10.1111/jth.15694.
- Greening DW, Glenister KM, Kapp EA, Moritz RL, Sparrow RL, Lynch GW, Simpson RJ. Comparison of human platelet membrane-cytoskeletal proteins with the plasma proteome: towards understanding the platelet-plasma nexus. PROTEOMICS – Clin Appl. 2008 2022 Nov 30;2(1):63–77. doi: 10.1002/prca.200780067.
- Miroshnychenko O, Chalkley RJ, Leib RD, Everts PA, Dragoo JL. Proteomic analysis of platelet-rich and platelet-poor plasma. Regener Ther. 2020;15:226–235. doi: 10.1016/j.reth.2020.09.004.
- Burkhart JM, Vaudel M, Gambaryan S, Radau S, Walter U, Martens L, Geiger J, Sickmann A, Zahedi RP. The first comprehensive and quantitative analysis of human platelet protein composition allows the comparative analysis of structural and functional pathways. Blood. 2012;120(15):e73–82. doi: 10.1182/blood-2012-04-416594.
- Burkhart JM, Gambaryan S, Watson SP, Jurk K, Walter U, Sickmann A, Heemskerk JWM, Zahedi RP. What can proteomics tell us about platelets? Circ Res. 2014 2022 Dec 16;114(7):1204–1219. doi: 10.1161/CIRCRESAHA.114.301598.
- Alfonso-Garrido J, Garcia-Calvo E, Luque-Garcia JL. Sample preparation strategies for improving the identification of membrane proteins by mass spectrometry. Anal Bioanal Chem. 2015;407(17):4893–4905. doi: 10.1007/s00216-015-8732-0.
- Chanpeng P, Svasti S, Paiboonsukwong K, Smith DR, Leecharoenkiat K. Platelet proteome reveals specific proteins associated with platelet activation and the hypercoagulable state in β-thalassmia/hbe patients. Sci Rep. 2019;9(1):6059. doi: 10.1038/s41598-019-42432-2.
- Karmakar S, Banerjee D, Chakrabarti A. Platelet proteomics in thalassemia: factors responsible for hypercoagulation. PROTEOMICS – Clin Appl. 2016 2022 Nov 28;10(3):239–247. doi: 10.1002/prca.201500049.
- Lewandrowski U, Wortelkamp S, Lohrig K, Zahedi RP, Wolters DA, Walter U, Sickmann A. Platelet membrane proteomics: a novel repository for functional research. Blood. 2009;114(1):e10–9. doi: 10.1182/blood-2009-02-203828.
- Hansen MS, Bang NU. Plasma protein regulation of platelet function and metabolism. Mol Cell Biochem. 1979;24(3):143–158. doi: 10.1007/BF00220733.
- McCafferty C, Busuttil-Crellin X, Cai T, Monagle P, Goldenberg NA, Ignjatovic V. Plasma proteomic analysis reveals age-specific changes in platelet- and endothelial cell–derived proteins and regulators of plasma coagulation and fibrinolysis. J Pediatr. 2020 2022 Nov 29;221:S29–36. doi: 10.1016/j.jpeds.2020.01.051.
- John AS, Wang Y, Chen J, Osborn W, Wang X, Lim E, Chung D, Stern S, White N, Fu X, et al. Plasma proteomic profile associated with platelet dysfunction after trauma. J Thromb Haemostasis. 2021 2022 Nov 29;19(7):1666–1675. doi: 10.1111/jth.15316.
- Cini C, Yip C, Attard C, Karlaftis V, Monagle P, Linden M, Ignjatovic V. Differences in the resting platelet proteome and platelet releasate between healthy children and adults. J Proteomics. 2015;123:78–88. doi: 10.1016/j.jprot.2015.04.003.
- Lakka Klement G. Platelet biomarkers in tumor growth. Curr Proteomics. 2011;8(3):169–180. doi: 10.2174/157016411797247486.
- Al-Tamimi M, Mu F-T, Moroi M, Gardiner EE, Berndt MC, Andrews RK. Measuring soluble platelet glycoprotein VI in human plasma by ELISA. Platelets. 2009;20(3):143–149. doi: 10.1080/09537100802710286.
- Yamashita Y, Naitoh K, Wada H, Ikejiri M, Mastumoto T, Ohishi K, Hosaka Y, Nishikawa M, Katayama N. Elevated plasma levels of soluble platelet glycoprotein VI (GPVI) in patients with thrombotic microangiopathy. Thromb Res. 2014;133(3):440–444. doi: 10.1016/j.thromres.2013.11.023.
- Montague SJ, Delierneux C, Lecut C, Layios N, Dinsdale RJ, Lee CSM, Poulter NS, Andrews RK, Hampson P, Wearn CM, et al. Soluble GPVI is elevated in injured patients: shedding is mediated by fibrin activation of GPVI. Blood Adv. 2018 2023 Feb 2;2(3):240–251. doi: 10.1182/bloodadvances.2017011171.
- Amin HM, Ahmad S, Walenga JM, Hoppensteadt DA, Leitz H, Fareed J. Soluble P-Selectin in human plasma: effect of anticoagulant matrix and its levels in patients with cardiovascular disorders. Clin Appl Thromb/Hemost. 2000 2023 Feb 2;6(2):71–76. doi: 10.1177/107602960000600204.
- Ay C, Simanek R, Vormittag R, Dunkler D, Alguel G, Koder S, Kornek G, Marosi C, Wagner O, Zielinski C, et al. High plasma levels of soluble P-selectin are predictive of venous thromboembolism in cancer patients: results from the Vienna cancer and thrombosis study (CATS). Blood. 2008 2023 Feb 2;112(7):2703–2708. doi: 10.1182/blood-2008-02-142422.
- Müller R, Rink G, Uzun G, Bakchoul T, Wuchter P, Klüter H, Bugert P. Increased plasma level of soluble P-selectin in non-hospitalized COVID-19 convalescent donors. Thromb Res. 2022;216:120–124. doi: 10.1016/j.thromres.2022.06.014.
- Berezin AE, Berezin AA. Platelet-derived vesicles: diagnostic and predictive value in cardiovascular diseases. J Unexplored Med Data. 42019:4. doi: 10.20517/2572-8180.2019.05.
- Alberro A, Iparraguirre L, Fernandes A, Otaegui D. Extracellular vesicles in blood: sources, effects, and applications. Int J Mol Sci. 2021;22(15):8163. doi: 10.3390/ijms22158163.
- Vismara M, Manfredi M, Zarà M, Trivigno SMG, Galgano L, Barbieri SS, Canobbio I, Torti M, Guidetti GF. Proteomic and functional profiling of platelet-derived extracellular vesicles released under physiological or tumor-associated conditions. Cell Death Discov. 2022;8(1):467. doi: 10.1038/s41420-022-01263-3.
- Eustes AS, Dayal S. The role of platelet-derived extracellular vesicles in immune-mediated thrombosis. Int J Mol Sci. 2022;23(14):7837. doi: 10.3390/ijms23147837.
- Stalker TJ, Traxler EA, Wu J, Wannemacher KM, Cermignano SL, Voronov R, Diamond SL, Brass LF. Hierarchical organization in the hemostatic response and its relationship to the platelet-signaling network. Blood. 2013 2022 Dec 22;121(10):1875–1885. doi: 10.1182/blood-2012-09-457739.
- Parsons MEM, Szklanna PB, Guerrero JA, Wynne K, Dervin F, O’connell K, Allen S, Egan K, Bennett C, McGuigan C, et al. Platelet releasate proteome profiling reveals a core set of proteins with low variance between healthy adults. PROTEOMICS. 2018 2023 Feb 2;18(15):1800219. doi: 10.1002/pmic.201800219.
- Baidildinova G, Nagy M, Jurk K, Wild PS, ten Cate H, van der Meijden PEJ. Soluble platelet release factors as biomarkers for cardiovascular disease. Front cardiovasc med. 2021 2021 Jun 21;8. doi: 10.3389/fcvm.2021.684920.
- Golebiewska EM, Poole AW. Platelet secretion: from haemostasis to wound healing and beyond. Blood Rev. 2015;29(3):153–162. doi: 10.1016/j.blre.2014.10.003.
- Li JL, Zarbock A, Hidalgo A. Platelets as autonomous drones for hemostatic and immune surveillance. J Exp Med. 2017;214(8):2193–2204. doi: 10.1084/jem.20170879.
- Lebas H, Yahiaoui K, Martos R, Boulaftali Y. Platelets are at the nexus of vascular diseases. Front cardiovasc med. 2019 2019 Sept 11;6. doi: 10.3389/fcvm.2019.00132.
- Lee EC, Cameron SJ. Cancer and thrombotic risk: the platelet paradigm. Front cardiovasc med. 2017 2017 Nov 7;4. doi: 10.3389/fcvm.2017.00067.
- Maiocchi S, Alwis I, Wu MCL, Yuan Y, Jackson SP. Thromboinflammatory functions of platelets in ischemia–reperfusion injury and its dysregulation in diabetes. Semin Thromb Hemost. 2018;44(02):102–113. doi: 10.1055/s-0037-1613694.
- Macaulay IC, Carr P, Gusnanto A, Ouwehand WH, Fitzgerald D, Watkins NA. Platelet genomics and proteomics in human health and disease. J Clin Invest. 2005;115(12):3370–3377. doi: 10.1172/JCI26885.
- Parguiña AF, Rosa I, García Á. Proteomics applied to the study of platelet-related diseases: aiding the discovery of novel platelet biomarkers and drug targets. J Proteomics. 2012;76:275–286. doi: 10.1016/j.jprot.2012.04.043.
- Londin ER, Hatzimichael E, Loher P, Edelstein L, Shaw C, Delgrosso K, Fortina P, Bray PF, McKenzie SE, Rigoutsos I. The human platelet: strong transcriptome correlations among individuals associate weakly with the platelet proteome. Biol Direct. 2014;9(1):3. doi: 10.1186/1745-6150-9-3.
- Marcus K, Immler D, Sternberger J, Meyer HE. Identification of platelet proteins separated by two-dimensional gel electrophoresis and analyzed by matrix assisted laser desorption/ionization-time of flight-mass spectrometry and detection of tyrosine-phosphorylated proteins. Electrophoresis. 2000 2022 Dec 22;21(13):2622–2636. doi: 10.1002/1522-2683(20000701)21:13<2622:AID-ELPS2622>3.0.CO;2-3.
- McRedmond JP, Park SD, Reilly DF, Coppinger JA, Maguire PB, Shields DC, Fitzgerald DJ. Integration of proteomics and genomics in platelets: a profile of platelet proteins and platelet-specific genes. Mol Cell Proteom. 2004;3(2):133–144. doi: 10.1074/mcp.M300063-MCP200.
- O’neill EE, Brock CJ, von Kriegsheim AF, Pearce AC, Dwek RA, Watson SP, Hebestreit HF. Towards complete analysis of the platelet proteome. Proteomics. 2002 2022 Dec 22;2(3):288–305. doi: 10.1002/1615-9861(200203)2:3<288:AID-PROT288>3.0.CO;2-0.
- Kyrle PA, Hron G, Eichinger S, Wagner O. Circulating P-selectin and the risk of recurrent venous thromboembolism. Thromb Haemost. 2007;97(06):880–883. doi: 10.1160/TH07-02-0115.
- Ten Cate V, Prochaska JH, Schulz A, Koeck T, Pallares Robles A, Lenz M, Eggebrecht L, Rapp S, Panova-Noeva M, Ardeschir Ghofrani H. Protein expression profiling suggests relevance of noncanonical pathways in isolated pulmonary embolism. Blood. 2021 2022 Dec 22;137(19):2681–2693. doi: 10.1182/blood.2019004571.
- Baidildinova G, ten Cate V, Nagler M, Panova-Noeva M, Rapp S, Köck T, Prochaska JH, Heitmeier S, Gerdes C, Schwers S, et al. Subtype-specific plasma signatures of platelet-related protein releasate in acute pulmonary embolism. Thromb Res. 2022;220:75–87. doi: 10.1016/j.thromres.2022.10.005.
- Campello E, Spiezia L, Radu CM, Bulato C, Castelli M, Gavasso S, Simioni P. Endothelial, platelet, and tissue factor-bearing microparticles in cancer patients with and without venous thromboembolism. Thromb Res. 2011;127(5):473–477. doi: 10.1016/j.thromres.2011.01.002.
- Lei BUW, Prow TW. A review of microsampling techniques and their social impact. Biomed Microdevices. 2019;21(4):81. doi: 10.1007/s10544-019-0412-y.
- Rauzi F, Smyth E, Emerson M. Refinement of mouse protocols for the study of platelet thromboembolic responses in vivo. Thromb Haemost. 2017;117(12):2283–2290. doi: 10.1160/TH17-04-0250.
- Xing J, Loureiro J, Patel MT, Mikhailov D, Gusev AI. Evaluation of a novel blood microsampling device for clinical trial sample collection and protein biomarker analysis. Bioanalysis. 2020 2022 Dec 16;12(13):919–935. doi: 10.4155/bio-2020-0063.
- Martínez-Botía P, Meinders M, De Cuyper IM, Eble JA, Semple JW, Gutiérrez L. Dissecting platelet proteomics to understand the pathophysiology of immune thrombocytopenia: studies in mouse models. Blood Adv. 2022 2 Feb 2023;6(11):3529–3534. doi: 10.1182/bloodadvances.2021006438.
