Abstract
Heart failure (HF) with preserved ejection fraction (HFpEF) is associated with high burden of comorbidities known to increase the mean platelet volume (MPV). This parameter has been associated with morbidity and mortality in HF. However, the role of platelets and the prognostic relevance of MPV in HFpEF remain largely unexplored. We aimed to evaluate the clinical usefulness of MPV as a prognostic marker in HFpEF. We prospectively enrolled 228 patients with HFpEF (79 ± 9 years; 66% females) and 38 controls of similar age and gender (78 ± 5 years; 63% females). All subjects underwent two-dimensional echocardiography and MPV measurements. Patients were followed-up for a primary end point of all-cause mortality or first HF hospitalization. The prognostic impact of MPV was determined using Cox proportional hazard models. Mean MPV was significantly higher in HFpEF patients compared with controls (MPV: 10.7 ± 1.1fL vs. 10.1 ± 1.1fL, p = .005). HFpEF patients (n = 56) with MPV >75th percentile (11.3 fL) displayed more commonly a history of ischemic cardiomyopathy. Over a median follow-up of 26 months, 136 HFpEF patients reached the composite endpoint. MPV >75th percentile was a significant predictor of the primary endpoint (HR: 1.70 [1.08; 2.67], p = .023) adjusted for NYHA class, chronic obstructive pulmonary disease, loop diuretics, renal function, and hemoglobin. We demonstrated that MPV was significantly higher in HFpEF patients compared with controls of similar age and gender. Elevated MPV was a strong and independent predictor of poor outcome in HFpEF patients and may be relevant for clinical use.
Plain Lanuage Summary
What is the context?
Heart failure with preserved ejection fraction (HFpEF) is associated with several comorbidities known to increase the mean platelet volume (MPV).
MPV is a measure of platelet size and a potential marker of platelet reactivity. An increased MPV results from an increased platelet turnover.
MPV has been associated with morbidity and mortality from heart failure.
No study has previously compared MPV between HFpEF and controls and investigated the prognostic relevance of MPV in HFpEF disease.
What is new?
In this study, we compared the MPV between HFpEF patients and controls of similar age and gender, prospectively enrolled between 2015 and 2021. We evaluated the prognostic role of elevated MPV in HFpEF patients.
Our main results:
The MPV was higher in HFpEF patients compared to controls of similar age and gender.
HFpEF patients with elevated MPV displayed more commonly a history of ischemic cardiomyopathy.
Elevated MPV was a strong and independent predictor of poor outcome in HFpEF patients.
What is the impact?
MPV may be relevant for clinical use to predict clinical outcome in HFpEF patients.
Elevated MPV reflecting platelet activity supports the potential role of platelets in HFpEF’s pathophysiology.
Introduction
Heart failure with preserved ejection fraction (HFpEF) is a major cause of cardiovascular (CV) morbidity and mortality [Citation1]. Although widely prevalent, HFpEF remains a poorly understood syndrome with only few efficient therapies. The growth of the HFpEF population can probably be explained by the increasing burden of advanced age and comorbidities. Indeed, a paradigm for HFpEF development has been proposed. Comorbidities, such as obesity, diabetes, chronic kidney disease, and chronic obstructive pulmonary disease, would lead to a systemic inflammatory state, thereby impacting the endothelium physiology while engaging complex molecular pathways that ultimately converge to myocardial fibrosis and left ventricle (LV) dysfunction [Citation2]. Interestingly, a recent unbiased network analysis involving a large cohort of HF patients (BIOSTAT-CHF project) demonstrated that biomarker profiles specific for HFpEF are related to inflammatory processes including cytokine response, extracellular matrix reorganization, as well as platelet activation [Citation3]. However, the precise role of platelets in HFpEF pathophysiology is still poorly understood.
The mean platelet volume (MPV), which is calculated by dividing the plateletcrit by the total number of platelets, is a measure of platelet size and a potential marker of platelet reactivity. An increased MPV results from an increased platelet turnover. The peripheral platelet consumption is instrumental in increasing the number of newly produced immature platelets, which are larger and more reactive than their mature counterparts [Citation4,Citation5].
An increased mean MPV has been associated with comorbidities that are recognized to be associated with HFpEF, including coronary disease, hypertension, diabetes, atrial fibrillation, renal dysfunction, hypertriglyceridemia, obesity, and metabolic syndrome [Citation6–12]. Moreover, increased mean MPV has been described to be elevated in patients with congestive HF [Citation13,Citation14]. MPV may thus be a biomarker of the risk and prognosis of common heart diseases [Citation15]. An increased mean MPV additionally represents a risk factor for overall cardiovascular mortality, which is an independent predictor of mortality in decompensated heart failure. Thus, this parameter allows for identifying patients at higher risk of death due to ischemic heart disease [Citation6,Citation13]. However, data on platelet indices in HFpEF are still limited, and the prognostic relevance of MPV in HFpEF remains largely unexplored.
This study sought to evaluate MPV in both HFpEF and controls of similar age and gender and to assess the association between MPV, clinical, biological, and imaging characteristics and its prognostic value in HFpEF.
Methods
Study population
Between December 2015 and June 2021, all consecutive patients with HFpEF were prospectively evaluated for inclusion into this study in a single center, Cliniques universitaires Saint-Luc (Brussels, Belgium) [Citation16].
The following criteria had to be fulfilled for study inclusion: New York Heart Association (NYHA) functional class ≥II, typical signs of HF, N-terminal pro-brain natriuretic peptide (NT‐proBNP) >350 pg/mL, or hospitalization for HF in the previous 12 months, left ventricular ejection fraction (LVEF) ≥50%, as well as relevant structural heart disease (LV hypertrophy/left atrial [LA] enlargement) or diastolic dysfunction assessed by echocardiography (LA > 34 ml/m2; E/e’ ratio >14; tricuspid regurgitation >2.8 ms, septal e’ velocity <7 cm/s, or Lateral e’ velocity <10 cm/s) [Citation17]. The average duration of the disease was 28 months. The exclusion criteria were as follows: severe valvular disease, infiltrative or hypertrophic cardiomyopathy, acute coronary syndrome within the previous 30 days, chronic obstructive pulmonary disease of either GOLD 3 or 4, congenital heart disease, pericardial disease, atrial fibrillation with a ventricular response >140 bpm, severe anemia (hemoglobin: <8 g/dL), active cancer, and auto-immune diseases. Overall, 228 patients met the inclusion criteria, thereby constituting the final study population.
Patients were compared with 38 controls of similar age and gender without a history of cardiovascular disease, significant past medical history, or chronic disease. All controls were prospectively recruited by advertisements in the local community and underwent a full clinical examination, electrocardiogram, echocardiography, and exercise stress test, all of which had to be normal prior to inclusion.
All subjects underwent blood sampling and complete transthoracic echocardiography.
The investigation was full in line with the principles outlined in the Declaration of Helsinki. The local ethics committee approved the study protocol, and all patients provided their written informed consent before enrolling into the study (Clinical trial NCT03197350).
Echocardiography
All subjects underwent a complete two‐dimensional transthoracic echocardiography at inclusion (iE33 system Philips) in order to assess LV and right ventricle (RV) structure, systolic and diastolic function, and measurements of left and right atrial volumes, in addition to a valvular evaluation. Pulmonary pressures were estimated using tricuspid regurgitation velocity. All measurements were averaged over three beats in patients suffering from atrial fibrillation.
Mean platelet volume analysis
Blood samples were obtained with venipuncture at inclusion and collected in microtubes containing lithium heparin for biochemistry tests and ethylenediamine tetraacetic acid (K3 EDTA) for the complete blood count. Biochemistry tests were performed using an automated chemistry platform, the Cobas® 8000 (Roche diagnostics, Basel, Switzerland), while the complete blood count was assessed on the XN-9000 (Sysmex, Kobe, Japan) chain. All analyses were carried out at the clinical laboratory of the Cliniques Universitaires Saint-Luc. The parameters assessed were as follows: absolute count and percentage of leukocytes, neutrophils, lymphocytes, and monocytes, as well as the neutrophil-to-lymphocyte ratio, platelet count, and MPV. All blood samples were collected, handled, and processed in the same way and by the same study nurse. Tubes were completely filled (3.4 ml) and mixed at room temperature. Samples were analyzed within 1 h of the collection. MPV was calculated on the XN-9000 from the following equation: MPV femtoliters (fL) = plateletcrit (%)/platelet count (x103/µL) × 10 000. Platelet count was measured using the impedance method. The reference intervals in our institution are as 150–450 × 103/µL for platelet count and 9.3–12.1 fL for MPV. Internal quality control was performed at three levels (XN Check level 1, 2 and 3; Sysmex) before, after daily maintenance, and at noon. Internal quality control results of MPV were also compared to so-called peer groups (laboratories using the same analyzer, lot number of QC material, and measurement method). External quality control from Sciensano (Ixelles, Belgium) is performed twice a year for platelet count but not for MPV. The intra-assay and inter-assay coefficients of variation for platelet count were 3.9%, 0.9%, 0.8% and 2.7%, 1.8%, 1.1% for low, medium, and high concentrations of control material, respectively.
Follow-up
Patients were prospectively followed up by ambulatory visits and phone calls at 6-month intervals. Clinical and survival status was obtained based on follow-up visits and using phone contact with the patients, their relatives, or their physician as necessary. The primary end point was a composite of either all-cause mortality or hospitalization for HF, whichever came first. Hospitalization was defined as patients diagnosed with HF (symptoms and signs) and requiring intravenous diuretics, being either treated in the emergency room or admitted to the hospital. The secondary end point was all-cause mortality. The end points have been adjudicated and validated by three trained physicians.
Statistical analysis
Statistical analyses were performed using the SPSS Version 26 (SPSS Corp., Somers, New York) and R Version 4.1.2 software (http://www.r-project.org). All tests were two-sided, with a statistical significance set at p < .05. Continuous variables were expressed as mean ±1 standard deviation (SD) if normally distributed or as median (25th and 75th percentiles) if not. Categorical variables were expressed as counts and percentages. Biomarkers were log‐transformed in order to establish normality. The HFpEF patient population was divided into two groups according to the 75th percentile of MPV, in line with a previous study [Citation18]. Clinical, biological, and imaging parameters between-group comparison were performed using either independent t- or chi‐squared tests as appropriate.
Event‐free survival and overall survival (OS) of HFpEF patients was estimated using log-rank test and Cox regression analysis. All baseline and imaging variables were initially proposed for inclusion into a univariate Cox proportional hazard model. Significant variables (p < .10) were entered into a multivariate Cox regression model. To avoid collinearity, the correlation coefficients between covariates were further examined. In the case of collinearity (r > 0.50), only the strongest of two covariates was proposed for inclusion into the multivariate model. To evaluate the additive prognostic value of MPV, we tested it with the MAGGIC (Meta-Analysis Global Group in Chronic) Score, a validated predictive risk tool for mortality prediction in HFPEF which incorporates multiple demographics and clinical and laboratory variables [Citation19]. All of these variables were available for patients. Kaplan–Meier curves based on the 75th percentile of MPV group was used to illustrate event‐free survival and OS of HFpEF patients. The maximally selected rank test was used to identify the MPV cutoff associated with event-free survival and OS of HFpEF patients.
Results
Baseline characteristics
Overall, 228 consecutive HFpEF patients (79 ± 9 years; 66% females) and 38 controls of similar age and gender (78 ± 5; 63% females) were prospectively included in the study. The baseline and imaging patient characteristics are summarized in . As expected, HFpEF patients displayed a higher incidence of cardiovascular risk factors and comorbidities compared with controls. These patients exhibited lower hemoglobin levels and lower estimated glomerular filtration rates (eGFR). Median NT‐proBNP, neutrophil-to-lymphocyte ratio, high-sensitivity troponin T (hsTnT), C-reactive protein (CRP), fibroblast growth factor 23 (FGF‐23), and soluble suppression of tumorigenicity 2 (ST2) were significantly higher in patients with HFpEF. Moreover, median total cholesterol, low-density lipoprotein cholesterol (LDL-C), and triglycerides were significantly higher in controls, most probably due to a difference in statin intake.
Table I. Baseline characteristics of controls and patients with HFpEF of similar age and gender.
HFpEF patients exhibited higher LA volumes, higher E wave velocities, higher E/e′ ratio, and higher pulmonary pressures, likely reflecting more diastolic dysfunction than controls.
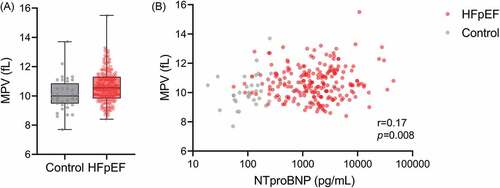
Furthermore, the MPV was significantly higher in HFpEF patients versus controls (MPV: 10.7 ± 1.1fL vs. 10.0 ± 1.1fL, p = .005) (). However, MPV was poorly correlated with NT-proBNP in the whole population ().
Comparison of clinical and imaging parameters according to the 75th percentile
summarizes the clinical and imaging parameters of HFpEF patients according to the 75th percentile of MPV. Interestingly, HFpEF patients with MPV >75th percentile displayed no comorbidities known to be associated with MPV apart from a history of ischemic cardiomyopathy. However, no differences in anticoagulation and antiplatelet treatments were found.
Table II. Baseline characteristics of patients with HFpEF according to MPV ≤ and >75th percentile.
Patients with MPV >75th percentile exhibited a higher level of intact FGF-23, lower level of high-density lipoprotein cholesterol (HDL-C), and lower platelet count. There were no significant differences in renal function and markers of inflammation or in myocardial injury and congestion.
Mean platelet volume and clinical outcome
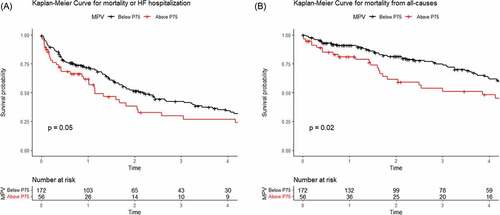
Over a median follow-up of 26 months (11.5–56.7 months), 87 patients (38%) died, and 107 patients (47%) were hospitalized for HF. Overall, 136 patients (60%) reached the primary composite end point of all‐cause mortality or HF hospitalization, whichever came first. depicts the Kaplan–Meier curves for the primary and secondary end points (all-cause mortality) according to the 75th percentile of MPV, respectively, illustrating that patients with the highest MPV level displayed the worse prognosis. In addition, the threshold of MPV associated with a higher risk of all-cause mortality or HF hospitalization was 11.2 fL using maximally selected rank test statistics. Interestingly, this value is close to the 75th percentile (11.3 fL).
Figure 2. A. Kaplan–Meier curves for the primary endpoint according to 75th percentile of mean platelet volume (MPV) with preserved ejection fraction (HFpEF) patients. B. Kaplan–Meier curves for the secondary end point according to 75th percentile.
In univariate Cox regression analysis, age, NYHA functional class III to IV, body mass index (BMI), chronic obstructive pulmonary disease (COPD), use of loop diuretics and thiazides, E/e’ ratio, estimated systolic pulmonary arterial pression (eSPAP), MPV >75th percentile, eGFR, hemoglobin, and NT-proBNP were primary end point predictors. In multivariate Cox regression analysis, NYHA functional class III to IV, COPD, loop diuretics, MPV >75th percentile (HR: 1.70 [1.08; 2.67], p = .023), eGFR, and hemoglobin turned out to be independent primary endpoint predictors (χ2 = 63.9, p < .001; ).
Table III. Cox regression analysis for the primary endpoint (all-cause mortality or HF hospitalization).
For the secondary end point, age, gender, BMI, COPD, loop diuretics, thiazide, eSPAP, E/e’ ratio, MPV >75th percentile, eGFR, hemoglobin, and NT-proBNP were predictors of all-cause mortality in univariate Cox regression. In multivariate Cox regression analysis, only BMI, loop diuretics, MPV >75th Percentile (HR: 2.06 [1.24–3.41], p = .005) and eSPAP were still independent predictors of all-cause mortality (χ2 = 41.9, p < .001; ).
Table IV. Cox regression analysis for the secondary end point (all-cause mortality).
Interestingly, MPV >75th percentile (HR 1.66 [1.05–2.62], p = .03) provided an additional prognostic value over the MAGGIC Score (HR 1.1 [1.06–1.15], p < .001), at least to predict mortality (χ2 = 30.1, p < .001).
Discussion
The current study has demonstrated MPV to be significantly higher in HFpEF patients compared with controls of similar age and gender. Furthermore, HFpEF patients with elevated MPV exhibited only one relevant difference in clinical features, notably the presence of ischemic heart disease. By contrast, there was no interaction between anticoagulation and antiplatelet treatments nor were there differences in cardiovascular risk factors known to be associated with elevated MPV. High MPV levels were not associated with renal function, inflammation markers, myocardial injury, or congestion markers, which are usually observed in HFpEF. However, high MPV levels were associated with high levels of intact FGF-23, which is a marker of cardiac fibrosis [Citation20]. These results imply that MPV might be linked to atherosclerosis and cardiac fibrosis, independently of renal function and congestion markers.
In addition, elevated MPV turned out to be both a strong and independent marker of poor outcome in HFpEF, whereas prior ischemic cardiomyopathy did not. To our knowledge, these findings have not been previously reported. According to our results, platelets might play an essential role in HFpEF’s pathology.
Mean platelet volume reflecting platelet activation in HFpEF
HFpEF is a clinical syndrome that has already been associated with a systemic proinflammatory state induced by comorbidities, with an impact on endothelium physiology, thereby engaging complex molecular pathways that ultimately converge to myocardial fibrosis and LV dysfunction [Citation2]. Tromp et al. [Citation3] showed that biomarker profiles that were associated with HFpEF compared with heart failure with reduced ejection fraction (HFrEF) are related to inflammatory processes including cytokine response, extracellular matrix reorganization, and platelet activation, supporting a potential role of platelets in HFpEF’s pathophysiology.
MPV is universally available with routine blood counts from automated hemograms, and it not only reflects platelet size but also reflects their turnover. Platelets are produced within the bone marrow by megakaryocytes, which are large precursor cells. Typically, younger platelets are larger than older ones. MPV is often considered a reflection of the average platelet age. Thus, in response to a significant platelet consumption resulting in a decreased platelet count, the bone marrow produces a higher number of megakaryocytes, which turn out to be fragmenting into larger platelets with a higher MPV. Elevated MPV is likewise a marker of platelet activation or reactivity [Citation21,Citation22]. This latter parameter has been associated with a variety of prothrombotic and proinflammatory diseases [Citation23]. Interestingly, it appears that platelet size depends on the intensity of systemic inflammation. Low-grade inflammation as opposed to high-grade inflammation, characteristic of CV disease and chronic HF, would contribute to MPV elevation. Chronic inflammation in HFpEF is characterized by the elevation of proinflammatory markers, such as interleukin 1, interleukin 6 (IL-6), and tumor necrosis factor-alpha, known to have a role in the regulation of thrombopoiesis [Citation2,Citation24]. Among these proinflammatory factors, IL-6 plays an important role in cardiac remodeling and in the pathophysiology of cardiac decompensation in HFpEF [Citation2,Citation25,Citation26]. Several studies have affirmed that IL-6 stimulated megakaryocytopoiesis and platelet production [Citation27,Citation28]. Moreover, IL-6 alters platelet function, rendering them more sensitive to activation by thrombin and platelet activating factor [Citation29,Citation30]. Thus, elevated MPV in HFpEF is probably related to the inflammatory context of the disease. However, we did not find any significant correlation between MPV and available markers of inflammation in our HFpEF population (neutrophil-to-lymphocytes ratio, soluble ST2, CRP). In addition, an elevated platelet size was found to be related with function and aggregation [Citation31,Citation32]. Larger platelets have been demonstrated to display increased reactivity, producing a higher level of activation biomarkers in plasma, including soluble P-selectin that is known to be associated with HFpEF prognosis [Citation33–35]. Moreover, there seems to be a link between platelet size and platelet content. Larger platelets are supposed to contain more granules, thereby becoming more reactive, the most numerous of which are alpha granules. In these granules, fibrinogen, von Willebrand factor, thrombospondin, thromboxane A2, and transforming growth factor (TGF-β) are notably stored. Interestingly, the TGF-β isoform levels have been shown to be increased in myocardial diseases [Citation36]. Furthermore, the highest cellular TGF-β concentrations are found in platelets, thereby contributing to 40–50% of systemic TGF-βs [Citation37]. Among several isoforms, the active TGF-β1 form has been specifically related to fibrosis, hypertrophy, and diastolic dysfunction in a mouse model of pressure overload induced by trans-aortic constriction, which likely mimics HFpEF [Citation38]. HFpEF patients have been previously shown to display higher circulating TGF-β1 levels than HFrEF patients [Citation39]. These data clearly support the hypothesis that both platelet size and activity are likely related to cardiac fibrosis in HFpEF. There is a probable close connection between MPV and TGF-β secretion level. However, data on TGF-β expression and platelet activity in HFpEF patients remain largely unexplored.
Clinical and biological parameters associated with elevated MPV
Surprisingly, not all clinical features known to increase MPV, including hypertension, diabetes, atrial fibrillation, and obesity, were shown to be associated with high MPV levels in HFpEF patients. Patients with elevated MPV displayed only a higher prevalence of prior ischemic cardiomyopathy, lower platelet counts, and lower HDL-C levels, thus reflecting macrovascular and atherothrombotic disease in HFpEF. Indeed, enhanced peripheral consumption caused by atherosclerotic disease does increase counts of immature platelets, which are larger and more reactive than mature platelets. Considering these results, several hypotheses emerge as follows: a high platelet volume is either a consequence of prothrombotic, proinflammatory events, or a reflection of increased platelet activity. Increased MPV is likely associated with greater activity owing to higher expression of adhesion molecules, with platelets undergoing faster activation, which eventually results in platelet hyperactivity. This hypothesis is supported by a study that showed that MPV levels were increased 2–3 days following acute coronary syndrome, probably reflecting platelet release that occurs prior to the cardiac event, with platelets having an average life span of 7 days [Citation40]. These large platelets could actually promote the thrombotic event rather than simply being its cause. In addition, HDL-C levels, which were reported to be inversely associated with the risk of atherosclerosis development, have been shown to be negatively correlated with MPV while reducing platelet aggregation [Citation41,Citation42]. Moreover, MPV has been previously shown to reflect the response to anti-platelet agents, although we did not find any effect of treatment on MPV in our HFpEF population [Citation40]. Therefore, in the future, it might be interesting to further explore the role of immature platelets in macrovascular disease in HFpEF and their relationship with MPV.
Prognostic and risk stratification
As demonstrated, high MPV turns out to be a strong and independent predictor of worse clinical prognosis in the HFpEF population, whereas prior ischemic heart disease does not. Elevated MPV was not impacted by classical predictors known to be associated with a worse prognosis in HFpEF, such as renal function and NT-proBNP. The prognostic impact of MPV has already been demonstrated in HF, whereas there is only one study that has previously demonstrated MPV’s prognostic impact in HFpEF [Citation43]. Indeed, Dahlen et al. [Citation18] revealed MPV levels to increase with the E/e’ ratio, displaying a stronger effect on prognostic deterioration in the HFpEF phenotype compared with the HFrEF phenotype. These results suggest that the platelets’ role is probably essential in disease progression, which should thus be further investigated.
Limitations
Our study displays several limitations. This is a single-center sampling of HFpEF patients, which likely limits the generalizability of our findings and their extrapolation to other settings. Although MPV has already demonstrated some usefulness in predicting mortality in HFpEF, its measurement is associated with several limitations, thereby restricting the extrapolation of these results. Indeed, MPV evaluation in clinical laboratories is routinely assessed within complete blood count testing, and it still suffers from lack of standardization. Several factors may influence MPV evaluation, including blood collection conditions, measurement conditions, and methodology used for platelet size evaluation. Furthermore, it has been previously shown in large cohorts that MPV increased with aging probably explained by comorbidities increasing over time [Citation44,Citation45]. However, we demonstrated that the MPV values, with blood samples collected and analyzed under the same conditions, remained significantly higher in HFpEF compared with a controlled population of similar age and gender. Interestingly, age alone does not erase the expected difference between these two groups. In addition, due to the limitations of generalizing MPV cutoff values, we identified a value associated with higher risk very close to the 75%ile in our HFpEF cohort. Thus, it might be worthwhile for each center to validate this cutoff value on their HFpEF population. However, further studies are needed to reproduce these results, involving a larger sample under other conditions, and to further explore MPV-specific cutoff values for predicting HFpEF outcomes.
Conclusion
HFpEF patients displayed significantly higher MPV levels than controls. The ele found in HFpEF patients likely supports the hypothesis that platelets play a role in HFpEF’s pathophysiology. MPV was a strong predictor of poor outcome, even after adjusting for usual confounding factors, and this parameter could thus serve as a valuable marker in clinical practice, enabling us to identify high-risk patients within the HFpEF population.
Author contributions
NM analyzed and interpreted patients’ data and wrote the manuscript. NM and SL prospectively recruited patients and collected data. SH, SL, DG, MAV, AP, DVC, BG, and LB provided advice on data analyses and presentation. ACP was the principal investigator of the study. CB, ACP, and SH were major contributors in writing the manuscript. All authors read and approved the final manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Savarese G, Lund LH. Global public health burden of heart failure. Card Fail Rev. 2017;3(1):7–10. doi: 10.15420/cfr.2016:25:2.
- Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263–271. doi: 10.1016/j.jacc.2013.02.092.
- Tromp J, Westenbrink BD, Ouwerkerk W, van Veldhuisen DJ, Samani NJ, Ponikowski P, Metra M, Anker SD, Cleland JG, Dickstein K, et al. Identifying pathophysiological mechanisms in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2018;72(10):1081–1090. doi: 10.1016/j.jacc.2018.06.050.
- Karpatkin S. Heterogeneity of human platelets. II. Functional evidence suggestive of young and old platelets. J Clin Invest. 1969;48(6):1083–1087. doi: 10.1172/JCI106064.
- Kamath S, Blann AD, Lip GY. Platelet activation: assessment and quantification. Eur Heart J. 2001;22(17):1561–1571. doi: 10.1053/euhj.2000.2515.
- Slavka G, Perkmann T, Haslacher H, Greisenegger S, Marsik C, Wagner OF, Endler G. Mean platelet volume may represent a predictive parameter for overall vascular mortality and ischemic heart disease. Arterioscler Thromb Vasc Biol. 2011;31(5):1215–1218. doi: 10.1161/ATVBAHA.110.221788.
- Gang L, Yanyan Z, Zhongwei Z, Juan D. Association between mean platelet volume and hypertension incidence. Hypertens Res. 2017;40(8):779–784. doi: 10.1038/hr.2017.30.
- Zaccardi F, Rocca B, Pitocco D, Tanese L, Rizzi A, Ghirlanda G. Platelet mean volume, distribution width, and count in type 2 diabetes, impaired fasting glucose, and metabolic syndrome: a meta-analysis. Diabetes Metab Res Rev. 2015;31(4):402–410. doi: 10.1002/dmrr.2625.
- Tekin G, Tekin YK, Sivri N, Yetkin E. Mean platelet volume in patients with nonvalvular atrial fibrillation. Blood Coagul Fibrinolysis. 2013;24(5):537–539. doi: 10.1097/MBC.0b013e32835facb3.
- Uçar H, Gür M, Koyunsever NY, Şeker T, Türkoğlu C, Kaypakli O, Şahin DY, Elbasan Z, Çayli M. Mean platelet volume is independently associated with renal dysfunction in stable coronary artery disease. Platelets. 2014;25(4):274–278. doi: 10.3109/09537104.2013.805406.
- Ding Q, Wang F, Guo X, Liang M. The relationship between mean platelet volume and metabolic syndrome in patients with type 2 diabetes mellitus: a retrospective study. Medicine (Baltimore). 2021;100(13):e25303. doi: 10.1097/MD.0000000000025303.
- Nardin M, Verdoia M, Barbieri L, De Luca G. Impact of metabolic syndrome on mean platelet volume and its relationship with coronary artery disease. Platelets. 2019;30(5):615–623. doi: 10.1080/09537104.2018.1499885.
- Kandis H, Ozhan H, Ordu S, Erden I, Caglar O, Basar C, Yalcin S, Alemdar R, Aydin M. The prognostic value of mean platelet volume in decompensated heart failure. Emerg Med J. 2011;28(7):575–578. doi: 10.1136/emj.2009.088401.
- Varol E, Ozaydin M. Mean platelet volume in differentiating congestive heart failure from chronic obstructive pulmonary disease. Int J Cardiol. 2014;172(2):e299. doi: 10.1016/j.ijcard.2013.12.191.
- Choi DH, Kang SH, Song H. Mean platelet volume: a potential biomarker of the risk and prognosis of heart disease. Korean J Intern Med. 2016;31(6):1009–1017. doi: 10.3904/kjim.2016.078.
- Roy C, Slimani A, de Meester C, Amzulescu M, Pasquet A, Vancraeynest D, Beauloye C, Vanoverschelde JL, Gerber BL, Pouleur AC. Associations and prognostic significance of diffuse myocardial fibrosis by cardiovascular magnetic resonance in heart failure with preserved ejection fraction. J Cardiovasc Magn Reson. 2018;20(1):55. doi: 10.1186/s12968-018-0477-4.
- Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277–314. doi: 10.1016/j.echo.2016.01.011.
- Dahlen B, Schulz A, Gobel S, Trobs SO, Schwuchow-Thonke S, Spronk HM, Prochaska JH, Arnold N, Lackner KJ, Gori T, et al. The impact of platelet indices on clinical outcome in heart failure: results from the MyoVasc study. ESC Heart Fail. 2021;8(4):2991–3001. doi: 10.1002/ehf2.13390.
- Rich JD, Burns J, Freed BH, Maurer MS, Burkhoff D, Shah SJ. Meta-analysis global group in chronic (MAGGIC) heart failure risk score: validation of a simple tool for the prediction of morbidity and mortality in heart failure with preserved ejection fraction. J Am Heart Assoc. 2018;7(20):e009594. doi: 10.1161/JAHA.118.009594.
- Roy C, Lejeune S, Slimani A, de Meester C, Ahn as SA, Rousseau MF, Mihaela A, Ginion A, Ferracin B, Pasquet A, et al. Fibroblast growth factor 23: a biomarker of fibrosis and prognosis in heart failure with preserved ejection fraction. ESC Heart Fail. 2020;7(5):2494–2507. doi: 10.1002/ehf2.12816.
- Paulus JM. Platelet size in man. Blood. 1975;46(3):321–336. doi: 10.1182/blood.V46.3.321.321.
- Leader A, Pereg D, Lishner M. Are platelet volume indices of clinical use? A multidisciplinary review. Ann Med. 2012;44(8):805–816. doi: 10.3109/07853890.2011.653391.
- Korniluk A, Koper-Lenkiewicz OM, Kaminska J, Kemona H, Dymicka-Piekarska V. Mean platelet volume (MPV): new Perspectives for an old marker in the course and prognosis of inflammatory conditions. Mediators Inflamm. 2019;2019:9213074. doi: 10.1155/2019/9213074.
- Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: a link between thrombosis and inflammation? Curr Pharm Des. 2011;17(1):47–58. doi: 10.2174/138161211795049804.
- Abernethy A, Raza S, Sun JL, Anstrom KJ, Tracy R, Steiner J, VanBuren P, LeWinter MM. Pro-inflammatory biomarkers in stable versus acutely decompensated heart failure with preserved ejection fraction. J Am Heart Assoc. 2018;7(8). doi: 10.1161/JAHA.117.007385.
- Chia YC, Kieneker LM, van Hassel G, Binnenmars SH, Nolte IM, van Zanden JJ, van der Meer P, Navis G, Voors AA, Bakker SJL, et al. Interleukin 6 and development of heart failure with preserved ejection fraction in the general population. J Am Heart Assoc. 2021;10(11):e018549. doi: 10.1161/JAHA.120.018549.
- Ishibashi T, Kimura H, Uchida T, Kariyone S, Friese P, Burstein SA. Human interleukin 6 is a direct promoter of maturation of megakaryocytes in vitro. Proc Natl Acad Sci USA. 1989;86(15):5953–5957. doi: 10.1073/pnas.86.15.5953.
- Williams N, Bertoncello I, Jackson H, Arnold J, Kavnoudias H.The role of interleukin 6 in megakaryocyte formation, megakaryocyte development and platelet production. Ciba Found Symp. 1992;167:160–170. discussion 70-3.
- Burstein SA. Effects of interleukin 6 on megakaryocytes and on canine platelet function. Stem Cells (Dayton, Ohio). 1994;12(4):386–393. doi: 10.1002/stem.5530120405.
- Senchenkova EY, Komoto S, Russell J, Almeida-Paula LD, Yan LS, Zhang S, Granger DN. Interleukin-6 mediates the platelet abnormalities and thrombogenesis associated with experimental colitis. Am J Pathol. 2013;183(1):173–181. doi: 10.1016/j.ajpath.2013.03.014.
- Karpatkin S. Heterogeneity of human platelets. VI. Correlation of platelet function with platelet volume. Blood. 1978;51(2):307–316. doi: 10.1182/blood.V51.2.307.307.
- Martin JF, Trowbridge EA, Salmon G, Plumb J. The biological significance of platelet volume: its relationship to bleeding time, platelet thromboxane B2 production and megakaryocyte nuclear DNA concentration. Thromb Res. 1983;32(5):443–460. doi: 10.1016/0049-3848(83)90255-4.
- Breimo ES, Osterud B. Studies of biological functions in blood cells from individuals with large platelets. Platelets. 2003;14(7–8):413–419. doi: 10.1080/02697450310001632597.
- Karpatkin S, Freedman ML. Hypersplenic thrombocytopenia differentiated from increased peripheral destruction by platelet volume. Ann Intern Med. 1978;89(2):200–203. doi: 10.7326/0003-4819-89-2-200.
- Kanagala P, Arnold JR, Khan JN, Singh A, Gulsin GS, Squire IB, McCann GP, Ng LL. Plasma P-selectin is a predictor of mortality in heart failure with preserved ejection fraction. ESC Heart Fail. 2021;8(3):2328–2333. doi: 10.1002/ehf2.13280.
- Liu G, Ma C, Yang H, Zhang PY. Transforming growth factor beta and its role in heart disease. Exp Ther Med. 2017;13(5):2123–2128. doi: 10.3892/etm.2017.4246.
- Karolczak K, Watala C. Blood platelets as an important but underrated circulating source of TGFβ. Int J Mol Sci. 2021;22(9):4492. doi: 10.3390/ijms22094492.
- Meyer A, Wang W, Qu J, Croft L, Degen JL, Coller BS, Ahamed J. Platelet TGF-beta1 contributions to plasma TGF-beta1, cardiac fibrosis, and systolic dysfunction in a mouse model of pressure overload. Blood. 2012;119(4):1064–1074. doi: 10.1182/blood-2011-09-377648.
- Bielecka-Dabrowa A, Sakowicz A, Misztal M, von Haehling S, Ahmed A, Pietrucha T, Rysz J, Banach M. Differences in biochemical and genetic biomarkers in patients with heart failure of various etiologies. Int J Cardiol. 2016;221:1073–1080. doi: 10.1016/j.ijcard.2016.07.150.
- Asher E, Fefer P, Shechter M, Beigel R, Varon D, Shenkman B, Savion N, Hod H, Matetzky S. Increased mean platelet volume is associated with non-responsiveness to clopidogrel. Thromb Haemost. 2014;112(1):137–141. doi: 10.1160/TH13-10-0845.
- Varol E, Aksoy F, Bas HA, Ari H, Ozaydin M. Mean platelet volume is elevated in patients with low high-density lipoprotein cholesterol. Angiology. 2014;65(8):733–736. doi: 10.1177/0003319713504024.
- Valiyaveettil M, Kar N, Ashraf MZ, Byzova TV, Febbraio M, Podrez EA. Oxidized high-density lipoprotein inhibits platelet activation and aggregation via scavenger receptor BI. Blood. 2008;111(4):1962–1971. doi: 10.1182/blood-2007-08-107813.
- Erne P, Wardle J, Sanders K, Lewis SM, Maseri A. Mean platelet volume and size distribution and their sensitivity to agonists in patients with coronary artery disease and congestive heart failure. Thromb Haemost. 1988;59(2):259–263. doi: 10.1055/s-0038-1642766.
- Demirin H, Ozhan H, Ucgun T, Celer A, Bulur S, Cil H, Gunes C, Yildirim HA. Normal range of mean platelet volume in healthy subjects: insight from a large epidemiologic study. Thromb Res. 2011;128(4):358–360. doi: 10.1016/j.thromres.2011.05.007.
- Lippi G, Meschi T, Borghi L. Mean platelet volume increases with aging in a large population study. Thromb Res. 2012;129(4):e159–60. doi: 10.1016/j.thromres.2011.12.031.