?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Thrombocytopenia represents one of the most prevalent hematologic complications observed in patients infected with the human immunodeficiency virus (HIV). In this study, we sought to analyze the clinical characteristics and treatment outcomes of patients with coexisting HIV and thrombocytopenia. Specifically, we retrospectively examined the medical records of 45 patients diagnosed with HIV/AIDS and thrombocytopenia at the Yunnan Infectious Diseases Specialist Hospital between January 2010 and December 2020, all of whom received highly active antiretroviral therapy (HAART) with/without glucocorticoids. The median follow-up period was 79 days, ranging between 14 and 368 days, the total platelet count was higher after receiving treatment than before (Z = −5.662, P < .001). Among the cohort, 27 patients (60.0%) responded to treatment, with 12 patients (44.44%) experiencing relapse during the follow-up period. The response rate (80.00%) of newly diagnosed ITP were significantly higher than of persistent ITP (28.57%) and chronic ITP (38.46%) (
2 = 9.560, P = .008) and the relapse rate of the newly diagnosed ITP (30.00%) was significantly lower than the persistent ITP and chronic ITP (100.00%, 80.00%) (
2 = 6.750, P = .034). Notably, we found that the number of CD4+ T cells, duration of HIV infection, selection of HAART and type of glucocorticoids administered displayed no statistically significant effect on platelet count, treatment response, or relapse rate. However, we observed a significant decrease in platelet count in hepatitis C virus-positive individuals coinfected with HIV compared to those with HIV alone (Z = −2.855, P = .003). Our findings suggest that patients diagnosed with HIV and thrombocytopenia exhibit a low response rate to treatment and have an increased likelihood of relapse.
Introduction
HIV infection is characterized by progressive damage to the body’s immune system, which results in a number of opportunistic infections and immunological and hematological complications. Citation1 An association between immune thrombocytopenia (ITP) and the acquired immune deficiency syndrome (AIDS) was first recognized in 1982.Citation2 ITP is the most common cause of thrombocytopenia in HIV-infected patients, and thrombocytopenia can occur at any stage of HIV infection.Citation3 Prior to the advent of highly active antiretroviral therapy (HAART), the incidence of HIV-associated thrombocytopenia was estimated at 10–30%. With the advent of HAART, the incidence of HIV-associated thrombocytopenia has been significantly reduced.Citation4
In HAART era, thrombocytopenia still represents a clinically important complication among HIV patients for a number of reasons. First, thrombocytopenia is associated with an increase in the risk of serious bleeding events, although few fatal bleeding events have been reported.Citation5–7 Second, thrombocytopenia is the initial clinical manifestation in about 10% of HIV cases and is frequently one of the first manifestations of advancing disease.Citation8,Citation9 Additionally, thrombocytopenia has been associated with decreased survival and other complications of HIV disease.Citation10–12 The cause of HIV-induced thrombocytopenia is unclear, and several studies have shown that progressively higher HIV viral loads and lower CD4+ T cell counts increase the incidence and severity of thrombocytopenia as the disease progresses.Citation5,Citation13 Thrombocytopenia in HIV patients has secondary causes, such as thrombocytopenia caused by antiviral drugs and HIV-related opportunistic diseases, and co-infection with HCV is a high-risk factor for thrombocytopenia in patients with HIV.Citation14 Some HAART drugs such as zidovudine and ritonavir also have the risk of causing thrombocytopenia. There are six major classes of drugs approved for the treatment of HIV, including Nucleoside reverse transcriptase inhibitors (NRTls), non-nucleoside reverse transcriptase inhibitors (NNRTIs), protease inhibitors (Pls), fusion inhibitor (FI), integrase inhibitors (INSTIs) and CCR5 inhibitors.
There are few reports on HIV combined thrombocytopenia in China, and this retrospective study analyzed the clinical data of 45 patients with HIV/AIDS combined with thrombocytopenia admitted to the Department II of Infectious Diseases of Yunnan Infectious Diseases Specialist Hospital from 2010 to 2020, summarized the clinical symptoms and treatment effects of HIV/AIDS combined with thrombocytopenia, exploring factors affecting treatment efficacy of HIV-related immune thrombocytopenia.
Method
Patients
Total of 45 patients diagnosed with HIV/AIDS combined with thrombocytopenia in the Department II of Infectious Diseases of Yunnan Infectious Diseases Specialist Hospital between January 2010 and December 2020 were collected. The diagnosis of HIV infection was in accordance with “Interpretation of Consolidated Guidelines on HIV Prevention, Testing, Treatment, Service Delivery and Monitoring: Recommendations for a Public Health Approach, WHO 2021.”Citation15 Thrombocytopenia diagnostic criteria, disease staging were referenced to the “Chinese guidelines for treatment of adult primary immune thrombocytopenia (2020).”Citation16 General conditions, clinical manifestations, signs, and antiviral regimens were collected for all cases. All patients agreed to use the clinical information for is study.
Co-infection
Co-infection is defined as the concurrent presence of HIV infection and infection with other pathogens. HBsAg positivity in serum is defined as being hepatitis B positive. Being positive for anti-HCV antibodies and HCV-RNA detection defines as hepatitis C positive. Syphilis was defined as a double positive result in serum test (TRUST+ and TPPA+). Positive smear microscopy or mycobacterial culture is defined as tuberculosis. EBV-DNA quantification >5.0E + 03 copies/ml defined as EBV infection.
Efficacy judgment
The primary end points were treatment response rate (including complete response (CR) and Response (R)). CR was defined as platelet count ≥100 × 109/L and absence of bleeding.Citation17 R as platelet count ≥30 × 109/L and at least twofold increase of the baseline count and absence of bleeding.Citation16,Citation17 No response (NR) as platelet count <30 × 109/L or less than twofold increase of the baseline platelet count or bleeding.Citation17 Relapse is defined as platelet count decreasing to less than 30 × 109/L or less than two times the basal value after effective treatment or reappearance of bleeding symptoms.Citation17 Definition of CR or R should contain two or more platelet count tests with interval at least 7 days. Definition of relapse with at least two tests with interval at least 1 day. Refractory ITPCitation16: Patients diagnosed with ITP after reevaluation of patients who have failed to respond to first- and second-line therapies, or who have had an ineffective splenectomy/post-operative recurrence.
Based on the duration of the disease course, ITP is classified into the following three phasesCitation17: (1) Newly diagnosed ITP: within 3 months from diagnosis. (2) Persistent ITP: between 3 and 12 months from diagnosis. Includes patients not reaching spontaneous remission or not maintaining complete response of therapy. (3) Chronic ITP: lasting for more than 12 months.
Statistical analysis
SPSS 23.0 software was used for statistical analysis. For inter-group comparison of measurement data, one-way ANOVA or t-test was used for normally distributed data, and Kruskal–Wallis H-test or Mann–Whitney U-test was used for non-normal data. Chi-square test was used for analysis of variance for count data, at the test level α = 0.05. Spearman Correlation test was used to test the correlation between the two groups.
Result
Clinical characteristics of participants
Among the 45 patients, 26 (57.78%) were male and 19 (42.22%) were female. The median age was 43 (8–71) years old, and 46.67% (21/45) were under 40 years old. Two (4.44%) patients were classified as men who have sex with men (MSM) and 25 (55.56%) heterosexual. Among all patients, 8(17.78%) also had history of intravenous drug use (IVDU). Ten patients (22.22%) had unknown routes of infection (Four of these patients had history of blood transfusions or surgery). Patients had platelet count of 10 (0–64, M, range) × 109/L at admission, 37 patients (82.22%) with platelet count <20 × 109/L, the hemoglobin count was 132 (60–160, M, range)g/L, 14 patients (31.11%) had hemoglobin below 90 g/L, and neutrophil count was 2.86 (1.05–19.06 (M, range)) × 109/L. The common bleeding sites were skin ecchymosis and epistaxis. The clinical characteristics of patients are shown in . The total follow-up duration was 79 (14–368) days, the platelet count of final follow-up was 37 (2–309, M, range) × 109/L, significantly higher than that at admission (Z = −5.662, P < .001).
Table I. Clinical characteristics of 45 patients with HIV combined with ITP.
Bone marrow cytology examination results
All patients underwent bone marrow aspiration cytology, bone marrow cell smear were observed under ordinary light after wright’s stain. Microscope myeloid cells were actively proliferating in 43 patients and hypoproliferating in two patients. All 45 cases showed impaired maturation of megakaryocytes, including 37 cases with increased number of megakaryocytes, four cases with normal and four cases with decreasing number of megakaryocytes.
Factors influencing the therapeutic effect of HIV-related thrombocytopenia
(1) HAART
Among the 45 patients in our study, thrombocytopenia was noted as the initial clinical symptom in 29 patients (64.44%). The median duration between the onset of thrombocytopenia and HIV diagnosis was 55 days (8–1836). Sixteen patients (35.56%) had initiated HAART prior to hospital admission, with a median treatment duration of 554 (2–2310) days, the median duration between HIV diagnosis and the development of thrombocytopenia was 427 days (15–3927) in those patients. There was no difference in treatment protocol between the two groups (χ2 = 4.857, P = .088). There was no significant difference between the two groups in terms of the platelet count at initial HIV diagnosis (Z = −0.498, P = .618), treatment response rate (χ2 = 0.005, P = .944), or relapse rate (χ2 = 0.267, P = .606), . We observed no correlation between the duration of HIV infection and the degree of platelet reduction in our study. No significant effect on final platelet count with or without HAART prior to hospital admission (Z = −0.962,P = .336). See .
Figure 1. (a) There was no significant difference in platelet counts before and after treatment between patients who had used HAART before admission and those who had reduced platelets as the initial symptom; (b) There was no significant difference in platelet counts before and after treatment between patients who used different HAART. the platelet counts after treatment were significantly higher than before treatment in all groups. (c, d) There were no significant differences in treatment efficiency and recurrence rates between HAART use before admission, different HAART regimens, and different ITP treatment regimens.
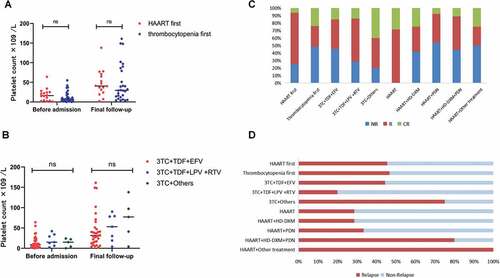
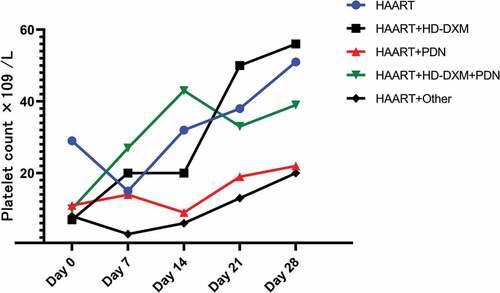
The HAART regimens of 33 patients (73.33%) consisted of lamivudine + Tenofovir + Efavirenz (3TC+TDF+EFV), while 7 patients (15.56%) received lamivudine + Tenofovir + Lopinavir and Ritonavir (3TC+TDF+LPV+RTV), the remaining 5 patients (11.11%) received Lamivudine + Others (including two cases of lamivudine + Abacavir + Efavirenz, one case each of Lamivudine + Tenofovir + Ritonavir, Lamivudine + Abacavir + Lopinavir and Ritonavir, and Lamivudine + Stavudine + Efavirenz). Before treatment, there were no significant differences in platelet counts among the three groups (H = 1.505, P = .471). No significant difference in platelet counts was observed among patients receiving different HAART regimens after a median follow-up of 79 days (H = −1.727, P = .422), . After treatment, platelet counts in all groups showed a significant increase compared to the pre-treatment levels (Z = −4.853, P < .001; Z = −2.028, P = .043; Z = −4.000, P < .001). There were no statistically significant differences in the treatment response rates and recurrence rates among the three groups (χ2 = 0.680, P = .712. χ2 = 2.723, P = .256), See .
Considering the potential adverse effect of Ritonavir on platelet counts, we also conducted an analysis of the treatment efficacy of HAART with or without ritonavir. We found that there was no statistically significant difference in platelet counts before and after treatment between the eight patients (17.78%) who received ritonavir and the 37 patients (82.22%) who did not receive ritonavir in this study (Z = −1.354, P = .176. Z = −1.099, P = .282). However, the platelet counts of both groups showed a significant increase after treatment compared to before (P < .001, P < .001). There were no statistically significant differences in the treatment response rates and recurrence rates among the two groups (χ2 = 0.912, P = .340. χ2 = 2.411, P = .121). See details in Supplemental Tables S1.
(2) ITP treatment
After being diagnosed with HIV infection, all patients received HAART. In addition to general supportive therapy, seven patients (15.56%) were treated with HAART alone for thrombocytopenia, twelve patients (26.67%) received HAART combined with high-dose dexamethasone (HD-DXM, 40 mg/day for 4 days), thirteen patients (28.89%) received HAART in combination with conventional prednisone (PDN, typically administered at 0.5 to 2 mg/kg body weight daily for 4 weeks at maximum dose followed by tapering), nine patients (20.00%) received HAART combined with HD-DXM and PDN, and four patients (8.89%) received HAART in combination with other treatments (IVIG, danazol, vincristine, recombinant human thrombopoietin). Patients treated with HAART alone had higher initial platelet counts than the other groups (H = 13.300, p = .010). See details in Supplemental Table S2.
At the time of the last follow-up visit, with 27 patients (60.0%) achieving an R response, among them, eight patients (17.8%) achieved complete remission (CR), while 18 patients (40.0%) did not respond to treatment. The median time to achieve R was 26 (12–44) days after treatment. The platelet counts (H = 6.715, p = .152), time to achieve R (H = 2.359, p = .670), and response rates (χ2 = 7.001, p = .136) did not show significant differences among the five treatment groups, . During the follow-up period, 12 patients (44.44%) experienced relapse, time to relapse was 11.5 (9–35) days after achieving R. There was no statistically significant difference in the relapse rate among patients treated with different therapies (χ2 = 2.336, p = .674). The platelet count at relapse was 19 (4–77, M, range) × 109/L, which was not significantly different from 20.5 (4–55, M, range) × 109/L at the time of initial diagnosis (Z = −0.089, P = .929). All treatment groups, except for the HAART+Other group (Z = −1.826, P = .068), demonstrated a significant increase in platelet count after treatment compared to admission (P < .05). No thrombotic events were observed during the follow-up period in any of the patients. The treatment outcomes of all 45 patients during the follow-up period are presented in .
(3) ITP phases
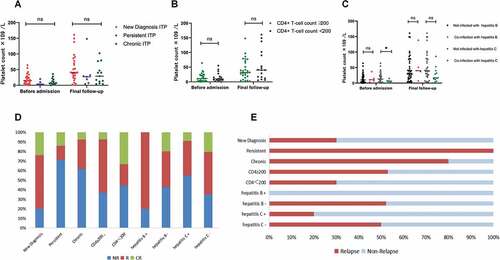
Among 45 patients, 25 (55.6%) were newly diagnosed with ITP, 7 (15.6%) had persistent ITP, and 13 (28.8%) had chronic ITP. Both persistent and chronic ITP patients had history of received first-line treatment regimens including glucocorticoids and IVIG. There was no statistically significant difference in platelet counts among the three groups of patients before treatment (H = 4.028, P = .133). After treatment, the platelet count of newly diagnosed ITP patients was higher than the other two groups, but there was no statistically significant difference (H = 4.356, P = .113), . In terms of treatment efficacy, the response rate (80.00%) and CR rate (24.00%) of newly diagnosed ITP were significantly higher than those of persistent ITP (28.57% and 14.29%, respectively) and chronic ITP (38.46% and 7.69%, respectively) (χ2 = 9.560, P = .008). In addition, the relapse rate of the newly diagnosed ITP (30.00%) was significantly lower than the other two groups (100.00%, 80.00%) (χ2 = 6.750, P = .034). There was no significant difference in the response and relapse rates between persistent and chronic ITP, .
Figure 3. (a) There was no statistically significant difference in platelet counts before and after treatment at different ITP phases. (b) There was no significant correlation between CD4+ T-cell count and platelet count. (c) Platelet count in HIV patients co-infected with hepatitis C was significantly lower before treatment than that in HIV patients without co-infected with hepatitis C. The platelet counts of patients co-infected with hepatitis B or hepatitis C were all higher after treatment than before. (d, e) the newly diagnosed ITP showed a higher response rate and a lower relapse rate than persistent and chronic ITP. CD4+ T-cell counts, co-infected with hepatitis B virus, and co-infected with hepatitis C virus had no significant effect on the treatment effect and relapse rate of patients with HIV-associated thrombocytopenia.
(4) CD4+ T cell count
The CD4+ T-cell count was 262 (50–1311, M, range) cells/µL in all patients, with 18 patients having CD4+ T-cell count <200 cells/µL, there was no significant correlation between CD4+ T-cell count and platelet count (Pearson correlation = −0.075, P = .624) at the initial diagnosis. Among patients with CD4+ T-cell count <200 cells/µL, the platelet count was 9 (1–55, M, range)×109/L, while it was 10 (1–64, M, range) × 109/L in patients with CD4+ T-cell count ≥200 cells/µL. The platelet count did not show statistically significant difference between the two groups prior to hospital admission (Z = −0.383, P = .702), . At the final follow-up, the CD4+ T-cell count was 294 (87–1378, M, range) cells/µL in all patients, which was significantly higher than the initial diagnosis (P = .038), but we still did not find significant correlation between CD4+ T-cell count and platelet count (Pearson correlation = −0.270, P = .072). Among patients with CD4+ T-cell count ≥200 cells/µL, 17 (62.96%) reached R (nine relapsed), while 10 (60.00%) patients with CD4+ T-cell count <200 cells/µL reached R (three relapsed). These findings indicate that the initial CD4+ T-cell count did not significantly affect the treatment response and relapse rate (χ2 = 0.247, P = .619; χ2 = 1.342, P = .247), .
(5) HIV co-infection with hepatitis B or C
Among the 45 HIV patients, five had co-infection with hepatitis B virus, eleven with hepatitis C virus, three with syphilis, three with mycobacterium tuberculosis, and one with EBV infection. None of the HIV patients with co-infection with hepatitis B or C received regular anti-hepatitis virus treatment prior to hospital admission. At initial diagnosis, the platelet count was 11 (3–37, M, range) × 109/L in HIV patients co-infected with hepatitis B and 10 (1–64, M, range) × 109/L in HIV patients who are negative for hepatitis B. There was no significant difference in platelet count between the two groups (Z = −0.470, P = .638). In HIV patients co-infected with hepatitis C, the platelet count at the initial diagnosis was 6 (1–18, M, range) × 109/L, which was significantly lower than that (12 (1–64, M, range)×109/L) of HIV patients without hepatitis C virus infection (Z = −2.855, P = .003). HIV-related thrombocytopenia patients co-infected with hepatitis B or C virus both showed significant increase in platelet count after treatment compared to pre-treatment levels (Z = −2.023, P = .043; Z = −2.669, P = .008), .
Five HIV patients with co-infection with hepatitis B had hepatitis B viral load below the minimum detection value, and three patients received HAART prior to hospital admission. After receiving treatment, 4 (80.0%) HIV patients with hepatitis B reached R, and no relapse was observed during the follow-up period. Hepatitis B infection had no effect on the response to treatment and relapse rate (χ2 = 1.361, P = .243; χ2 = 2.045, P = .153). One HIV patient with co-infection with hepatitis C received HAART prior to hospital admission, the viral load of hepatitis C in 11 patients with HIV co-infection was 7 210 000 (1000–34000000, M, range) IU/mL, and there was no significant correlation between viral load and platelet count (Pearson correlation = 0.134, P = .695). For those co-infected with hepatitis C virus, only five cases (41.7%) achieved R, and one patient (20.0%) relapsed during follow-up. Although the treatment response rate was lower than that of hepatitis C-uninfected HIV patients (64.71%), there was no statistically significant difference in the treatment response rate and relapse rate between the two groups (χ2 = 0.363, P = .547; χ2 = 2.166, P = .141), . See details in Supplemental Tables S3.
Discussion
In this study, we retrospectively analyzed the clinical data of 45 HIV patients combined with thrombocytopenia. All patients were treated with HAART with/without glucocorticoids, 18 (40.00%) of the 45 patients did not respond to treatment. In addition, we did not find a correlation between CD4+ T cells, time of HIV infection and platelet count, which may be related to the fact that some patients had already started HAART before thrombocytopenia. The newly diagnosed ITP showed a higher treatment response rate than persistent and chronic ITP, and a lower relapse rate. Similar to previous studies,Citation18 our study found that platelet counts were significantly lower in hepatitis C co-infected HIV patients compared to hepatitis C-uninfected HIV patients and there was no significant correlation between hepatitis C viral load and platelet count.
In this study, among the 45 HIV patients included, nearly two-thirds of the patients presented with thrombocytopenia as the initial symptom. Additionally, 16 patients had received HAART prior to admission. However, there were no significant differences in platelet counts at admission and during follow-up between these two groups of patients, indicating that a considerable proportion of patients may experience a decrease in platelet count despite HAART use. This may be related to inadequate HIV viral load reduction, irregular medication adherence, and/or comorbid opportunistic infections. Therefore, it is necessary to combine other treatment regimens for HIV patients with severe thrombocytopenia to achieve effective treatment.
For patients with detectable HIV viral loads, first-line treatment approaches involve optimizing HAART followed by standard ITP options used to treat those without HIV infection.Citation19,Citation20 But there are several reports of thrombocytopenia due to NRTIs, patients on zidovudine (AZT)-based therapy were more likely to have thrombocytopenia (16.3%) than patients on tenofovir (TDF)-based therapy (14.8%).Citation21 Marchionatti et al. also reported that zidovudine-based ART regimens produced a higher proportion of thrombocytopenia.Citation22 Abacavir may alter platelet activation and influence thrombus formation through multiple pathways, it may lead to some extent to the clearance of platelets from circulation.Citation23 There have also been reports of severe thrombocytopenia following the use of lopinavir/ritonavir.Citation24 There were no patients in our cohort who received zidovudine, but eight patients received HAART including ritonavir. The platelet counts of these eight patients did not show statistically significant differences before and after treatment compared to the other patients, which may be due to the small sample size of patients receiving ritonavir and the use of other drugs. Only one patient used abacavir in this study, so no statistical analysis was performed. The majority of patients received HAART based on lamivudine and tenofovir, and from the CD4+ T cell count at the last follow-up, this treatment regimen achieved satisfactory results in reducing HIV load. However, no correlation was found between CD4+ T cell counts and platelet reduction in this study; this is inconsistent with previous reports,Citation25 which may be related to some patients starting HAART before admission. Because lower HIV viral load and HAART exposure were significantly associated with decreased risk of thrombocytopenia,Citation26 thrombocytopenic HIV patients should start HAART therapy excluding zidovudine as soon as possible and changing HAART drugs promptly if drug-related thrombocytopenia is observed.
However, for HIV-related thrombocytopenia, lower response rates and higher relapse rates are still unresolved issues. In this study, over one-third of patients did not respond to treatment. Especially in persistent and chronic ITP, the treatment response rate was low and the relapse rate was high. In the HAART + other group, there was no significant increase in platelet count after treatment compared to before treatment, which may be related to the fact that all patients in this group had refractory recurrent thrombocytopenia. Almost all patients in our study used HAART + glucocorticoids as the first treatment regimen, overall response rate was 60.00%, the type of glucocorticoid had no significant effect on treatment efficiency and recurrence rate. Compared to the patients (82.1% and 67.4%) without HIV infection in prior studies,Citation27 HIV-infected patients with thrombocytopenia in this study demonstrated a lower response rate to HD-DXM (58.88%) and PDN (46.15%). In addition, 44.44% patients in our study required secondary treatment due to loss of response, and the relapse rate was not significantly associated with the patient’s treatment regimen. We speculate that this may be related to the presence of cross-reactive antibody between HIV viral proteins and PLT glycoproteins, the inhibitory effect of HIV on megakaryocyte growth and the bone marrow suppressive effects of certain HAART medications.Citation28,Citation29 Therefore, we need to search for more effective treatment regimens for HIV-related thrombocytopenia. In a series case reports by Mark et al., eltrombopag can improve platelet counts in refractory HIV-associated ITP patients.Citation20 The combination of HAART with second-line drugs for ITP may be a beneficial approach to consider.
Co-infection with HCV is a high risk factor for thrombocytopenia in patients with HIV. In a retrospective study conducted in Taiwan that excluded other infectious factors and included 1,803 patients with HCV and 1,652 patients with HBV, HCV was associated with higher thrombocytopenia incidence than HBV, prevalence of platelet <100 × 109/L was 11.86% and 6.35% in HCV and HBV patients without cancer history, respectively.Citation30 Ivanova L et al. reported that patients co-infected with HCV–HIV had a thrombocytopenia prevalence of 47.6% and patients mono-infected with HCV had only 3.2%.Citation31 In addition, the lower log of the HCV and HIV viral load was significantly associated with thrombocytopenia.Citation32 Our study is consistent with previous reports that HIV patients who co-infected with HCV had lower platelet counts compared to patients infected with only HIV prior to treatment, but we did not find a correlation between HCV viral load and platelet count. In this study, co-infection with HCV did not affect the treatment response of HIV-associated thrombocytopenia.
There are few clinical studies on HIV-combined thrombocytopenic patients in China, since Yunnan is a province with a high prevalence of HIV, we compared and analyzed the clinical data of 45 local patients in Yunnan. The main limitation of our study is retrospective and the lack of HIV viral load-related data. Approximately 40% of the patients in this study did not achieve treatment response and had high relapse rate, so we should continue to search for new approaches to treat HIV-related ITP.
Authorship
YaXian Tan, Lei Che, Hui Bi, and ZePing Zhou drafted the manuscript, calculated statistics, and prepared the figures. Shanshan Fan and HaiYan Min were involved in patient management. All authors were involved in data collection and proofreading.
Supplemental Material
Download PDF (425.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/09537104.2023.2200836.
Additional information
Funding
References
- Duguma N, Tesfaye KG, Adissu MW, Bimerew LG. Hematological parameters abnormalities and associated factors in HIV-positive adults before and after highly active antiretroviral treatment in Goba Referral Hospital, southeast Ethiopia: a cross-sectional study. SAGE Open Med. 2021;9:20503121211020175. doi:10.1177/20503121211020175.
- Morris L, Distenfeld A, Amorosi E, Karpatkin S. Autoimmune thrombocytopenic purpura in homosexual men. Ann Intern Med. 1982;96(6_part_1):714–7. doi:10.7326/0003-4819-96-6-714.
- Tamir Z, Seid A, Haileslassie H. Magnitude and associated factors of cytopenias among antiretroviral therapy naive human immunodeficiency virus infected adults in Dessie, Northeast Ethiopia. PLoS One. 2019;14(2):e0211708. doi:10.1371/journal.pone.0211708.
- Schoonen WM, Kucera G, Coalson J, Li L, Rutstein M, Mowat F, Fryzek J, Kaye JA. Epidemiology of immune thrombocytopenic purpura in the general practice research database. Br J Haematol. 2009;145(2):235–44. doi:10.1111/j.1365-2141.2009.07615.x.
- Sloand EM, Klein HG, Banks SM, Vareldzis B, Merritt S, Pierce P. Epidemiology of thrombocytopenia in HIV infection. Eur J Haematol. 1992;48(3):168–72. doi:10.1111/j.1600-0609.1992.tb00591.x.
- Marks KM, Clarke RM, Bussel JB, Talal AH, Glesby MJ. Risk factors for thrombocytopenia in HIV-infected persons in the era of potent antiretroviral therapy. J Acquir Immune Defic Syndr. 2009;52(5):595–9. doi:10.1097/QAI.0b013e3181b79aff.
- Liebman HA. Viral-associated immune thrombocytopenic purpura. Hematology Am Soc Hematol Educ Program. 2008;2008(1):212–8. doi:10.1182/asheducation-2008.1.212.
- Mientjes GH, van Ameijden EJ, Mulder JW, Hoek JARVD, Coutinho RA, Borne AEVD. Prevalence of thrombocytopenia in HIV-infected and non-HIV infected drug users and homosexual men. Br J Haematol. 1992;82(3):615–9. doi:10.1111/j.1365-2141.1992.tb06476.x.
- Stasi R, Willis F, Shannon MS, Gordon-Smith EC. Infectious causes of chronic immune thrombocytopenia. Hematol Oncol Clin North Am. 2009;23(6):1275–97. doi:10.1016/j.hoc.2009.08.009.
- Sullivan PS, Hanson DL, Chu SY, Jones JL, Ward JW. Epidemiology of anemia in human immunodeficiency virus (HIV)-infected persons: results from the multistate adult and adolescent spectrum of HIV disease surveillance project. Blood. 1998;91(1):301–8. doi:10.1182/blood.V91.1.301.
- Burbano X, Miguez MJ, Lecusay R, Rodriguez A, Ruiz P, Morales G, Castillo G, Baum M, Shor-Posner G. Thrombocytopenia in HIV-infected drug users in the HAART era. Platelets. 2001;12(8):456–61. doi:10.1080/09537100120093956.
- Wachtman LM, Skolasky RL, Tarwater PM, Esposito D, Schifitto G, Marder K, McDermott MP, Cohen BA, Nath A, Sacktor N, et al. Platelet decline: an avenue for investigation into the pathogenesis of human immunodeficiency virus -associated dementia. Arch Neurol. 2007;64(9):1264–72. doi:10.1001/archneur.64.9.1264.
- Servais J, Nkoghe D, Schmit JC, Arendt V, Robert I, Staub T, Moutschen M, Schneider F, Hemmer R. HIV-associated hematologic disorders are correlated with plasma viral load and improve under highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;28(3):221–5. doi:10.1097/00042560-200111010-00003.
- Teh BS, Lu HH, Lynch GR, Banez E, Kroll MH. AIDS-related Kaposi’s sarcoma involving bone and bone marrow. South Med J. 1999;92(1):61–4. doi:10.1097/00007611-199901000-00012.
- Who consolidated guidelines on hiv prevention: testing, treatment, service delivery and monitoring: recommendations for a public health approach; 2021.
- Liu XG, Bai XC, Chen FP, Cheng YF, Dai KS, Fang MY, Feng JM, Gong YP, Guo T, Guo XH, et al. Chinese guidelines for treatment of adult primary immune thrombocytopenia. Int J Hematol. 2018;107(6):615–23. doi:10.1007/s12185-018-2445-z.
- Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, Bussel JB, Cines DB, Chong BH, Cooper N, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–93. doi:10.1182/blood-2008-07-162503.
- Ciernik IF, Cone RW, Fehr J, Weber R. Impaired liver function and retroviral activity are risk factors contributing to HIV-associated thrombocytopenia. Swiss HIV cohort study. Aids. 1999;13(14):1913–20. doi:10.1097/00002030-199910010-00014.
- Grainger ST JD. A practical guide to the use of eltrombopag in children with chronic immune thrombocytopenia. Pediatr Hematol Oncol. 34(2), 73–89. doi:10.1080/08880018.2017.1313918.
- Kowalczyk M, Rubinstein PG, Aboulafia DM. Initial experience with the use of thrombopoetin receptor agonists in patients with refractory HIV-Associated immune thrombocytopenic purpura: a case series. J Int Assoc Provid AIDS Care. 2015;14(3):211–6. doi:10.1177/2325957414557266.
- Talargia F, Getacher L. Thrombocytopenia and associated factors among HIV infected patients in pre- and post-anti-retroviral therapy, North East Ethiopia. J Blood Med. 2021;12:741–8. doi:10.2147/JBM.S323086.
- Marchionatti A, Parisi MM. Anemia and thrombocytopenia in people living with HIV/AIDS: a narrative literature review. Int Health. 2021;13(2):98–109. doi:10.1093/inthealth/ihaa036.
- Taylor KA, Smyth E, Rauzi F, Cerrone M, Khawaja AA, Gazzard B, Nelson M, Boffito M, Emerson M. Pharmacological impact of antiretroviral therapy on platelet function to investigate human immunodeficiency virus-associated cardiovascular risk. Br J Pharmacol. 2019;176(7):879–89. doi:10.1111/bph.14589.
- Colebunders R, De Schacht C, Vanwolleghem T, Callens S. Lopinavir/ritonavir- and indinavir-induced thrombocytopenia in a patient with HIV infection. Int J Infect Dis. 2004;8(5):315–316.
- Li B, Zhang L, Liu Y, Xiao J, Wang X, Wei Y, Fan L, Duan Y, Li G, Kong Y, et al. Manifestations and related risk factors of thrombocyte abnormalities in HIV-Positive patients before and after the initiation of ART. Infect Drug Resist. 2021;14:4809–19. doi:10.2147/IDR.S334046.
- Nka AD, Sosso SM, Fokam J, Bouba Y, Teto G, Simo Rachel R, Tiga A, Yimga J, Nukenine EN, Nanfack AJ, et al. Thrombocytopenia according to antiretroviral drug combinations, viremia and CD4 lymphocytes among HIV-infected patients in Cameroon: a snapshot from the City of Yaoundé. BMC Res Notes. 2019;12(1):632. doi:10.1186/s13104-019-4664-7.
- Wei Y, Ji XB, Wang YW, Wang J-X, Yang E-Q, Wang Z-C, Sang Y-Q, Bi Z-M, Ren C-A, Zhou F, et al. High-dose dexamethasone vs prednisone for treatment of adult immune thrombocytopenia: a prospective multicenter randomized trial. Blood. 2016;127(3):296–302; quiz 370. doi:10.1182/blood-2015-07-659656.
- Bettaieb A, Fromont P, Louache F, Oksenhendler E, Vainchenker W, Duedari N, Bierling P. Presence of cross-reactive antibody between human immunodeficiency virus (HIV) and platelet glycoproteins in HIV-related immune thrombocytopenic purpura. Blood. 1992;80(1):162–9. doi:10.1182/blood.V80.1.162.162.
- Costantini A, Giuliodoro S, Mancini S, Butini L, Regnery CM, Silvestri G, Greco F, Leoni P, Montroni M. Impaired in-vitro growth of megakaryocytic colonies derived from CD34 cells of HIV-1-infected patients with active viral replication. Aids. 2006;20(13):1713–20. doi:10.1097/01.aids.0000242817.88086.8c.
- Afdhal N, McHutchison J, Brown R, Jacobson I, Manns M, Poordad F, Weksler B, Esteban R. Thrombocytopenia associated with chronic liver disease. J Hepatol. 2008;48(6):1000–7. doi:10.1016/j.jhep.2008.03.009.
- MSKA IL. The comparative analysis of morphological structre of a liver in patients with HIV-HCV coninfection and HCV-monoinfection in Russia. 16th International AIDS Conference; 2006; Toronto Canada.
- Basharkhah S, Sabet F, Ghezeldasht SA, Mosavat A, Jahantigh HR, Barati E, Shamsian K, Saleh‐moghaddam M, Sharebyani H, Hassannia T, et al. Prediction of HCV load using genotype, liver biomarkers, and clinical symptoms by a mathematical model in patients with HCV infection. Microbiol Immunol. 2019;63(11):449–57. doi:10.1111/1348-0421.12735.