Abstract
The emergence of rituximab biosimilars offers the prospect of significant savings to the healthcare system. However, these drugs have never been evaluated for treating immune thrombocytopenia (ITP). This was an observational, matched study. We included adults who received a rituximab biosimilar for ITP. Each rituximab-naïve biosimilar patient was matched with two controls from the historic ITP-ritux registry. For non-naïve patients, we compared the response to the biosimilar with that observed with the reference product. Response status was defined according to international criteria. We included 107 patients; 55 receiving Rixathon™ and 52 Truxima™. Three months after the first infusion of rituximab biosimilars, the overall response rate was 47/74 (63.5%) versus 76/142 (53.5%) for the matched controls receiving the reference product (p = .13). The 3-month overall response rate was 76.5% for Rixathon™ versus 51.5% for the matched control group (p = .01) and 21/40 (52.5%) for Truxima™ versus 41/74 (55.4%) for the matched controls (p = .81). For non-naïve patients, the response pattern was similar to that observed previously with the reference product. Safety was analogous to that observed with the reference product. Rituximab biosimilars seemed safe and effective for ITP treatment.
Plain Language Summary
What is the context?
Immune thrombocytopenia (ITP) is an autoimmune disease defined by a low platelet count without any other cause of thrombocytopenia. Patients with ITP may experience severe bleedings.
Rituximab, a biotechnological therapy, is a valid second-line treatment option for ITP.
Biotechnological therapies are expensive. Because the patent expiratory date of the reference product of Rituximab expired, highly similar drugs called biosimilars have been developed and used in ITP treatment without any direct evaluation in this particular disease.
What is new?
In this study, we evaluate the efficacy and safety of rituximab biosimilars versus the reference product for treating adult ITP
We included adults who received a rituximab biosimilar for ITP. Each rituximab-naïve biosimilar patient was matched with two controls from a historic registry that included ITP patients treated by the reference product. For non-naïve patients, we compared the response to the biosimilar with that observed with the reference product.
For naïve and non-naïve patients, the response pattern was similar to that observed previously with the reference product. Safety was analogous to that observed with the reference product.
What is the impact?
This study provides further evidence that rituximab biosimilars are safe and effective for immune thrombocytopenia treatment.
Introduction
Immune thrombocytopenia (ITP) is an autoimmune disease defined by a low platelet count (< 100 × 109/L) without any other cause of thrombocytopenia. Because of the implication of B cells and auto-antibodies in the pathophysiology, anti-CD20 monoclonal antibodies such as rituximab have become a valid second-line treatment option for ITP and are now recommended in most international guidelines.Citation1,Citation2 The previously publishedCitation3 largest prospective cohort of rituximab-treated ITP patients, the “ITP-ritux” registry, reported an overall initial response rate of nearly 60% after treatment with the original (or reference) rituximab product MabThera™ (Roche™, Welwyn Garden City, UK) in Europe and Rituxan (Genentech™, San Francisco, CA) in the United States.
The exponentially growing use of expansive biotechnological therapies such as monoclonal antibodies have led to a marked inflation of costs in many countriesCitation4 and thus inequities in access to these treatments. For instance, in 2019, MabThera™ was the seventh top drug in sales worldwide, for a total of 6.5 billion US dollars.Citation4 To deal with the increasing issue of unaffordable therapies, biosimilars have been developed. A biosimilar is a biological product that is highly similar to and has no clinically meaningful difference from an existing reference product that has reached patent expiratory date. Because of the high complexity of biotechnological products, one cannot expect an exact copy of this kind of drug which might differ from one batch to another. Unlike for small-molecule generics, biosimilar approval procedure allows variations in pharmaceutical attributes, and a review of glycosylated biosimilars approved in the European Union (EU) and Japan in 2016 has highlighted structural variances between biosimilars and their reference products.Citation5 In the past few years, regulation authorities have authorized several biosimilars according to stringent recommendationsCitation6–8 for their development and evaluation. Two rituximab biosimilars (i.e. Rixathon™ and Truxima™) were approved by French authorities after rigorous preclinical evaluation and large randomized controlled trials involving patients with follicular lymphomaCitation9,Citation10 and rheumatoid arthritis.Citation11,Citation12 According to French law, approved biosimilars can be used for all the reference product’s indications once the efficacy and safety for one of them has been validated.Citation13 However, because of the differences that remain between a biosimilar and its reference product, hospital pharmacist has no authority to deliver a rituximab biosimilar, if the reference product is prescribed, whereas they can for generic product. Nevertheless, rituximab biosimilars have replaced the reference product in most French hospitals for all rituximab indications, including ITP, without direct evaluation in this specific condition. Indeed, to our knowledge, no study has compared the efficacy and safety of any approved rituximab biosimilar to the rituximab original in ITP.
We conducted this work to evaluate the efficacy and safety of rituximab biosimilars versus the reference product for treating adult primary ITP.
Methods
Study design and population
This was an observational, multicenter, retrospective and prospective, matched pharmacoepidemiological cohort study comparing patients exposed to rituximab biosimilars with patients exposed to the reference product for the treatment of primary ITP.
We included consecutive adults who received at least one rituximab biosimilar infusion for the treatment of ITP between 1 January 2018 and 31 December 2019 in 10 tertiary medical centers in France. Data for all participants were analyzed in the safety analysis. We excluded from the efficacy analysis patients with ITP considered “secondary” because it was associated with another condition such as systemic lupus erythematosus, antiphospholipid syndrome or active malignant hematologic disease. We excluded patients if a new ITP treatment (excluding corticosteroids and intravenous immunoglobulins [IVIg]) was begun in the 8 weeks before the first infusion of the rituximab biosimilar.
For patients who were naïve of rituximab (MabThera™), we chose controls from the historic “ITP-ritux” registry. Characteristics of this cohort have been published elsewhere.Citation3,Citation14 Briefly, ITP-ritux was a multicenter prospective registry designed to evaluate the efficacy and safety of rituximab for ITP in “real-life” settings. In total, 248 adults receiving rituximab for primary ITP were enrolled from July 2010 to July 2012 in 31 centers across France and followed until 2017. At that time, MabThera™ was the only anti-CD20 product available in France.
ITP diagnosis and management
ITP diagnosis was based on international guidelines.Citation15 As an observational study, the anti-CD20 indication, infusion protocol, ITP management, and follow-up were performed as the standard of care in each center. All medical records were reviewed for clinical and laboratory values at ITP diagnosis, during the phase before rituximab biosimilar treatment, at infusion and during post-rituximab biosimilar follow-up. To uniformly assess the severity of bleeding events, each center used a modified bleeding score widely used for adult ITP in France and previously described by our group.Citation16 As compared with the initially described score, this study did not take into account age in the score. Bleeding severity was graded from 1 to 19 based on clinical evaluation, decreased hemoglobin level, and the requirement for blood or platelet transfusion. Severe bleeding was defined by a score>8.
Matching procedure
Each rituximab-naïve biosimilar patient was matched to two controls by an exact, random, with-replacement, 1:2 matching procedure. We used sex, age (± 10 years), ITP duration (less or more than 1 year), history of splenectomy and ITP response to corticosteroids as matching variables. These variables were chosen a priori because they are associated with the ITP response to rituximab treatment in the literature.Citation3,Citation17–19
Outcome assessment
Response was defined according to international guidelines: complete response (CR) was defined as platelet count >100 × 109/L and partial response (PR) as platelet count 30 to 100 × 109/L with at least a doubling of the pretreatment count.Citation15 Overall response (OR) was defined as CR or PR. To manage ITP during follow-up, patients requiring another treatment, including a new rituximab cycle, were considered non-responders, regardless of platelet count: only rescue therapy with a short course of corticosteroids or IVIg infusions administered <8 weeks after the date of the first rituximab infusion was authorized. Any adverse events were collected.
To assess treatment efficacy, we compared the overall initial response rate between rituximab-naïve biosimilar patients and matched controls. For rituximab non-naïve patients, we compared the response to rituximab biosimilar with that observed previously with the reference product for the same patient. Safety was assessed in the same way for every patient included and was compared to data in the ITP-ritux registry. We performed a subgroup safety analysis to search for patients switching from the reference rituximab to a biosimilar.
The main efficacy outcome was defined as the 3-month post-first infusion response.
Statistical statement
Statistical analyses and the matching procedure were performed with R 4.0.3. Quantitative and categorical variables are described with median (interquartile range) and number (percentage) respectively. Remaining differences between the baseline characteristics of the matched populations were assessed by calculating the standardized differences. We used conditional logistic regression adjusting on the matching variables to calculate p values in the matched analysis for the categorical outcome. For continuous outcomes in the matched population, we used a paired Student t test. All tests were two-sided, and p < .05 was considered statistically significant.
Ethical statement
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. Patients were informed that data were collected in their medical records for research, but written consent was not necessary according to French law. The institutional review board of Henri Mondor hospital approved the research (00011558–2020–094).
Results
Selected populations and patient characteristics
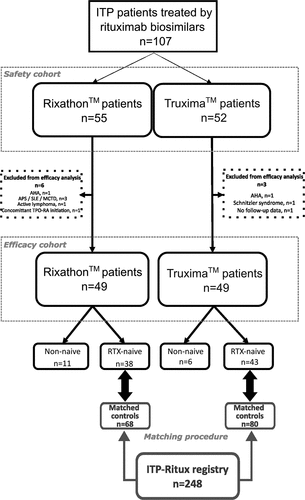
We included 107 patients with ITP treated by rituximab biosimilars from 2017 to 2020 in 10 tertiary care hospitals in France. The median age of patients was 50.8 (32.6–68.4) years, and 70 (65.4%) were female. Among them, 55 (51.4%) received Rixathon™ and 52 (48.6%) Truxima™. These patients defined the safety cohort. We excluded nine patients (six in the Rixathon™ group and three in the Truxima™ group) to define the efficacy cohort. Among the 49 patients included in the Rixathon™ efficacy cohort, 38 (77.5%) were rituximab-naïve, and 43/49 (87.8%) in the Truxima™ efficacy cohort were rituximab-naïve. A flowchart of the selected populations and more details about reasons for exclusion are in .
Figure 1. Flow-chart of the study.
The characteristics of the selected safety cohort are in . Those of patients included in the ITP-ritux registry are also presented for information. Briefly, 28 (73.7%) patients in the Rixathon™ group and 24 (75.0%) in the Truxima™ group were female. The median age at biosimilar infusion was 47.5 (33.3–67.1) and 55.1 (32.1–69.9) years, respectively. Almost every biosimilar patient (53/55 and 48/52 for Rixathon™ and Truxima™ groups, respectively) received the treatment with the 1-g (fixed dose) day-1 and day-15 protocol, whereas most of the patients in the historical ITP-ritux registry (176/248) had received the protocol of 375-mg/m2 once a week for 4 weeks. After excluding corticosteroids and IVIg treatments, the median number of previous treatment lines received before the biosimilar infusion was 1 (0–1.75) in the Rixathon™ group, 1 (0–1.25) in the Truxima™ group and 0 (0–1) in the ITP-ritux registry. In particular, the number of patients who previously received thrombopoietin-receptor agonists (TPO-RAs) was higher among the biosimilar patients (15 [27.3%] in the Rixathon™ group and 15 [28.8%] in the Truxima™ group) than in the historical ITP-ritux registry (30 [12.2%]).
Table I. Characteristics of the included safety population with immune thrombocytopenia (ITP) receiving rituximab biosimilars.
Crude efficacy of the rituximab biosimilars among the naïve patients
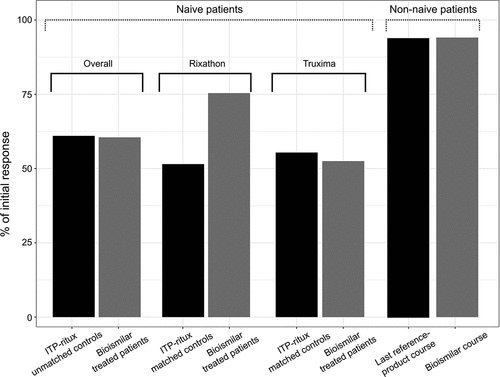
Three months after the first infusion, among the naïve patients, the crude (before matching) OR rate (ORR) was 49/81 (60.5%) after pooling the two types of biosimilars. The reported initial ORR in the ITP-ritux registry was 152/248 (61.3%).Citation3 Overall, 27/38 (71.0%) of Rixathon™ patients and 22/43 (51.2%) of Truxima™ patients exhibited OR. In the Rixathon™ group, 15/38 (39.4%) and 12/38 (31.6%) of patients met the definition of PR and CR, respectively. In the Truxima™ group, 7 (16.3%) and 15 (34.8%) met the definition of PR and CR, respectively. Three months after the first infusion, the median platelet count was 89 × 109/L (53.5–156.5) in the Rixathon™ group and 82 × 109/L (50.3–197) in the Truxima™ group. With a median follow-up of 13.8 (11.7–19.4) months in the cohort, 10 patients with an initial response showed relapse during the follow-up after a median of 7.5 (6.8–10.7) months ().
Efficacy of the rituximab biosimilars in the matched analysis
According to our random matching procedure, two controls were matched with 34/38 (89.4%) patients in the Rixathon™ group and 40/43 (93.0%) in the Truxima™ group. Characteristics of the matched populations are in . The 3-month response rate was not available for six matched Truxima™ controls.
Table II. Characteristics and outcomes for the Rixathon™ and matched control populations.
Table III. Characteristics and outcomes for the Truxima™ and matched control populations.
At 3 months post-infusion, the ORR was 47/74 (63.5%) for patients receiving the two biosimilars versus 76/142 (53.5%) for the matched controls receiving the reference product (p = .13).
For the Rixathon™ group, the ORR at 3 months was 26/34 (76.5%) versus 35/68 (51.5%) for the matched reference-product group (p = .01). At 12 months after the first infusion, the remaining response rate was 18/28 (64.3%) for the Rixathon™ group versus 24/57 (42.1%) for the reference product group (p = .04) ().
For the Truxima™ group, the ORR at 3 months was 21/40 (52.5%) versus 41/74 (55.4%) for the matched reference-product group (p = .81). At month 12 after the first infusion, the remaining ORR was 15/32 (46.8%) for the Truxima™ group versus 27/68 (39.7%) for the matched control group (p = .45) ().
Efficacy of the rituximab biosimilars in the non-naïve population
We assessed the treatment efficacy for 17 non-naïve patients (11 receiving Rixathon™ and 6 Truxima™): 13 previously received one course of rituximab, 3 received two courses of rituximab and 1 received three courses of rituximab. All of these previous sequences involved the reference rituximab product and all of these patients except one had shown transient response with the reference product. The median duration of response for the last reference product course was 1.7 years.
Every non-naive patient except the one who previously received three courses of rituximab showed response to the biosimilar at month 3. The one patient who responded poorly to the reference product during the previous treatment sequence was also in response three months after the biosimilar infusion. All the others had a similar response as the one during their previous sequence with the reference product ().
Safety of the rituximab biosimilars
All the included patients received a pre-infusion treatment of paracetamol, corticosteroids, and antihistaminic. In the safety cohort (n = 107), we observed 9 (8.4%) immediate adverse events during the first infusion. One event (severe dyspnea) led to prematurely stopping the infusion. All the other events were mild and did not require stopping the infusion early. In the historical ITP-ritux registry, 38/248 (15.3%) patients experienced minor intolerance to rituximab (reference product) infusion and 3 had to stop the infusion early.Citation3
We observed four late-onset post-rituximab neutropenia episodes during follow-up: two after Truxima™ and two after Rixathon™. Two events were complicated by fever and sepsis that required hospitalization and granulocyte colony-stimulating factor treatment. The other events resolved spontaneously without any complications. Three of these neutropenia episodes occurred in the 20 patients who previously received MabThera™. In the ITP-ritux registry, only 1/248 patients experienced late-onset post-rituximab neutropenia.
Only one patient (in the Rixathon™ group) experienced documented hypogammaglobulinemia after the treatment. He did not require IVIg infusions.
Among the patients receiving Truxima™, three experienced more delayed and nonspecific adverse events after treatment: one patient had a coronary spasm 25 days after the first injection, one patient had a pulmonary embolism 35 days after the first infusion, and one patient experienced persistent urticaria for several days after the second infusion. We observed a seizure in a known epileptic patient 21 days after the first infusion in the Rixathon™ group.
We observed only six episodes of post-biosimilar infection (three in the Rixathon™ group and three in the Truxima™ group). One 89-year-old patient died of severe COVID-19 more than 20 months after the last biosimilar injection. Except for this case and the two episodes of febrile neutropenia previously described, none of the other infections were severe or required hospitalization.
Discussion
We report the first evaluation of two rituximab biosimilars (Rixathon™ and Truxima™) for treating ITP that used a retrospective cohort of 107 patients. As compared with a historical prospective cohort that received the reference product, our findings suggest that these drugs seem as safe and as effective.
According to their approval procedure,Citation8 both of these biosimilars have been previously evaluated head-to-head to the reference product in large randomized controlled trials of treatment for follicular lymphomaCitation9,Citation10 and rheumatoid arthritis,Citation11,Citation12 with reassuring results, but not for other indications. Because biosimilars represent an important hope of cost saving for healthcare systems, we need reliable data on their use in all real-life settings, including the treatment of rare conditions such as ITP.
The economic benefits for the currently challenged healthcare systems, which have to deal with a significant increase in healthcare costs, are of primary importance.Citation20 The design of our retrospective study does not allow for precisely calculating the cost savings obtained with the use of rituximab biosimilars. However, the significant price difference between the products (the median cost for 2 g of MabThera™ in the participating centers was 3000€ and the median cost for 2 g of the biosimilar drugs during the period of study was 1094€) suggests a significant cost saving for the healthcare system.
We observed an overall similar efficacy of rituximab biosimilar products and even a significantly better efficacy for Rixathon™ than the reference product. However, in the absence of a prospective randomized controlled study, stating that one treatment is superior to the other is difficult. Although we matched the two populations using the main criteria known to influence the response to rituximab, we cannot eliminate the biases inherent in a retrospective study. However, our study provides reassuring data for clinicians and patients on the efficacy of rituximab biosimilars. Among the possible measured confounders, more biosimilar patients received TPO-RAs previous to the rituximab biosimilar than controls. This observation suggests that these patients might have a more “resistant” ITP. However, TPO-RAs were not readily available in France and were reserved for patients with chronic ITP in theory with failure of splenectomy when the prospective ITP-ritux registry was set up, whereas during the more recent period of use of rituximab biosimilars, TPO-RAs were widely used as second- or third-line treatment and can no longer be considered a severity criterion. For example, our group has recently reported that failure of TPO-RAs treatment prior to splenectomy is not associated with splenectomy failure.Citation21 However, although there is no data in the literature suggesting it, we cannot exclude that TPO-Ras exposure modified the response to rituximab. Another difference between the matched populations was the protocol used for the rituximab infusions. Almost all patients receiving rituximab biosimilars were under the “rheumatoid arthritis” therapeutic schedule (fixed dose of 1 g on day 1 and day 15), whereas most of the matched controls received the “lymphoma protocol” (375 mg/m2 once a week for 4 weeks). However, we previously demonstrated in a prospective trial a closely similar pattern of response using the two different schedules that explains why the two infusions protocol is now largely usedCitation3,Citation22 in France.
Even though the number of adverse events is too low to draw any conclusions, we unexpectedly observed four episodes of late-onset post-biosimilar rituximab neutropenia, three occurring in patients who previously received the reference product. Late-onset post-rituximab neutropenia is a well-known side effect of rituximab, with a highly variable incidence depending on the abnormality treated. This complication, whose pathophysiological mechanism is unknown, even though it seems associated with B-cell recovery,Citation23 is described as more frequent in patients receiving treatment for lymphoid hemopathy, whose management also includes chemotherapy, than in patients receiving treatment for an autoimmune disease.Citation24 In the ITP-ritux registry,Citation3 only 1 (0.4%) patient experienced this side effect, but a more recent studyCitation25 found an 18% post-rituximab neutropenia rate in patients receiving treatment for autoimmune diseases including ITP. The rate of neutropenia under a rituximab biosimilar was not higher in phase 3 studies comparing Rixathon™ to MabThera™Citation9,Citation11 in patients receiving treatment for lymphoma or rheumatoid arthritis, but, to our knowledge, no study has evaluated the frequency of this adverse effect in patients switching from MabThera™ to Rixathon™ or other biosimilars.
Our study has some limitations. The B-cell depletion was not monitored in our study because it is not part of standard of care in France as it has been previously shown that rituximab causes an initial B depletion in all patients, whether they are responders or not.Citation26 However, the ability of rituximab biosimilars to induce B-cell depletion has already been extensively studied and compared to the reference product in other settings.Citation9–11 Another limitation is that the retrospective data collection for the biosimilar patients might have altered the completeness of the reported outcomes. We compared two cohorts (biosimilar and reference product) who did not receive treatment during the same period: biosimilar patients from 2017 to 2020 and ITP-ritux registry patients from 2010 to 2012.
This study has also some strengths: we were able to collect data from 10 different centers and from 107 ITP patients, so a relatively frequent adverse outcome would have been captured by the study. We were able to match and compare biosimilar patients to patients included in a prospective cohort that received the reference product. Finally, our real-life setting is reassuring for patients and physicians who use this off-label drug in most countries and need reliable data on its use.
In conclusion, rituximab biosimilars seem an effective and safe alternative to the rituximab reference product for treating primary ITP. Physicians should remain vigilant about the risk of late-onset post-rituximab neutropenia, especially in patients switching from the reference product to the biosimilar. The use of rituximab biosimilars for ITP treatment is a reliable option and offers hope for decreasing the cost of treatment for the challenged healthcare system.
Acknowledgments
The authors thank Marine Cecchi (CHU de la Timone, AP-HM Marseille), Valérie PREDAN (CHU Dijon-Bourgogne) and Laetitia Languille (CHU Henri Mondor, APHP Créteil) for their invaluable help in data collection.
Disclosure statement
M Mahevas received funds for research from GSK and fees from Amgen and Novartis for lectures. B Godeau served as an expert for Amgen, Novartis, Grifols and Sobi; M Michel received honoraria (advisory boards, speaker fees) from Novartis, Amgen, UCB, Argenx, Alexion and Sanofi. .BBonnotte participated in medical boards for Novartis, Amgen, and Boeringher, received financial support for travelling to congresses from Novartis and Astra-Zeneca and received honoraria from Alexion, Astra-Zeneca, LFB, Novartis, and Roche Chugai. JF Viallard served as an expert for AMGEN, NOVARTIS, GSK, Grifols. The other authors declare no potential conflict of interest.
Additional information
Funding
References
- Neunert C, Terrell DR, Arnold DM, Buchanan G, Cines DB, Cooper N, Cuker A, Despotovic JM, George JN, Grace RF, et al. American society of hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829–8. doi:10.1182/bloodadvances.2019000966.
- HAS. PNDS, Purpura thrombopénique immunologique de l’enfant et de l’adulte. Haute Aut Santé. 2017.
- Khellaf M, Charles-Nelson A, Fain O, Terriou L, Viallard J-F, Cheze S, Graveleau J, Slama B, Audia S, Ebbo M, et al. Safety and efficacy of rituximab in adult immune thrombocytopenia: results from a prospective registry including 248 patients. Blood. 2014;124(22):3228–36. doi:10.1182/blood-2014-06-582346.
- Urquhart L. Top drugs and companies by sales in 2018. Nat Rev Drug Discov. 2019;18:245. doi:10.1038/d41573-019-00049-0.
- Grampp G, Ramanan S. The diversity of biosimilar design and development: implications for policies and stakeholders. BioDrugs Clin Immunother Biopharm Gene Ther. 2015;29(6):365–72. doi:10.1007/s40259-015-0147-0.
- Food And Drug Administration. Scientific considerations in demonstrating biosimilarity to a reference product guidance for industry. 2015;27.
- World Heath Organization. WHO | Similar biotherapeutic products. WHO.
- Guideline on similar biological medicinal products containing monoclonal antibodies – non-clinical and clinical issues, EMA. 2012.
- Jurczak W, Moreira I, Kanakasetty GB, Munhoz E, Echeveste MA, Giri P, Castro N, Pereira J, Akria L, Alexeev S, et al. Rituximab biosimilar and reference rituximab in patients with previously untreated advanced follicular lymphoma (ASSIST-FL): primary results from a confirmatory phase 3, double-blind, randomised, controlled study. Lancet Haematol. 2017;4(8):e350–61. doi:10.1016/S2352-3026(17)30106-0.
- Ogura M, Sancho JM, Cho S-G, Nakazawa H, Suzumiya J, Tumyan G, Kim JS, Lennard A, Mariz J, Ilyin N, et al. Efficacy, pharmacokinetics, and safety of the biosimilar CT-P10 in comparison with rituximab in patients with previously untreated low-tumour-burden follicular lymphoma: a randomised, double-blind, parallel-group, phase 3 trial. Lancet Haematol. 2018;5(11):e543–53. doi:10.1016/S2352-3026(18)30157-1.
- Smolen JS, Cohen SB, Tony H-P, Scheinberg M, Kivitz A, Balanescu A, Gomez-Reino J, Cen L, Zhu P, Shisha T. A randomised, double-blind trial to demonstrate bioequivalence of GP2013 and reference rituximab combined with methotrexate in patients with active rheumatoid arthritis. Ann Rheum Dis. 2017;76(9):1598–602. doi:10.1136/annrheumdis-2017-211281.
- Yoo DH, Suh C-H, Shim SC, Jeka S, Cons-Molina FF, Hrycaj P, Wiland P, Lee EY, Medina-Rodriguez FG, Shesternya P, et al. A multicentre randomised controlled trial to compare the pharmacokinetics, efficacy and safety of CT-P10 and innovator rituximab in patients with rheumatoid arthritis. Ann Rheum Dis. 2017;76(3):566–70. doi:10.1136/annrheumdis-2016-209540.
- État des lieux sur les médicaments biosimilaires, ANSM, Mai 2016. 2016.
- Deshayes S, Khellaf M, Zarour A, Layese R, Fain O, Terriou L, Viallard J-F, Cheze S, Graveleau J, Slama B, et al. Long-term safety and efficacy of rituximab in 248 adults with immune thrombocytopenia: results at 5 years from the French prospective registry ITP-ritux. Am J Hematol. 2019;94(12):1314–24. doi:10.1002/ajh.25632.
- Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, Bussel JB, Cines DB, Chong BH, Cooper N, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–93. doi:10.1182/blood-2008-07-162503.
- Khellaf M, Michel M, Schaeffer A, Bierling P, Godeau B. Assessment of a therapeutic strategy for adults with severe autoimmune thrombocytopenic purpura based on a bleeding score rather than platelet count. Haematologica. 2005;90:829–32.
- Godeau B, Porcher R, Fain O, Lefrère F, Fenaux P, Cheze S, Vekhoff A, Chauveheid M-P, Stirnemann J, Galicier L, et al. Rituximab efficacy and safety in adult splenectomy candidates with chronic immune thrombocytopenic purpura: results of a prospective multicenter phase 2 study. Blood. 2008;112(4):999–1004. doi:10.1182/blood-2008-01-131029.
- Marangon M, Vianelli N, Palandri F, Mazzucconi MG, Santoro C, Barcellini W, Fattizzo B, Volpetti S, Lucchini E, Polverelli N, et al. Rituximab in immune thrombocytopenia: gender, age, and response as predictors of long-term response. Eur J Haematol. 2017;98(4):371–7. doi:10.1111/ejh.12839.
- Bussel JB, Lee CS, Seery C, Imahiyerobo AA, Thompson MV, Catellier D, Turenne IG, Patel VL, Basciano PA, Elstrom RL, et al. Rituximab and three dexamethasone cycles provide responses similar to splenectomy in women and those with immune thrombocytopenia of less than two years duration. Haematologica. 2014;99(7):1264–71. doi:10.3324/haematol.2013.103291.
- Healthcare expenditure statistics.
- Mageau A, Terriou L, Ebbo M, Souchaud‐debouverie O, Orvain C, Graveleau J, Lega J-C, Ruivard M, Viallard J-F, Cheze S, et al. Splenectomy for primary immune thrombocytopenia revisited in the era of thrombopoietin receptor agonists: new insights for an old treatment. Am J Hematol. 2022;97(1):10–17. doi:10.1002/ajh.26378.
- Mahévas M, Ebbo M, Audia S, Bonnotte B, Schleinitz N, Durand J-M, Chiche L, Khellaf M, Bierling P, Roudot‐thoraval F, et al. Efficacy and safety of rituximab given at 1,000 mg on days 1 and 15 compared to the standard regimen to treat adult immune thrombocytopenia. Am J Hematol. 2013;88(10):858–61. doi:10.1002/ajh.23518.
- Dunleavy K, Hakim F, Kim HK, Janik JE, Grant N, Nakayama T, White T, Wright G, Kwak L, Gress R, et al. B-cell recovery following rituximab-based therapy is associated with perturbations in stromal derived factor-1 and granulocyte homeostasis. Blood. 2005;106(3):795–802. doi:10.1182/blood-2004-08-3198.
- Wolach O, Bairey O, Lahav M. Late-onset neutropenia after rituximab treatment: case series and comprehensive review of the literature. Medi (Baltimore). 2010;89(5):308–18. doi:10.1097/MD.0b013e3181f2caef.
- Malpica Castillo LE, Palmer S, Zhu A, Deal AM, Chen S-L, Moll S. Incidence and time course of neutropenia in patients treated with rituximab-based therapy for non-malignant immune-mediated hematologic diseases. Am J Hematol. 2020;95(5):E117–20. doi:10.1002/ajh.25751.
- Cooper N, Stasi R, Cunningham-Rundles S, Feuerstein MA, Leonard JP, Amadori S, Bussel JB. The efficacy and safety of B-cell depletion with anti-CD20 monoclonal antibody in adults with chronic immune thrombocytopenic purpura. Br J Haematol. 2004;125(2):232–9. doi:10.1111/j.1365-2141.2004.04889.x.