Abstract
Clopidogrel combined with aspirin is widely used in coronary artery disease (CAD) patients, while some patients exhibit high platelet activity when receiving the combined treatment. Current environmental and genetic factors could only explain part of the variability in clopidogrel efficacy. Human platelets harbor abundant miRNAs which might affect clopidogrel efficacy by regulating the expression of key proteins in the clopidogrel antiplatelet signaling pathway. This study aimed to investigate the association between platelet miRNA levels and clopidogrel efficacy. Here we recruited 508 CAD patients who underwent clopidogrel antiplatelet therapy and determined the platelet reactivity index (PRI) to evaluate antiplatelet reactivity responses to clopidogrel. Subsequently, 22 patients with extreme clopidogrel response were selected for platelet small RNA sequencing. Another 41 CAD patients taking clopidogrel were collected to verify the differentially expressed candidate miRNAs. We found the metabolic types of the CYP2C19 enzyme (based on CYP2C19 * 2 and * 3 polymorphisms) could significantly affect the PRI of CAD patients with or without percutaneous coronary intervention (PCI) in Chinese. A total of 43 miRNAs were differentially expressed in the platelets from the 22 extreme clopidogrel response samples, and 109 miRNAs were differentially expressed in the 13 CYP2C19 extensive metabolizers with extreme clopidogrel response. Platelet miR-199a-5p levels were correlated negatively with PRI after clopidogrel therapy. Studies in cultured cells revealed that miR-199a-5p inhibited the expression of VASP, a key effector protein downstream of the P2Y12 receptor. In conclusion, we found the expression of VASP could be inhibited by miR-199a-5p, and decreased platelet miR-199a-5p was associated with high on-clopidogrel platelet reactivity in CAD patients.
Plain Language Summary
What is the context?
● Clopidogrel combined with aspirin is widely used in coronary artery disease (CAD) patients, while some patients exhibit high platelet activity when receiving the combined treatment.
● Current environmental and genetic factors could only explain part of the variability in clopidogrel efficacy.
● Human platelets harbor abundant miRNAs which might affect clopidogrel efficacy by regulating the expression of key proteins in the clopidogrel antiplatelet signaling pathway.
What is new?
● We found that decreased platelet miR-199a-5p level was associated with high on-clopidogrel platelet reactivity.
● Overexpression of miR-199a-5p significantly down-regulated the expression of VASP protein in cultured cells.
What is the impact?
● The current study provided new insights into the exploration of interindividual variability in clopidogrel response from the perspective of miR-199a-5p and VASP interaction.
Introduction
Acute fatal cardiovascular events occur or recur in approximately 100 out of every 1,000 adults according to the report from the American Heart Association, and the formation of atherothrombosis in coronary artery disease (CAD) patients is a vital cause of acute cardiovascular events.Citation1 Activation and aggregation of platelets play crucial roles in the process of “atherothrombosis.”Citation2 Dual antiplatelet therapy (DAPT) with a P2Y12 antagonist and aspirin significantly reduces atherothrombotic events and has been widely used in patients with acute coronary syndrome (ACS) and those undergoing percutaneous coronary intervention (PCI).Citation3,Citation4
Clopidogrel (a kind of P2Y12 antagonist) is an inactive pro-drug and about 85% of clopidogrel is hydrolyzed into inactive metabolite by carboxylesterase 1 (CES1) after oral administration. Approximately 15% of clopidogrel is metabolized into an active metabolite by several hepatic cytochrome P450 (CYP) enzymes, such as CYP2C19, CYP3A4, CYP1A2, and CYP2B6. There is a wide interindividual variability of platelet response to clopidogrel,Citation5,Citation6 and many patients receiving standard antiplatelet therapy with clopidogrel and aspirin did not exhibit effective inhibition of platelet activity, so-called high on-treatment platelet reactivity (HTPR).Citation7,Citation8 HTPR is an important risk factor for clinical adverse events such as stent thrombosis, recurrent myocardial infarction, and cardiovascular death in CAD patients.Citation9–11 Evidence has indicated that CAD patients with CYP2C19 loss-function mutations (CYP2C19 * 2 or CYP2C19 * 3) show a significantly increased risk of ischemic cardiovascular events (owing to the occurrence of HTPR) with clopidogrel therapy.Citation12–15 In our previous publications, we also found the metabolic types of CYP2C19 enzyme (based on CYP2C19 * 2 and * 3 polymorphisms) could significantly affect the platelet reactivity index (PRI) of CAD patients taking clopidogrel in Chinese.Citation16–18 While the current known genetic variations and clinical factors could only explain the individual differences in clopidogrel efficacy in a small part.Citation8,Citation19–22 Therefore, further exploration of biomarkers affecting individual differences in clopidogrel efficacy remains a top priority for optimizing the clinical application of clopidogrel.
MiRNAs are~22 nucleotides (nt) endogenous RNAs that act as key regulators of gene expression.Citation23,Citation24 Mature miRNAs could bind to the 3’-untranslated region (3’-UTR) of target mRNAs and induce mRNAs degradation or translational inhibition. Increasing evidence indicates that miRNAs have emerged as novel biomarkers in the field of disease diagnosis, prognosis prediction, and personalized medicine.Citation25,Citation26 MiRNAs may regulate platelet function of CAD patientsCitation27,Citation28 and platelets harbor abundant miRNAs which might affect clopidogrel efficacy by regulating the expression of key proteins involved in platelet activation (such as P2Y12 receptor; vasodilator-stimulated phosphoprotein, VASP; platelet glycoprotein IIb/IIIa receptor, GP IIb/IIIa, and so on). Low expression of platelet miR-223 in CAD patients was found to be associated with high on-clopidogrel platelet reactivity possibly by targeting the 3’-UTR of P2RY12 (encodes the clopidogrel target P2Y12 receptor).Citation29–31 However, the relation between plasma miR-223 and platelet response to clopidogrel remains controversial. Zhang et al found that decreased circulating miR-223 level could predict HTPR,Citation32 while another study argued against the notion of low plasma miR-223 as a marker of platelet responsiveness to DAPT.Citation33 Besides, the level of miR-26a in platelets was also found to be associated with HTPR, and the expression of its potential target protein VASP was significantly elevated in patients with HTPR,Citation34 which needs further exploration. In addition, a clinical association study found several other platelet miRNAs (miR-223, miR-221, and miR-21) were independently associated with clopidogrel antiplatelet responsiveness, all three miRNAs were predicted to target P2RY12 3’-UTR without further validation.Citation35 A polymorphism of miR-605 was also found to affect clopidogrel therapy through modulation of P2RY12 and CYP2B6 expression.Citation36 It remains to be explored whether any other platelet miRNAs affect clopidogrel therapy.
Methods
Study population and design
The study program was registered with the China Clinical Trial Registration Center (http://www.chictr.org.cn; Registration number: ChiCTR-OPN-15006260) and was approved by the Ethics Committee of the Institute of Clinical Pharmacology, Central South University (Approval number: CTXY-140002-13). Men and women who were 18 ~ 80 years of age were eligible for inclusion if they had been hospitalized for CAD. All eligible patients received a loading dose (LD) of 300 mg clopidogrel for 12–24 h or a maintenance dose (MD) of 75 mg clopidogrel for more than 5 days. Exclusion criteria included: 1) Anemia (hemoglobin < 100 g/L); 2) Patients with severe liver disease; 3) Patients with systemic bleeding and coagulopathy; 4) Patients with malignant tumors or other fatal diseases; 5) History of food or drug allergy; 6) History of drug abuse, alcohol abuse or mental illness; 7) Patients who had received GP IIb/IIIa receptor antagonist before enrollment.
A total of 508 CAD patients (male: 330; 65.0%) were recruited in our study and all the patients received clopidogrel for antiplatelet therapy. Among all the patients, 269 patients underwent PCI before sample collection or during their hospitalization after sample collection. Platelet reactivity index (PRI) was determined to reflect the clopidogrel response for all the patients. According to the distribution of PRI values in the overall patients, 22 patients with extreme PRI values were selected for platelets miRNA sequencing analysis. Platelets from the above 22 patients were purified with human CD45 MicroBeads reagent (Miltenyi Biotec, USA), and total RNA was extracted for miRNA sequencing. Purified platelets from another 41 CAD patients were prepared for validation. And the mechanism of clinically validated miRNA miR-199a-5p on the efficacy of clopidogrel was preliminarily investigated.
VASP phosphorylation analysis and PRI calculation
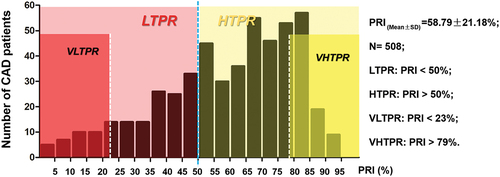
Peripheral blood samples of the CAD patients were collected in a 3.8% sodium citrate vacutainer tube. The phosphorylation of VASP (VASP-P) in platelet was monitored and PRI was calculated according to a standardized flow cytometric assay (PLT VASP/P2Y12 kit, France) using a Cytomics FC500 flow cytometer (Beckman, USA).Citation37 Mean fluorescence intensity (MFI) values of the T1, T2, and T3 tubes of each sample (which can be recorded as MFI1, MFI2, and MFI3, respectively) were recorded. Flow analysis diagrams of the representative samples were shown in Figure S1. PRI values were calculated according to the following formula: PRI = (MFI1-MFI2)/(MFI1-MFI3) × 100%. High on-treatment platelet reactivity (HTPR) was defined as PRI index >50% as previously proposed,Citation38–40 and low on-treatment platelet reactivity (LTPR) was defined as PRI < 50%. Besides, very high on-treatment platelet reactivity (VHTPR) was defined as above the 25th percentile of PRI in HTPR (PRI above 79% in this study) and very low on-treatment platelet reactivity (VLTPR) was defined as below the 75th percentile of PRI in LTPR (PRI below 23% in this study).
Genotyping
CYP2C19 * 2 and * 3 polymorphisms show significant racial differences in humans. Here we genotyped these two polymorphisms in 508 Chinese CAD patients to explore the association between the PRI values of the patients and the above two polymorphisms. Genomic DNA was extracted from peripheral blood leukocytes using Wizard® Genomic DNA Purification Kit (Promega, USA). The genotyping of CYP2C19 * 2 and * 3 polymorphisms was performed via direct Sanger sequencing after amplification by polymerase chain reaction (PCR). The primers used for the genotyping of CYP2C19 * 2 and * 3 polymorphisms were shown in Table S1. CYP2C19 metabolic types of all the involved CAD patients were classified into extensive metabolizers (EM: Both CYP2C19 * 2 noncarriers and * 3 noncarriers), intermediate metabolizers (IM: CYP2C19 * 2 heterozygotes or * 3 heterozygotes) and poor metabolizers (PM: CYP2C19 * 2 homozygotes or * 3 homozygotes or both * 2 carriers and * 3 carriers) according to the genotyping results of CYP2C19 * 2 and * 3 polymorphisms.
LDP and RNA preparations
Approximately 12 ml blood was drawn into a sodium citrate tube and centrifuged at 180 g for 15 minutes to obtain platelet-rich plasma (PRP). Ethylene diamine tetraacetic acid (EDTA) was added to the PRP at a final concentration of 2 mM. The PRP was further purified by human CD45 MicroBeads reagent to obtain leukocyte depleted plasma (LDP) as previously described.Citation41 The LDPs were centrifuged at 2000 g to obtain purified platelet preparations. Quantitative real-time PCR amplification of CD45 (a marker of leukocytes) and CD41 (a marker of platelets) mRNA was performed to monitor the purified platelet preparations as previously describedCitation30 (Figure S2; Primers used for the detection were also shown in the Table S2). Total RNA extraction from the preparations was performed with TRIzol (Invitrogen).
MiRNA sequencing
22 patients with extreme phenotypes of clopidogrel response (11 VHTPR and 11 VLTPR, respectively) were selected for platelet small RNA expression sequencing. The amount and the fragment distribution of the total platelet RNA were verified by a hypersensitive Agilent 2100 pic600. NEBNext® Multiplex Small RNA Library Prep Set for Illumina® (NEB, USA) was used to generate the sequencing libraries according to the manufacturer’s recommendations. The library preparations were sequenced on an Illumina Hiseq 2500/2000 platform after cluster generation. The whole small RNA sequencing and data analysis were performed by Novogene. DESeq2 software package was used to analyze the differentially expressed miRNAs in two groups of extreme clopidogrel response samples and p < .05 was considered to be statistically significant. MiRanda database was used to predict the target genes of the differentially expressed miRNA. According to the results predicted by the miRanda, we performed Gene Ontology (GO; three basic categories of Go term: Biological process, cellular component and molecular function) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis for the target gene sets of differentially expressed miRNA.
Correlation analysis
We collected the blood samples from 41 CAD patients taking a loading dose (300 mg) clopidogrel for 12–24 hours and determined the PRI values of the patients according to the above instructions in the validation study. LDPs of the patients and total RNA of purified platelets were obtained. Total RNA to mRNA complementary DNA (cDNA) and miRNA cDNA were reverse transcripted using PrimeSctiptTM RT reagent Kit and Mir-XTM miRNA First-Strand Synthesis Kit respectively. Quantitative Real-time PCR was used to determine the relative expression of candidate miRNAs and key genes (P2RY12, VASP, and ITGB3; P2RY12 encodes P2Y12 receptor which is the target of clopidogrel active metabolite; VASP encodes VASP protein and phosphorylation of VASP effectively inhibits the activation of platelet GP IIb/IIIa receptor which plays critical roles in the activation and aggregation of platelets; ITGB3 encodes integrin β3 of the GP IIb/IIIa receptor) in platelet activation. GAPDH was used to normalize the relative expression levels of mRNAs and U6 was used to normalize the relative expression levels of miRNAs. The correlations between the expression of candidate miRNAs and PRI or the expression of key candidate genes were analyzed.
The dual-luciferase reporter assay system
TargetScan database predicted that miR-199a-5p could potentially target the 3’-UTR of VASP mRNA and the database was also used to identify the location of miR-199a-5p binding sites in the 3’-UTR of VASP. A fragment of approximately 300 bp around the corresponding recognition region (with mutations or not) of VASP was cloned into multiple cloning sites (MCS) of the pmirGLO vector. The miR-199a-5p mimics and the constructed pmirGLO-VASP-WT/Mut were co-transfected into HEK293 cells to verify the specific binding of miR-199a-5p and VASP 3’-UTR. Dual-luciferase reporter assay system (Promega, USA) was used to detect the luciferase activity according to the technical manual.
Overexpression of miR-199a-5p
The human protein atlas database was used to analyze the expression of VASP protein in various cell lines. We overexpressed miR-199a-5p using Lipofectamine® RNAiMAX to verify the regulation of miR-199a-5p on VASP in A549 cells, a cell line showing high VASP expression. PrimeScript™ RT reagent Kit (TaKaRa, Japan) was used to reverse miR-199a-5p, and quantitative real-time PCR was carried out to detect the expression level of miR-199a-5p. Western Blot was used to detect the expression of VASP protein in cells, and the human VASP polyclonal antibody was purchased from ABclonal (Catalog: A14217; Wuhan, China).
Statistical analysis
Data of continuous variables were presented as variables mean ± SD, and categorical variables were shown as numbers and percentages. Chi-square test was used for the comparison of categorical variables. Independent sample t-test or one-way ANOVA was used for the comparison of continuous variables following normal distributions, while non-parametric test (Mann-Whitney U test or Kruskal-Wallis test) was used for the comparison of continuous variables not following normal distributions. The statistical analysis was performed in SPSS version 26.0 (IBM, USA) and a two-tailed p < .05 was considered to be statistically significant except for special marks.
Results
General characteristics of the study population
A total of 508 CAD patients receiving 300 mg LD clopidogrel for 12–24 hours or 75 mg MD clopidogrel above 5 days were enrolled in the study. The levels of platelet VASP-P in venous blood samples of all the patients were monitored within 24 hours after the sample collection, and PRI was calculated. The general characteristics of the overall 508 patients, 22 patients for small RNA Sequencing, and the 41 additional patients for miRNA validation were presented in . The histogram of PRI distribution in the patients was shown in .
Table I. General characteristics of the overall CAD patients and the patients for small RNA sequencing.
CYP2C19 genotypes and PRI
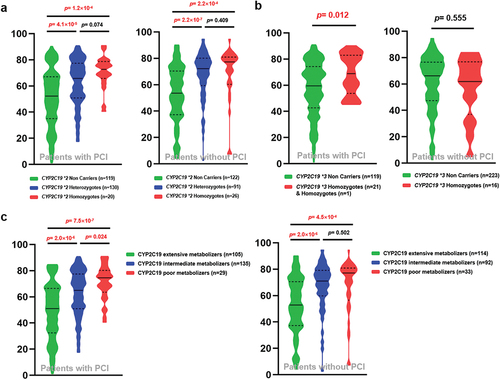
CYP2C19 genotypes based on the CYP2C19 * 2 and CYP2C19 * 3 polymorphisms showed significant elevated PRI in carriers of the mutant alleles as described in our previous publication.Citation16–18 Among all the 508 patients, 269 patients underwent PCI before sample collection or during their hospitalization after sample collection, and 239 patients did not undergo PCI. The majority of the patients undergoing PCI were acute coronary syndrome (ACS) patients with indications for PCI. There was no difference in the PRI distribution between CAD patients who underwent PCI and those who did not (Figure S3). More importantly, we found that the CYP2C19 * 2 polymorphism significantly increased the PRI of CAD patients with or without PCI, and PRI values gradually increased as the CYP2C19 * 2 mutational allele increased (). CYP2C19 * 3 polymorphism significantly affected PRI of CAD patients with PCI ( left), while this phenomenon was not found in CAD patients without PCI ( right). Combining the genotypes of CYP2C19 * 2 and * 3 polymorphisms, we found that the metabolic types of CYP2C19 enzyme could significantly affect the PRI of CAD patients with or without PCI ().
Figure 2. Influence of CYP2C19 enzyme on the PRI of Chinese CAD patients taking clopidogrel. (a) Association between CYP2C19 × 2 polymorphism and the PRI of CAD patients undergoing PCI (left) and CAD patients without PCI (right); (b) Association between CYP2C19 × 3 polymorphism and the PRI of CAD patients undergoing PCI (left) and CAD patients without PCI (right); (c) Association between the metabolic types of CYP2C19 enzyme and the PRI of CAD patients undergoing PCI (left) and CAD patients without PCI (right). p < .025 was considered significant.
MiRNA expression profiling
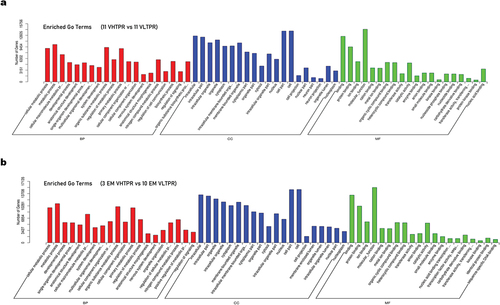
Total RNA used for miRNA profiling was extracted from the purified platelet preparations of 11 VHTPR and 11 VLTPR patients. The read counts of miRNAs obtained from the sequencing data were normalized by the method of TPM (transcripts per million reads). The miRNAs expression levels in each purified platelet preparation were ranked from high to low based on the TPM expression of the corresponding miRNAs, and the top 100 miRNAs were shown in Table S3. We found a total of 43 differentially expressed miRNAs (VHTPR vs VLTPR: 33 miRNAs up-regulated and 10 miRNAs down-regulated) between the two groups by differential expression analysis (Table S4). The volcano plots and cluster analysis of differentially expressed miRNAs were shown in . KEGG enrichment () and GO enrichment () analyses were performed on the obtained candidate target genes. The candidate target genes covered the three basic categories (Biological process, BP; Cellular component, CC; Molecular Function, MF) in the GO analysis with slight differences and many genes were enriched in the Rap1 signaling pathway. Activation of the Rap1b GTP binding proteins can lead to activation of the platelet GP IIb/IIIa receptor, an indispensable process in platelet aggregation.
Figure 3. Differential expression analysis of miRnas in extreme clopidogrel response LDP samples. The volcano plots (a) and cluster analysis (b) of differentially expressed miRnas (11 VHTPR vs 11 VLTPR). KEGG enrichment analysis (c) of the candidate target genes of the differentially expressed miRnas (11 VHTPR vs 11 VLTPR). The volcano plots (d) and cluster analysis (e) of differentially expressed miRnas (3 EM VHTPR vs 10 EM VLTPR). KEGG enrichment analysis (f) of the candidate target genes of the differentially expressed miRnas (3 EM VHTPR vs 10 EM VLTPR).
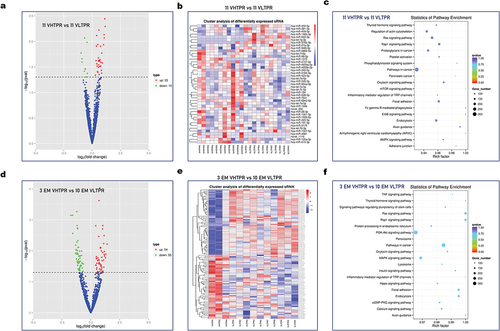
Figure 4. GO enrichment analysis of the obtained candidate target genes (a) 11 VHTPR vs 11 VLTPR; (b) 3 EM VHTPR vs 10 EM VLTPR). BP: Biological process; CC: Cellular component; MF: Molecular Function.
Considering the important influence of CYP2C19 genotypes on PRI in the patients, we performed a stratified analysis limited to the 13 CYP2C19 EM samples (3 VHTPR and 10 VLTPR) out of the 22 sequenced samples. We found that a total of 109 miRNAs (VHTPR vs VLTPR: 54 miRNAs up-regulated, 55 miRNAs down-regulated) were differentially expressed between the two groups (Table S5). The volcano plots and cluster analysis of the differentially expressed miRNAs were shown in . KEGG enrichment () and GO enrichment () analysis were also performed on the obtained candidate target genes. Similar to the previous results, the candidate target genes also covered the three basic categories (BP, CC, and MF) of GO analysis. It was worth noting that most of the potential target genes were enriched in the PI3K-Akt signaling pathway, one of the most important downstream pathways after P2Y12 activation.
Validation of differentially expressed candidate miRnas
Overlapping the differentially expressed miRNAs (43 miRNAs for the 11 VHTPR vs 11 VLTPR analysis, and 109 miRNAs in the stratified analysis with 3 EM VHTPR vs 10 EM VLTPR) with concomitant consideration of the top 100 miRNAs in platelets, we obtained 35 differentially and highly expressed miRNAs in platelets (Overlapping the 43 differentially expressed miRNAs with the top 100 miRNAs in platelets, 15 differentially expressed miRNAs was obtained. Similarly, 27 differentially expressed miRNAs were obtained when overlapping the 109 differentially expressed miRNAs with the top 100 miRNAs in platelets. Seven of the overlapping miRNAs were shared for both analyses; Figure S4). Six of the 35 miRNAs (including hsa-let-7f-5p, hsa-miR-98-5p, hsa-miR-199a-5p, hsa-let-7a-5p, hsa-miR-409-3p and hsa-let-7d-5p) were predicted to target key genes (P2RY12, VASP and ITGB3) involved in the platelet activation pathway and were validated. The target gene prediction results of the 6 candidate miRNAs were shown in Table S6.
LDP samples from another 41 CAD patients taking 300 mg loading dose clopidogrel (12-24 h) were collected for validation. Platelet VASP-P levels of the patients were determined and PRI was calculated. Relative expression of the 6 candidate miRNAs and mRNA of their potential target genes in platelets were detected.
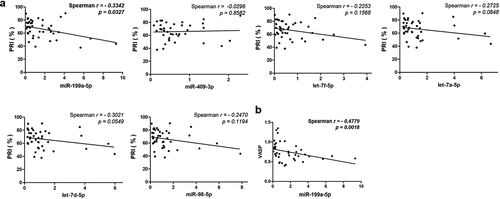
In the validation samples, we observed that the expression of miR-199a-5p in platelets correlated negatively with PRI (Spearman r: −0.3342, p = 0.0327, ), and no significant correlations between the other 5 miRNAs (let-7d-5p, let-7a-5p, miR-98-5p, let-7f-5p and miR-409-3p) and PRI was observed (). Besides, we found a significant negative correlation between the expression of miR-199a-5p and VASP mRNA (the predicted target gene of miR-199a-5p) expression in platelets (Spearman r = −0.4779, p = .0018; ).
Figure 5. Validation of candidate miRNAsin LDPs samples from another 41 CAD patients. (a) Correlation between 6 candidate miRNA levels in LDPs samples and the PRI of the corresponding patients. (b) A significant negative correlation between the expression of miR-199a-5p and VASP mRNA expression in platelets.
Dual-luciferase reporter assays
The potential miR-199a-5p binding sequence in the 3”-UTR of VASP was predicted using TargetScan software. The DNA sequences (~300 bp) around the potential binding site (Wide type, WT; Mutant, Mut) of VASP 3”-UTR were amplified and inserted into the polyclonal region of the pmirGLO vector (). The maps of the constructed pmirGLO-VASP-WT/Mut dual-luciferase reporters were shown in and the sequences of the reporters were verified by Sanger sequencing (). The mimics of miR-199a-5p and pmirGLO-VASP-WT or pmirGLO-VASP-Mut plasmid were co-transfected into HEK293 cells. The results showed that miR-199a-5p could significantly down-regulated the relative luciferase activity of pmirGLO-VASP-WT but not the pmirGLO-VASP-Mut ().
Figure 6. The regulation of miR-199a-5p on VASP. (a) The binding sequence of miR-199a-5p in the 3 ́-UTR of VASP. (b) The maps of the constructed pmirGLO-VASP-WT/Mut dual-luciferase reporters. (c) The partial sequences of two constructed vectors. (d) Dual-luciferase reporter assays to explore the regulation of miR-199a-5p on VASP. (e) Overexpression of miR-199a-5p down-regulates VASP expression in cells. All the experiments were repeated three times, independently.
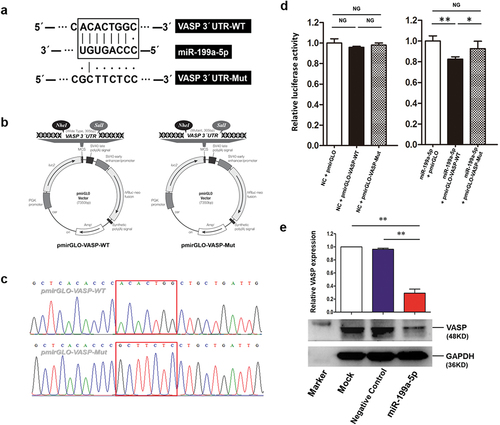
Overexpression of miR-199a-5p down-regulates VASP expression in cells
The human protein atlas database was used to query the relative expression of VASP mRNA in various cell lines. A549 cells showed a high abundance of VASP mRNA expression and were selected for the following study. We overexpressed miR-199a-5p in A549 cells to investigate the regulation of miR-199a-5p on VASP expression. The results indicated that overexpression of miR-199a-5p significantly down-regulated VASP protein expression in A549 cells ().
Discussion
In this study, we screened for differentially expressed platelet miRNA in patients with VHTPR and VLTPR. Though a group of differentially expressed miRNAs was identified by RNA sequencing, only the platelet expression of miR-199a-5p was validated. We observed that platelet miR-199a-5p expression level correlated negatively with PRI, an indicator of clopidogrel response. Subsequent reporter gene assay and cell study suggest VASP be the direct target of miR-199a-5p.
Though the majority of the patients we recruited in our study were on dual antiplatelet therapy with aspirin and clopidogrel, the antiplatelet mechanism of aspirin is different from that of clopidogrel. Aspirin acts through inhibition of thromboxane A2 (TXA2) synthesis but not the ADP stimulated P2Y12 receptor.Citation5 The efficacy parameter PRI adopted in our study is calculated based on VASP phosphorylation, an effect specific for P2Y12 inhibition and preferred for inhibition in ADP-induced aggregation. Therefore, our findings may rule out the interference of aspirin to some extent.
For the patients recruited in this study, we confirmed the influence of CYP2C19 * 2 and CYP2C19 * 3 polymorphisms on PRI in our samples as reported previously,Citation16–18 which was consistent with previous literature.Citation19,Citation42,Citation43 This indicates that the samples we collected and the methods we established to determine clopidogrel response for the CAD patients were reliable.
We collected purified platelet preparations from 22 patients with extreme phenotypes of clopidogrel response (11 VHTPR and 11 VLTPR) for small RNA sequencing. However, the VHTPR and VLTPR samples were not matched well with CYP2C19 genotypes. This is because CYP2C19 loss of function (LOF) alleles are the major determinant of VHTPR during clopidogrel use, while the allele frequencies of the LOF were fairly high in the Chinese population. In fact, 56.9% (289 of 508) patients carried at least one CYP2C19 LOF allele in our study. CYP2C19 EMs rarely occur in the VHTPR group. CYP2C19 EM patients are more likely to produce higher levels of clopidogrel active metabolite and exhibit lower PRI values (or better clopidogrel response) compared with CYP2C19 IM or PM patients. To avoid the impact of CYP2C19 genotypes on PRI, we performed a stratified analysis for the 13 CYP2C19 EM samples (3 with VHTPR, 10 with VLTPR) from the 22 sequenced samples. Therefore, there were only 3 EM patients in 11 VHTPR sequencing samples.
In the validation stage in this study, we observed that platelets miR-199a-5p expression correlated negatively with PRI values in the 41 samples, which suggested that patients with decreased platelet miR-199a-5p expression were more likely to exhibit high on-clopidogrel platelet reactivity. We were the first to report the relationship between platelet miR-199a-5p and the antiplatelet efficacy of clopidogrel. Phosphorylation of VASP is a downstream effector as well as a specific indicator of P2Y12 receptor inhibition. We observed in our study that the platelet miR-199a-5p expression correlated negatively with VASP mRNA expression (r = –0.4779, p = .0018). Furthermore, we observed that miR-199a-5p inhibited the expression of VASP in cultured cells. The results from the reporter gene assay also indicate direct inhibition of miR-199a-5p on VASP mRNA. Our findings indicate that miR-199a-5p might affect the efficacy of clopidogrel through inhibition of VASP expression.
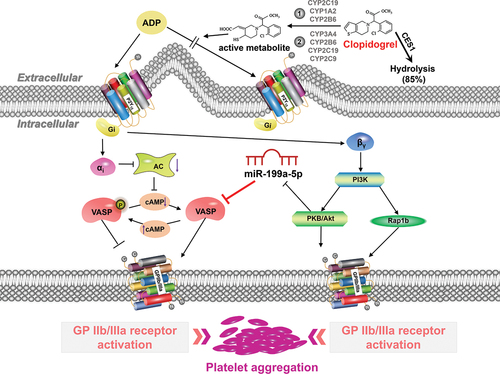
Based on the enrichment analysis (KEGG; 3 EM VHTPR vs 10 EM VLTPR) results of the obtained target genes, we found a large number of potential candidate target genes of the differentially expressed miRNAs enriched in the PI3K-Akt signaling pathway which might be activated by βγ subunit of G proteins after P2Y12 receptor stimulation. Activation of the PI3K-Akt signaling pathway leads to the activation of platelet GP IIb/IIIa receptors, which promotes the activation and aggregation of platelets. However, the exact mechanism by which the activated PI3K-Akt pathway regulates the GP IIb/IIIa receptor remains unclear.Citation44 There is evidence indicates that overexpression of active AKT induces rapid downregulation of miR-199a-5p.Citation45,Citation46 Based on our study, the expression of VASP was increased when the expression of miR-199a-5p decreased sharply. Simultaneously, the αi subunit of the G protein released after the activation of the P2Y12 receptor can inhibit the activity of the adenosine cyclase, which results in a reduction in cAMP in platelets. The reduction of cAMP can result in VASP dephosphorylation (conversion of VASP-P to VASP), and then GP IIb/IIIa receptors and platelets were activated in turn. Our study provided a possible mechanism mediating PI3K-Akt to platelet activation. However, further studies are needed to support our hypothesis. gives an overview of the mechanisms of action of clopidogrel according to the previous studiesCitation47,Citation48 and our findings.
Figure 7. The mechanism of action of clopidogrel. Clopidogrel is a pro-drug and approximately 15% of the clopidogrel is metabolized into an active metabolite by CYP enzymes (include 2 steps: CYP2C19, CYP1A2 and CYP2B6 enzymes contribute to the formation of intermediate 2-oxo-clopidogrel, while CYP3A4, CYP2B6, CYP2C19 and CYP2C9 enzymes promote the conversion from 2-oxo-clopidogrel to active metabolites) after oral administration. The active metabolite can irreversibly bind to the P2Y12 receptor on the surface of platelets and block the activation of the P2Y12 receptor by adenosine diphosphate (ADP, a kind of P2Y12 receptor agonist). P2Y12 is a Gi- protein-coupled receptor and it can liberate the Gi protein subunits αi and βγ when activated. The αi subunit inhibits adenylyl cyclase (AC) and reduces Camp-mediated phosphorylation of vasodilator-stimulated phosphoprotein (VASP-P). However, VASP-P can effectively inhibit the activation of platelet GP IIb/IIIa receptor which plays vital roles in the activation and aggregation of platelets. The βγ subunit activates phosphatidylinositol 3-kinase (PI3K) which leads to the activation of the GP IIb/IIIa receptor through activating serine/threonine protein kinase B (PKB/Akt) and Rap1b GTP binding proteins.
There are some limitations that should be strengthened. First, the findings of the current study were based on the clinical research of the Chinese population. Whether these findings could be extended to other populations requires further extensive exploration. Second, we failed to collect all the information of the patients such as height and bodyweight of the patients. Third, more balanced samples would have been ideal and it is best to select CYP2C19 EM patients to eliminate the effect of CYP2C19 as much as possible. However, there are some difficulties with sample collection, especially for VHTPR patients with CYP2C19 EM genotype. We believe that a more comprehensive and reliable analysis corrected by the confounding factors is anticipated in further clinical studies with expanded samples. Fourth, miRNAs that target other genes (except P2RY12, VASP, and ITGB3) in the clopidogrel antiplatelet signaling pathway might be ignored in the current study.
Conclusion
In summary, we observed that decreased platelet miR-199a-5p was associated with high on-clopidogrel platelet reactivity in CAD patients and the level of platelet miR-199a-5p might affect the efficacy of clopidogrel via direct inhibiting the expression of VASP in this preliminary study. Our study might provide new insights into the further exploration of interindividual variability in clopidogrel response from the perspective of miR-199a-5p and VASP interaction.
Abbreviations
| CAD | = | coronary artery disease |
| DAPT | = | dual antiplatelet therapy |
| PRI | = | Platelet reactivity index |
| HTPR | = | high on-treatment platelet reactivity |
| VHTPR | = | very high on-treatment platelet reactivity |
| LTPR | = | low on-treatment platelet reactivity |
| VLTPR | = | very low on-treatment platelet reactivity |
| PRP | = | platelet-rich plasma |
| LDP | = | leukocyte depleted plasma |
| VASP | = | vasodilator-stimulated phosphoprotein |
| VASP-P | = | phosphorylation of vasodilator-stimulated phosphoprotein |
| GP IIb/IIIa | = | glycoprotein IIb/IIIa receptor |
Supplemental Material
Download PDF (1.4 MB)Acknowledgments
Thanks for the contributions of all the individuals involved in our study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/09537104.2023.2200860
Additional information
Funding
References
- Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart disease and stroke statistics—2020 update: a report from the American heart association. Circulation. 2020;141(9):e139–11. doi:10.1161/CIR.0000000000000757.
- Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8(11):1227–34. doi:10.1038/nm1102-1227.
- Mehta SR, Yusuf S, Peters RJ, Bertrand ME, Lewis BS, Natarajan MK, Malmberg K, Rupprecht H, Zhao F, Chrolavicius S, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358(9281):527–33. doi:10.1016/s0140-6736(01)05701-4.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Clopidogrel in unstable angina to prevent recurrent events trial I. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med. 2001;345:494–502. doi:10.1056/NEJMoa010746.
- Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol. 2005;45(2):246–51. doi:10.1016/j.jacc.2004.09.067.
- Trenk D, Zolk O, Fromm MF, Neumann FJ, Hochholzer W. Personalizing antiplatelet therapy with clopidogrel. Clin Pharmacol Ther. 2012;92:476–85. doi:10.1038/clpt.2012.133.
- Tantry US, Bonello L, Aradi D, Price MJ, Jeong YH, Angiolillo DJ, Stone GW, Curzen N, Geisler T, Ten Berg J, et al. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. 2013;62(24):2261–73. doi:10.1016/j.jacc.2013.07.101.
- Trenk D, Kristensen SD, Hochholzer W, Neumann FJ. High on-treatment platelet reactivity and P2Y12 antagonists in clinical trials. Thromb Haemost. 2013;109(05):834–45. doi:10.1160/TH12-08-0588.
- Price MJ, Angiolillo DJ, Teirstein PS, Lillie E, Manoukian SV, Berger PB, Tanguay JF, Cannon CP, Topol EJ. Platelet reactivity and cardiovascular outcomes after percutaneous coronary intervention: a time-dependent analysis of the gauging responsiveness with a VerifyNow P2Y12 assay: impact on thrombosis and safety (GRAVITAS) trial. Circulation. 2011;124(10):1132–7. doi:10.1161/CIRCULATIONAHA.111.029165.
- Bliden KP, DiChiara J, Tantry US, Bassi AK, Chaganti SK, Gurbel PA. Increased risk in patients with high platelet aggregation receiving chronic clopidogrel therapy undergoing percutaneous coronary intervention: is the current antiplatelet therapy adequate? J Am Coll Cardiol. 2007;49(6):657–66. doi:10.1016/j.jacc.2006.10.050.
- Stone GW, Witzenbichler B, Weisz G, Rinaldi MJ, Neumann FJ, Metzger DC, Henry TD, Cox DA, Duffy PL, Mazzaferri E, et al. Platelet reactivity and clinical outcomes after coronary artery implantation of drug-eluting stents (ADAPT-DES): a prospective multicentre registry study. Lancet. 2013;382(9892):614–23. doi:10.1016/S0140-6736(13)61170-8.
- Park MW, Her SH, Kim CJ, SunCho J, Park GM, Kim TS, Choi YS, Park CS, Koh YS, Park HJ, et al. Evaluation of the incremental prognostic value of the combination of CYP2C19 poor metabolizer status and ABCB1 3435 TT polymorphism over conventional risk factors for cardiovascular events after drug-eluting stent implantation in East Asians. Genet Med. 2016;18(8):833–41. doi:10.1038/gim.2015.171.
- Mao L, Jian C, Changzhi L, Dan H, Suihua H, Wenyi T, Wei W. Cytochrome CYP2C19 polymorphism and risk of adverse clinical events in clopidogrel-treated patients: a meta-analysis based on 23,035 subjects. Arch Cardiovasc Dis. 2013;106(10):517–27. doi:10.1016/j.acvd.2013.06.055.
- Tantray JA, Reddy KP, Jamil K, Kumar YS. Pharmacodynamic and cytogenetic evaluation in CYP2C19*2 and CYP2C19*3 allelomorphism in South Indian population with clopidogrel therapy. Int J Cardiol. 2017;229:113–18. doi:10.1016/j.ijcard.2016.11.217.
- Sun H, Qu Q, Chen ZF, Tan SL, Zhou HJ, Qu J, Chen H. Impact of CYP2C19 variants on clinical efficacy of clopidogrel and 1-year clinical outcomes in coronary heart patients undergoing percutaneous coronary intervention. Front Pharmacol. 2016;7:453. doi:10.3389/fphar.2016.00453.
- Wang JY, Zhang YJ, Li H, Hu XL, Li MP, Song PY, Ma QL, Peng LM, Chen XP. CRISPLD1 rs12115090 polymorphisms alters antiplatelet potency of clopidogrel in coronary artery disease patients in Chinese Han. Gene. 2018;678:226–32. doi:10.1016/j.gene.2018.08.027.
- Li H, Zhang YJ, Li MP, Hu XL, Song PY, Peng LM, Ma QL, Tang J, Zhang W, Chen XP. Association of N6AMT1 rs2254638 polymorphism with clopidogrel response in Chinese patients with coronary artery disease. Front Pharmacol. 2018;9:1039. doi:10.3389/fphar.2018.01039.
- Zhu KX, Song PY, He L, Li MP, Du YX, Ma QL, Peng LM, Chen XP. Association of FMO3 rs1736557 polymorphism with clopidogrel response in Chinese patients with coronary artery disease. Eur J Clin Pharmacol. 2021;77(3):359–68. doi:10.1007/s00228-020-03024-6.
- Shuldiner AR, O’Connell JR, Bliden KP, Gandhi A, Ryan K, Horenstein RB, Damcott CM, Pakyz R, Tantry US, Gibson Q, et al. Association of cytochrome P450 2C19 genotype with the antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA. 2009;302(8):849–57. doi:10.1001/jama.2009.1232.
- Zhong WP, Wu H, Chen JY, Li XX, Lin HM, Zhang B, Zhang ZW, Ma DL, Sun S, Li HP, et al. Genomewide association study identifies novel genetic loci that modify antiplatelet effects and pharmacokinetics of clopidogrel. Clin Pharmacol Ther. 2017;101(6):791–802. doi:10.1002/cpt.589.
- Hochholzer W, Trenk D, Fromm MF, Valina CM, Stratz C, Bestehorn HP, Buttner HJ, Neumann FJ. Impact of cytochrome P450 2C19 loss-of-function polymorphism and of major demographic characteristics on residual platelet function after loading and maintenance treatment with clopidogrel in patients undergoing elective coronary stent placement. J Am Coll Cardiol. 2010;55(22):2427–34. doi:10.1016/j.jacc.2010.02.031.
- Stratz C, Bomicke T, Younas I, Kittel A, Amann M, Valina CM, Nuhrenberg T, Trenk D, Neumann FJ, Hochholzer W. Comparison of immature platelet count to established predictors of platelet reactivity during thienopyridine therapy. J Am Coll Cardiol. 2016;68(3):286–93. doi:10.1016/j.jacc.2016.04.056.
- Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N. Widespread changes in protein synthesis induced by microRnas. Nature. 2008;455(7209):58–63. doi:10.1038/nature07228.
- Bai YP, Zhang JX, Sun Q, Zhou JP, Luo JM, He LF, Lin XC, Zhu LP, Wu WZ, Wang ZY, et al. Induction of microRNA-199 by nitric oxide in endothelial cells is required for nitrovasodilator resistance via targeting of prostaglandin I2 synthase. Circulation. 2018;138(4):397–411. doi:10.1161/CIRCULATIONAHA.117.029206.
- Willeit P, Zampetaki A, Dudek K, Kaudewitz D, King A, Kirkby NS, Crosby-Nwaobi R, Prokopi M, Drozdov I, Langley SR, et al. Circulating microRnas as novel biomarkers for platelet activation. Circ Res. 2013;112(4):595–600. doi:10.1161/CIRCRESAHA.111.300539.
- Barwari T, Joshi A, Mayr M. MicroRNAs in cardiovascular disease. J Am Coll Cardiol. 2016;68(23):2577–84. doi:10.1016/j.jacc.2016.09.945.
- Kaudewitz D, Skroblin P, Bender LH, Barwari T, Willeit P, Pechlaner R, Sunderland NP, Willeit K, Morton AC, Armstrong PC, et al. Association of MicroRNAs and YRNAs with platelet function. Circ Res. 2016;118(3):420–32. doi:10.1161/CIRCRESAHA.114.305663.
- Pedersen OB, Hvas AM, Grove EL, Larsen SB, Pasalic L, Kristensen SD, Nissen PH. Association of whole blood microRNA expression with platelet function and turnover in patients with coronary artery disease. Thromb Res. 2022;211:98–105. doi:10.1016/j.thromres.2022.01.026.
- Landry P, Plante I, Ouellet DL, Perron MP, Rousseau G, Provost P. Existence of a microRNA pathway in anucleate platelets. Nat Struct Mol Biol. 2009;16(9):961–6. doi:10.1038/nsmb.1651.
- Shi R, Ge L, Zhou X, Ji WJ, Lu RY, Zhang YY, Zeng S, Liu X, Zhao JH, Zhang WC, et al. Decreased platelet miR-223 expression is associated with high on-clopidogrel platelet reactivity. Thromb Res. 2013;131(6):508–13. doi:10.1016/j.thromres.2013.02.015.
- Liu YL, Hu XL, Song PY, Li H, Li MP, Du YX, Li MY, Ma QL, Peng LM, Song MY, et al. Influence of GAS5/MicroRNA-223-3p/P2Y12 axis on clopidogrel response in coronary artery disease. J Am Heart Assoc. 2021;10(21):e021129. doi:10.1161/JAHA.121.021129.
- Zhang YY, Zhou X, Ji WJ, Shi R, Lu RY, Li JL, Yang GH, Luo T, Zhang JQ, Zhao JH, et al. Decreased circulating microRNA-223 level predicts high on-treatment platelet reactivity in patients with troponin-negative non-ST elevation acute coronary syndrome. J Thromb Thrombolysis. 2014;38(1):65–72. doi:10.1007/s11239-013-1022-9.
- Chyrchel B, Toton-Zuranska J, Kruszelnicka O, Chyrchel M, Mielecki W, Kolton-Wroz M, Wolkow P, Surdacki A. Association of plasma miR-223 and platelet reactivity in patients with coronary artery disease on dual antiplatelet therapy: a preliminary report. Platelets. 2015;26(6):593–7. doi:10.3109/09537104.2014.974527.
- Chen S, Qi X, Chen H, Li M, Gu J, Liu C, Xue H, Wang L, Geng Y, Qi P, et al. Expression of miRNA-26a in platelets is associated with clopidogrel resistance following coronary stenting. Exp Ther Med. 2016;12(1):518–24. doi:10.3892/etm.2016.3278.
- Peng L, Liu J, Qin L, Liu J, Xi S, Lu C, Yin T. Interaction between platelet-derived microRnas and CYP2C19*2 genotype on clopidogrel antiplatelet responsiveness in patients with ACS. Thromb Res. 2017;157:97–102. doi:10.1016/j.thromres.2017.07.011.
- Zhou WL, Mo ZZ, Xiao FY, Dai W, Wang G, Zhou G, Zhang W, Chen BL. MicroRNA-605 rs2043556 polymorphisms affect clopidogrel therapy through modulation of CYP2B6 and P2RY12 in acute coronary syndrome patients. Platelets. 2020;31(7):897–905. doi:10.1080/09537104.2019.1696455.
- Aleil B, Ravanat C, Cazenave JP, Rochoux G, Heitz A, Gachet C. Flow cytometric analysis of intraplatelet VASP phosphorylation for the detection of clopidogrel resistance in patients with ischemic cardiovascular diseases. J Thromb Haemost. 2005;3(1):85–92. doi:10.1111/j.1538-7836.2004.01063.x.
- Bonello L, Paganelli F, Arpin-Bornet M, Auquier P, Sampol J, Dignat-George F, Barragan P, Camoin-Jau L. Vasodilator-stimulated phosphoprotein phosphorylation analysis prior to percutaneous coronary intervention for exclusion of postprocedural major adverse cardiovascular events. J Thromb Haemost. 2007;5(8):1630–6. doi:10.1111/j.1538-7836.2007.02609.x.
- Bonello L, Camoin-Jau L, Arques S, Boyer C, Panagides D, Wittenberg O, Simeoni MC, Barragan P, Dignat-George F, Paganelli F. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicenter randomized prospective study. J Am Coll Cardiol. 2008;51(14):1404–11. doi:10.1016/j.jacc.2007.12.044.
- Bonello L, Pansieri M, Mancini J, Bonello R, Maillard L, Barnay P, Rossi P, Ait-Mokhtar O, Jouve B, Collet F, et al. High on-treatment platelet reactivity after prasugrel loading dose and cardiovascular events after percutaneous coronary intervention in acute coronary syndromes. J Am Coll Cardiol. 2011;58(5):467–73. doi:10.1016/j.jacc.2011.04.017.
- Nagalla S, Shaw C, Kong X, Kondkar AA, Edelstein LC, Ma L, Chen J, McKnight GS, Lopez JA, Yang L, et al. Platelet microRNA-mRNA coexpression profiles correlate with platelet reactivity. Blood. 2011;117(19):5189–97. doi:10.1182/blood-2010-09-299719.
- Varenhorst C, James S, Erlinge D, Brandt JT, Braun OO, Man M, Siegbahn A, Walker J, Wallentin L, Winters KJ, et al. Genetic variation of CYP2C19 affects both pharmacokinetic and pharmacodynamic responses to clopidogrel but not prasugrel in aspirin-treated patients with coronary artery disease. Eur Heart J. 2009;30(14):1744–52. doi:10.1093/eurheartj/ehp157.
- Mega JL, Hochholzer W, Frelinger AL 3rd, Kluk MJ, Angiolillo DJ, Kereiakes DJ, Isserman S, Rogers WJ, Ruff CT, Contant C, et al. Dosing clopidogrel based on CYP2C19 genotype and the effect on platelet reactivity in patients with stable cardiovascular disease. JAMA. 2011;306(20):2221–8. doi:10.1001/jama.2011.1703.
- Woulfe DS. Akt signaling in platelets and thrombosis. Expert Rev Hematol. 2010;3(1):81–91. doi:10.1586/ehm.09.75.
- Rane S, He M, Sayed D, Yan L, Vatner D, Abdellatif M. An antagonism between the AKT and beta-adrenergic signaling pathways mediated through their reciprocal effects on miR-199a-5p. Cell Signal. 2010;22(7):1054–62. doi:10.1016/j.cellsig.2010.02.008.
- Sayed D, Abdellatif M. AKT-ing via microRNA. Cell Cycle. 2010;9(16):3213–7. doi:10.4161/cc.9.16.12634.
- Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Alfonso F, Macaya C, Bass TA, Costa MA. Variability in individual responsiveness to clopidogrel: clinical implications, management, and future perspectives. J Am Coll Cardiol. 2007;49(14):1505–16. doi:10.1016/j.jacc.2006.11.044.
- Jiang XL, Samant S, Lesko LJ, Schmidt S. Clinical pharmacokinetics and pharmacodynamics of clopidogrel. Clin Pharmacokinet. 2015;54(2):147–66. doi:10.1007/s40262-014-0230-6.