Abstract
This prospective study was aimed to test changes in hemostasis in patients with GBM, occurring at baseline (before surgery, time 0, T0) and 2 (T2), 24 (T24), and 48-hour (T48) after surgery. We enrolled consecutive patients subjected to GBM resection (GBR group; N = 60), laparoscopic colon cancer resection (comparative CCR group; N = 40), and healthy blood donors (HBD group; N = 40). We performed 1. conventional coagulation tests 2. ROTEM (rotational thromboelastometry) parameters and 3. platelet function tests, including PFA-200 closure time when stimulated by collagen/epinephrine (COL-EPI) and ROTEM platelet, using three different activators (arachnoid acid in ARATEM, adenosine diphosphate in ADPTEM, and thrombin receptor-activating peptide-6 in TRAPTEM). Variables associated with unfavorable 1-year clinical outcome were investigated, too. We observed in GBR patients that platelet aggregometry, as assessed by ROTEM platelet parameters, was significantly impaired along with a shortened closure time. These changes were evident from T0 to T48. A decreased area under the aggregation curve in TRAPTEM was associated with improved survival (adjusted odd ratio (95% CI), 1.03 (1.01–1.06)). This study suggests that patients with GBM presented a decreased platelet aggregation from before surgery and thorough the postoperative period. Decreased platelet aggregation improved clinical outcome.
Plain Language Summary
What is the context?
Glioblastoma has an impact on the platelet number and the functional state of platelets. Platelets can be activated by tumor cells, and platelets count and function may impact patient survival. It has been showed an association between thrombocytosis and a decreased overall survival, with a small reduction in glioma risk associated with the long-term use of low-dose aspirin.
Platelet function before and during the perioperative period in patients with glioblastoma has not been systematically investigated. Limited data suggest that platelet function may be impaired before and throughout the perioperative period, and that impaired platelet function affects clinical outcome.
What is new?
In this prospective study, we systematically investigated how glioblastoma provokes systemic alterations of hemostasis. We enrolled 60 consecutive patients (sample size calculated) subjected to resection of glioblastoma multiforme, and other 40 consecutive patients undergoing laparoscopic resection of colon cancer, as a comparative group, in order to differentiate hemostasis and coagulation profiles of two tumors (glioblastoma and colon adenocarcinoma) with high prothrombotic power. Forty healthy volunteers were also included to establish local reference values.
We performed 1. conventional coagulation tests 2. ROTEM (rotational thromboelastometry) parameters and 3. platelet function tests, including PFA-200 closure time when stimulated by collagen/epinephrine (COL-EPI) and ROTEM platelet, using three different activators (arachnoid acid in ARATEM, adenosine diphosphate in ADPTEM, and thrombin receptor-activating peptide-6 in TRAPTEM). Variables associated with unfavorable 1-year clinical outcome were investigated, too. All these analyses were carried out at baseline (T0, time 0, before surgery) and 2 (T2), 24(T24) and 48-hour (T48) after surgery.
We observed in GBR patients that platelet aggregometry, as assessed by ROTEM platelet parameters, was significantly impaired along with a shortened closure time. These changes were evident from T0 to T48. A decreased area under the aggregation curve in TRAPTEM was associated with improved survival (adjusted odd ratio (95% CI), 1.03 (1.01–1.06)).
What is the impact?
This study provides further evidence that patients with GBM presented a decreased platelet aggregation from before surgery and thorough the postoperative period. Decreased platelet aggregation improved clinical outcome.
The cut-offs obtained can potentially to provide risk stratification for clinical outcome and to be hypothesis generating research to be confirmed by RCTs.
Introduction
Glioblastoma multiforme (GBM) can be grouped into the highest risk category of solid tumors favoring cancer-associated coagulopathyCitation1 with cancer-associated venous thrombosis and death.Citation2,Citation3 Tumor cells directly activate hemostasis by expressing tissue factor;Citation3 consequently, D-dimersCitation3,Citation4 and von Willebrand factor (vWF)Citation5 are increased in these patients. Platelets can also be activated by malignant cells, and platelet count and function may affect patient’s survival.Citation3,Citation6–8 GBM can provoke coagulopathy by other pathways related to its cerebral localization, since severe and isolated brain injury produces systemic alterations of hemostasis in non-cancer patients.Citation9–14 The release of brain-derived substances enriched with tissue factor and platelet-activating factors into the systemic circulation may trigger systemic coagulopathy.Citation3,Citation8,Citation9,Citation11,Citation14–16
It could be hypothesized that the etiology of hemostatic disturbances observed in patients with GBM is multifactorial and can be related to the solid tumor itself, brain location, and if hemostasis is assessed in the post-operative period, an acute cerebral injury may occur provoked by surgery. However, the mechanism by which GBM and elective surgery cause systemic coagulopathy remains poorly understood. Better characterization of these pathomechanisms could help in the development of novel diagnostic strategies and enable the identification of potential therapeutic targets to prevent or treat coagulopathy.
Platelet function before and during the perioperative period in GBM patients has not been systematically investigated. This study aimed to investigate the changes in hemostasis observed in patients with GBM before and after elective tumor resection by comparing the results of coagulation tests, thromboelastometry, and platelet function testing in patients with GBM with those measured in a patient population undergoing standardized elective laparoscopic resection of colon carcinoma (comparative group). A group of healthy adult blood donors was used as the control group to characterize the reference values. We also investigated the factors associated with unfavorable 1-year clinical outcomes.
Methods
Study design
This single-center, prospective, cross-sectional study was conducted between March 2019 and April 2021 at the Neurocritical Care, University hospital “Virgen del Rocío,” Seville, Spain. The study protocol was approved by the local ethical committee (IRB 00012104), and informed consent was obtained from all participants. This study is part of the project Acute Brain Coagulopathy Development study” (ABCD study; clinicalTrials.gov NCT 02652897), which is aimed at investigating hemostasis derangements in patients with severe brain injury.
To investigate how GBM provokes systemic alterations of hemostasis, we included three groups of consecutive patients as follows: patients undergoing elective brain tumor resection (GBR), patients undergoing elective laparoscopic colorectal cancer resection (CCR), and healthy blood donors (HBD) donating blood at the Andalusian Blood Transfusion Center. CCR group was investigated as a comparative group, in order to differentiate the hemostasis and coagulation profiles of two tumors (glioblastoma and colon adenocarcinoma) with high prothrombotic power. The possible differences in hemostasis and coagulation between these two groups of patients (glioblastoma and colon cancer) could be due to the type of tumor and the intracerebral location of the GBM.
We choose patients with colon cancer because, like patients with glioblastoma, studies have documented the development of a hypercoagulable state with elevated markers of coagulation and platelet hyperactivation. In fact, colorectal cancer is associated with a high risk of venous thrombosis, and the number of venous thromboembolism events is 4.5 times higher in colon cancer patients than in a control population.Citation17–19
The HBD group was sex-and age-matched with the first 20 patients who underwent GBR and were included in the study. Pertinent information regarding possible bleeding disorders or medication intake that might interfere with coagulation test results over a period of 15 days prior to surgery was obtained through personal interviews.
Laboratory assays
Blood samples were collected at four different time points in patients with GBM and CCR patients (HBD only at T0) as follows: within 24 h prior to surgery or time 0 (T0) and at 2 h (T2), 24 h (T24), and 48 h (T48) after surgery. Samples were drawn into vacuum tubes containing 3.2% buffered trisodium citrate (BD Vacutainer VR, Plymouth, PL67BP, UK). Von Willebrand factor and FXIII assays were performed using the Sysmex CS-2500 System (Sysmex, Norderstedt, Germany) with the BC von Willebrand and Berichrom F XIII reagent (Siemens Healthineers, Erlangen, Germany).
Thromboelastometry
Thromboelastometry assays were performed using a ROTEM delta system (Tem Innovations GmbH, Munich, Germany).Citation10,Citation20–23 In this study, only the clotting time in EXTEM (extrinsic activation, CT-EXTEM in s), maximum clot firmness in EXTEM (MCF-EXTEM in mm), maximum clot firmness in FIBTEM (fibrin contribution to clot firmness, MCF-FIBTEM in mm), residual clot firmness in percentage of MCF 60 min after CT (Lysis index 60: LI60 in %), and reduction of clot firmness in percentage of MCF-EXTEM at 50–60 min during run time (maximum lysis, ML) were assessed. We stratified subjects into 3 fibrinolysis groups according to their EXTEM-ML/LI60 values: hyperfibrinolysis (ML > 15% and/or LI60 < 82%), physiologic (ML: 4–15% and/or LI60 82–98%) and shutdown (ML < 4% and/or LI60 > 98%).Citation24,Citation25
Platelet function tests: ROTEM platelet and platelet function analyzer (PFA-200)
The ROTEM platelet module (Tem Innovations GmbH, Munich, Germany) is an add-on device to the ROTEM delta system, which measures platelet aggregation in whole blood samples using impedance aggregometry.Citation20 Platelets are activated by the specific agonists arachidonic acid (ARATEM), adenosine diphosphate (ADPTEM), and thrombin receptor-activating peptide 6 (TRAPTEM). The main parameter was the area under the aggregation curve (AUC in Ω min) from the start of the measurement until 6 min of runtime. The AUC reflects overall platelet aggregation.
In a platelet function analyzer (PFA-200, Siemens Healthineers, Erlangen, Germany), the assessment of primary hemostasis is based on the co-stimulation of platelets by high shear stress and the contact of platelets with a collagen membrane and epinephrine (COL-EPI). Closure time was measured as the time (s) required for the generated platelet plug to completely occlude the central aperture of the collagen membrane.
Statistical analysis
Descriptive data are shown as proportions for qualitative variables and mean (SD) or median (p25-p75) for quantitative variables, as appropriate. At baseline, demographic data, laboratory values, and ROTEM parameters were compared between the three groups using the Kruskal–Wallis or one-way ANOVA test, as appropriate. For post-hoc two-group comparisons, the t-test or Mann–Whitney U test for continuous variables and Chi-square or Fisher’s exact test for categorical variables were used. Correlations were calculated using Pearson’s test. For serial determinations obtained in the GBR and CCR groups, generalized linear mixed models were used. For global analysis of temporal changes, Pillai’s trace and Wilk’s lambda tests were used. Inter- and intra-participant comparisons were made for repeated measurements using ANOVA with polynomial contrast.
We further investigated the influence of demographic, operative, and laboratory variables on unfavorable 1-year outcomes in patients undergoing GBM resection using two logistic regression models with crude mortality and Glasgow outcome scale (GOS) as dependent variables (GOS levels 1, 2, and 3 were considered unfavorable outcomes). Values are expressed as adjusted odds ratios with the 95% confidence interval (AOR, 95% CI). Receiver operating characteristics (ROC) with the area under the curve (AUC) was performed for all variables associated with unfavorable clinical outcomes (T0) in the multivariate analysis. Discriminative optimum cutoffs were calculated as the Youden index along with their corresponding sensitivities, specificities, and positive and negative values.
Bonferroni correction for multiple comparisons was used and statistical significance was set at p value <.01. Statistical analyses were performed using GraphPad PRISM 7 (La Jolla, CA) and SPSS 25.0 (Chicago, IL) with license
The STROBE guidelines were used in this study. The anonymized data analyzed in the current study that support the findings are available from the corresponding author upon reasonable request.
Results
Consecutive patients who were initially scheduled for elective surgery (GBR = 71, CCR = 48) and HBD (n = 51) were enrolled in the study. After screening, 24 participants (GBR = 8, CCR = 7, and HBD = 9) were excluded from taking anti-aggregation or anticoagulant drugs. Six participants refused to sign the informed consent form. Finally, 60 patients were included in the GBR group, 40 in the CCR group, and 40 HBD were enrolled in the control group over a 2-year study period. The basic demographics, comorbidities, and surgical data are shown in and II, respectively. Patients who underwent GBM resection were successfully matched with the HBD group (p > .05).
Table I. Baseline characteristics of all study participants. Data are mean (SD) and n (%). Glioblastoma multiforme volume was calculated by using the simplified formula for volume of an ellipsoid v = abc/2. KPS, Karnofsky performance score; *tumor involving total or preferably this cerebral lobe. Bonferroni correction for multiple comparisons was used and statistical significance was set at p < .01.
At baseline (T0) assessment
When compared with HBD (), patients with GBR had significantly lower factor XIII activity and aPTT results () and higher vWF and D-dimer levels ( and ). Nevertheless, the PT and aPTT results were always within the reference ranges.
Figure 1. Time course of coagulation tests at baseline (time 0, T0), 2 (T2), 24 (T24), and 48-hour (T48) after surgery. Changes over time are plotted in patients undergoing glioblastoma multiforme resection (GBR, red lines), colon cancer resection (CCR, blue lines), and healthy blood donors (HBD, black bold line, T0). Inter-subject significant differences (p < .01, open brackets up) between both groups of patients or between patients and health blood donors, and intra-subject significant differences (p < .01, open brackets down) observed in each period in both groups of patients with respect to its baseline value, are shown. Graphs represent mean ± standard deviation. (a). Prothrombin time (s); (b). activated partial thromboplastin time aPTT (s); (c). Fibrinogen Clauss (g/L); (d). Factor XIII (%).
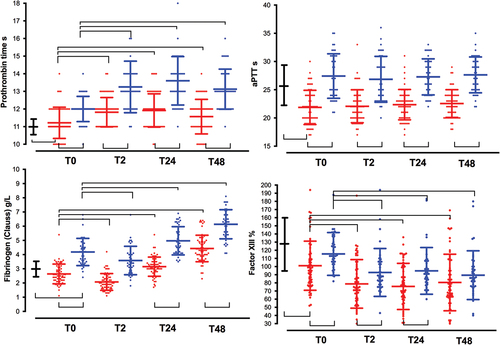
Figure 2. Time course of platelets count, von willebrand factor, and D-dimmers. See captions in . (a). Platelet count (x109/L); (b). von Willebrand factor (IU. dL−1); (c). D-dimers (ng.mL−1); (d). C-reactive protein (mg. L−1).
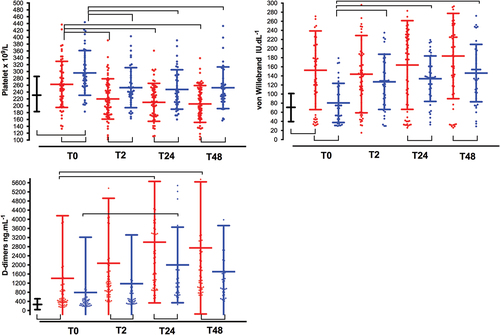
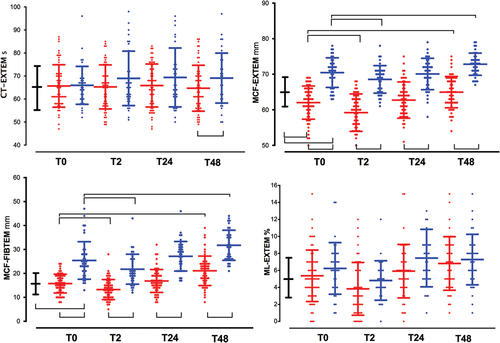
Differences in the clot firmness amplitude were detected between the three groups, but not with respect to the CT-EXTEM results. The mean MCF-EXTEM was significantly lower in patients with GBR than in patients with HBD; however, this difference was not observed with respect to MCF-FIBTEM. Clot firmness amplitudes were higher in patients who underwent CCR than in HBD patients, mirroring the high platelet counts and plasma fibrinogen concentrations found in these patients ( and ).
Figure 3. Time-course of ROTEM parameters. See captions in . (a). Clotting time in EXTEM (CT-EXTEM, s); (b). maximum clotting firmness in EXTEM (MCF-EXTEM, mm); (c). FIBTEM (MCF-FIBTEM, mm); (d). maximum lysis in EXTEM (ML-EXTEM, %).
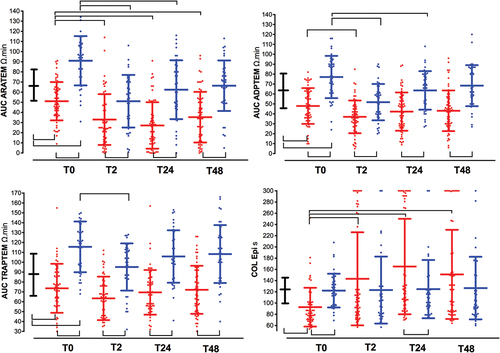
There was a significant worsening in platelet aggregation in response to all activators (ARATEM, ADPTEM, and TRAPTEM) in patients who underwent with GBR, but not in patients with CCR, when compared with HBD. In contrast, we observed a shortened closure time in PFA-200 (COLEPI) ( and ).
Figure 4. Time-course of platelet function tests. See captions in . The area under the aggregation curve (AUC) in response to stimulation with (a). arachidonic acid (AUC ARATEM, Ω·min); (b). adenosine diphosphate (AUC ADPTEM, Ω·min); (c). thrombin receptor-activating peptide-6 (AUC TRAPTEM, Ω·min); (d). the closure time in the PFA-200 collagen/epinephrine cartridge (Col Epi in s).
Time course (from T0 to T48 assessment)
Factor XIII activity and platelet count were reduced in patients who underwent GBR and CCR with respect to their respective baseline values, with lower values in the GBR group ( and Supplementary Table S1). In contrast, fibrinogen levels decreased discretely only in the immediate postoperative period (T2), remaining significantly above their respective baseline values during the postoperative period (T24, T48), with lower values observed in the GBR group ( and Supplementary Table S1).
Postoperative D-dimer values were significantly higher (p < .001) in patients who underwent GBR than those who underwent CCR ( and Supplementary Table S1), with a stepwise increment from their baseline values. The mean values of ML-EXTEM and LI60-EXTEM did not differ between groups at any time point ( and Supplementary Table S2). According with ML-EXTEM values, physiologic fibrinolysis was the most frequent phenotype at T0 (73.3%, 82.4% and 72.9%; p = .54 for GBR, CCR and HBD groups, respectively), T24 (82.5% and 87.5%; p = .83 for GBR and CCR groups, respectively) and T48 (86.4% and 91.7%; p = 0,22 for GBR and CCR groups, respectively). However, in the immediate postoperative period (T2) the phenotype fibrinolysis shutdown was more frequently observed in GBR than CCR patients (55.3% vs. 22.2%; p = .005). Hyperfibrinolysis (ML > 15% or LI60 < 82%) was not observed. No correlations were observed between D-dimers and LI60-EXTEM or ML-EXTEM when stratified by the GBR, CCR, and HBD groups or study period (T0➔T48) (p > .05).
MCF-EXTEM was always lower in GBR patients than those who underwent in CCR ( and Supplementary Table S2). Both groups of patients showed a decrease in clot amplitudes only in the immediate postoperative period (T2). CT-EXTEM values remained within the normal ranges in both groups of patients in all periods.
ROTEM platelet tests showed a reduced response to specific activators at baseline (GBR vs. HDB and CCR groups, inter subject comparisons) and from T0 to T48 (GBR vs. CCR groups, inter subject comparisons), as well as reduced activity of GBR group from T0 to postoperative periods (intra subject comparisons) ( and Supplementary Table S2). At baseline, a significantly shorter closure time with COLEPI was observed in the GBR group than in the HBD group ( and Supplementary Table S2). Von Willebrand factor levels were correlated with c-reactive protein (Pearson correlation coefficient (r) = 0.35; p = .005), but not with COLEPI ((r)=-0.20; p = .126).
Multivariate analyses and receiving operating characteristics (ROC) curves
Bivariate analysis of the risk factors associated with 1-year mortality and unfavorable clinical outcomes (GOS 1–3) is shown in .
Table II. Surgical variables and clinical outcomes. Data are showed as mean (SD), median [interquartile range] and n (%). GOS: Glasgow Outcome Scale.
Table III. Baseline (before surgery, T0) data of laboratory biochemistry, coagulation tests, rotational thromboelastometry (ROTEM), ROTEM platelet, and collagen/epinephrine (COL EPI, time closure, s) for patients with multiforme glioblastoma (N = 60 patients), colon cancer (N = 40 patients) and healthy blood donors (N = 40).
Multivariate logistic regression analysis showed that lower AUC-TRAPTEM (T0) results were associated with improved 1-year survival and 1-year favorable GOS (). AUC-TRAPTEM [0.759 (0.06) and 0.635 (0.07)] with optimum AUC cutoffs >64 and >42 Ω·min was the most discriminating value for patients who underwent GBR with increased 1-year mortality and unfavorable GOS (1–3), respectively.
Discussion
The main results of this study showed that patients who underwent GBR had the following presentations: a pattern of coagulation activation (increased D-dimer levels), increased platelet adhesion (shortened closure times in COL-EPI), and decreased platelet aggregation (reduced AUC in ARATEM, ADPTEM, and TRAPTEM) leading to a hypocoagulable state (reduced MCF-EXTEM). These changes were present before surgery (T0) and were even more pronounced throughout the postoperative period (T2, T24, and T48). We also observed that reduced platelet aggregation was associated with favorable clinical outcomes at the 1-year follow-up.
At baseline (T0), D-dimers were nearly 5-fold and 3-fold higher in GBR patients, than those measured in HBD and CCR patients () respectively, remaining significantly higher (GBR vs. CCR patients) throughout the postoperative period ( and supplementary Table S1), suggesting a continuously activated coagulationCitation4 with consumptive coagulopathy. D-dimers values were not associated with hyperfibrinolysis (D-dimers vs. ML% or LI60%, p > .05). We found that physiologic fibrinolysis was the most frequent phenotype, except in the immediate postoperative period (T2) when hypofibrinolysis/fibrinolysis shutdown (ML < 4%) was more frequently observed in patients with GBR (55.3% vs. 22.2%; p = .005, GBR vs. CCR patients). The combination of increased D-dimer and fibrinolysis shutdown is actually the best predictor of thrombosis.Citation26
As previously describedCitation16 we found a shortened aPTT at baseline and across all time period, which could be compatible with a hypercoagulable state, while PT ranged from 11 to 12 s, near to 11 ± 0.6 s observed in HDB. However, there was no significant decrease in CT-EXTEM between the different groups, so a hypercoagulable state was not detected by CT-EXTEM.Citation21 In fact, and based on the ROTEM findings, a hypocoagulable state was more plausible. At baseline, the ROTEM clot amplitude () was diminished in MCF-EXTEM, without significant changes in CT-EXTEM and MCF-FIBTEM, suggesting that the diminished MCF-EXTEM was more likely due to decreased platelet count, platelet dysfunction ( and ), and factor XIII depletion ( and ). It should be noted that at the baseline platelet count, plasma fibrinogen and MCF-FIBTEM were similar between the GBR and HBD groups, while platelet function tests were not (), suggesting that decreased platelet aggregation and factor XIII depletion were partially responsible for the decrease in baseline clot amplitude.
Our findings suggest a continuous activation of coagulation (increase in D-dimers) together with a decrease in clot amplitude. This last finding may be controversial, since patients with GBM suffer from a high rate of thrombotic events. Several coagulation pathways may also be involved.Citation15,Citation27 First, the tissue factor pathway inhibitor (TFPI), TFPI-1, and plasminogen activator inhibitor (PAI-1) have been shown to be increased in patients with GBM, whereas thrombomodulin is decreased. TFPI reduces the activity of both coagulation factor X and tissue factor/factor VIIa, PAI-1 inhibits fibrinolysis by inactivating plasminogen activators, and complex thrombomodulin and protein C are necessary to activate fibrinolysis. Taken together, these factors could lead to hypocoagulability, as observed in our patients who underwent GBR.
Second, how and when coagulation is assessed by ROTEM is of paramount importance. In our study, we found a state of hypocoagulability (based on decreased ROTEM amplitude), rather than hypercoagulability (based on decreased PT/aPTT). The procoagulant activity of GBM has been documented by ROTEM in 21 patients with cerebral tumors (8 gliomas).Citation28 All intra-operative data had median value within the ROTEM reference ranges, however a significant decreased CT-EXTEM and clot amplitude (MCF in EXTEM, INTEM and APTEM, except FIBTEM) and increased fibrinolisis (median ML > 50% in all the tests) were observed after spiking with tumor tissue extract own citrated whole blood. Authors concluded that there was a procoagulant state counterbalanced by strong hyperfibrinolysis and speculated that tumor extract induced and strong and early drop in platelets count and worsened platelet aggregation leading to reduced clot amplitude (MCF-EXTEM). We found reduced MCF in EXTEM, although we did not observe hyperfibrinolysis, nor did these authors find it in the basal ROTEM, without adding extracts of brain tissue.Citation28
Third, tumor cells can drive platelet activation, mainly by inducing thrombin generation via coagulation activation.Citation3 Thrombin is a main platelet activator and prolonged thrombin exposure is followed by time-dependent platelet activation, dysfunction, and disintegration.Citation29 Unlike other studies,Citation6,Citation30–32 we did not observe significant differences in baseline platelet count between the GBM and HBD groups (), nor platelet count was significantly associated with poorer results at the 1-year follow-up (). Although speculative, our findings are more consistent in that, high thrombin generation in GBM results in initial platelet activation (as manifested by shortened closure time in PFA) and subsequent exhaustionCitation33 (as manifested by impaired responses to ex vivo agonist stimulation in aggregometry assays of ROTEM platelets) (). Increased adhesion and decreased platelet aggregation were present before surgery and only in patients with GBM, probably related to an accelerated coagulation process due to the high levels of tissue factor and thrombin observed in glial tumors but not in patients with colon cancer.
Table IV. Univariate analysis of risk factors assessed at baseline, associated with 1-year mortality and 1-year of unfavorable GOS (1–3) in patients with glioblastoma multiforme (N = 60). Hb: hemoglobin. See captions in . Data are expressed as mean (standard deviation) or number (%).
Reduced platelet aggregation seems to be a pattern specific to GBR with probable clinical consequences. Reduced platelet aggregation observed in ARATEM and TRAPTEM tests was associated with improved 1-year survival (p = .03 and p = .003, respectively) and favorable clinical outcomes (TRAPTEM, p = .05), whereas platelet count and COL-EPI results on PFA-200 did not have an impact on 1-year survival or clinical outcomes (). In fact, a slight reduction in the risk of being diagnosed with glioma with the long-term use of low-dose aspirin has been documented.Citation7 These cutoffs obtained with ROTEM platelet tests may potentially provide risk stratification for clinical outcomes, and be hypothesis-generating research to be confirmed by randomized controlled trials.
Table V. Logistic regression analyses for variables associated with 1-year mortality and 1-year unfavorable clinical outcome (GOS 1–3). Adjusted odd ratio and 95% intervals confidence [AOR (CI95%)] are shown. ROC with AUC (SE) analyses with optimum cutoffs values (Youden index) for variables associated with unfavorable clinical outcome in bivariate analysis, are also shown.
This study has several limitations. Our cohort size was too small to have sufficient power to discriminate between the clinical subgroups based on coagulation and hemostasis thresholds as well as their influence on clinical outcomes. Although our data are consistent with GBM having a strong effect on platelet adhesion/aggregation and coagulation, there are likely multiple other non-included factors that affect hemostasis and coagulation in vitro and in vivo. Unlike other studiesCitation16,Citation34–36 increased D-dimer and von Willebrand factor values did not influence the clinical outcome (). In fact, von Willebrand levels were not correlated with COLEPI, but with the c-reactive protein, which suggest that von Willebrand levels were more related to inflammation. Lastly, we did not investigate the relationship between these lab values and thromboembolic events.
Conclusion
Our findings suggest that patients with GBM present with stimulation of coagulation, increased platelet adhesion, and decreased platelet aggregation, in the face of sustained, profound platelet activation. These alterations were present before surgery and were enhanced by the surgical insult, lasting at least 48 h after surgery. Notably, lower preoperative AUC-TRAPTEM and AUC-ARATEM scores were associated with improved 1-year survival and favorable clinical outcomes. Accordingly, reduced platelet aggregation in GBM might be considered an adaptive mechanism rather than platelet dysfunction. Further studies are needed to understand the pathophysiology of brain tumor resection and the role of platelet function in clinical outcomes in this setting.
Abbreviation
| aPTT | = | activated partial thromboplastin time (s). |
| ADPTEM AUC | = | Area under the aggregation curve in response to stimulation with adenosine diphosphate (AUC, Ω·min). |
| ARATEM AUC | = | Area under the aggregation curve in response to stimulation with arachidonic acid (AUC, Ω·min). |
| CCR | = | Group of patients undergoing elective laparoscopic resection of colon cancer. |
| CT-EXTEM | = | Clotting time assessed by thromboelastometry with extrinsic activation (s). |
| COLEPI | = | PFA-200 test using collagen/epinephrine activation (s). |
| FXIII | = | Factor XIII (%). |
| GBM | = | Glioblastoma multiforme. |
| GBR | = | Group of patients undergoing elective resection of glioblastoma multiforme. |
| GOS | = | Glasgow outcome scale. |
| HBD | = | Healthy blood donors. |
| LI60-EXTEM | = | Lysis index 60 defined as residual clot firmness amplitude in percentage of MCF at 60 minutes after CT (%). |
| MCF-EXTEM | = | Maximum clot firmness (mm) assessed by thromboelastometry with extrinsic activation (mm). |
| MCF-FIBTEM | = | Fibrin contribution to maximum clot firmness (mm). assessed by thromboelastometry with extrinsic activation. |
| ML-EXTEM | = | Maximum lysis defined as decrease in clot firmness amplitude in percentage of MCF during run time (%). |
| PT | = | Prothrombin time (s). |
| ROTEM | = | Rotational thromboelastometry. |
| TRAPTEM AUC | = | Area under the curve in response to stimulation with thrombin receptor-activating peptide-6 (AUC, Ω·min). |
| vWF | = | von Willebrand factor (IU dL−1). |
Supplementary Table S2
Download PDF (134.7 KB)Supplementary Table S1
Download PDF (116.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/09537104.2023.2216802.
Additional information
Funding
References
- Wun T, White RH. Epidemiology of cancer-related venous thromboembolism. Best Pract Res Clin Haematol. 2009;22:9–11. doi:10.1016/j.beha.2008.12.001.
- Timp JF, Braekkand SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122:1712–23. doi:10.1182/blood-2013-04-460121.
- Mandoj C, Tomao L, Conti L. Coagulation in brain tumors: biological basis and clinical implications. Front Neurol. 2019;10:181. doi:10.3389/fneur.2019.00181.
- Ay C, Vormittag R, Dunkler D, Simanek R, Chiriac AL, Drach J, Quehenberger P, Wagner O, Zielinski C, Pabinger I. D-dimer and prothrombin fragment 1 + 2 predict venous thromboembolism in patients with cancer: results from the vienna cancer and thrombosis study. J Clin Oncol. 2009;27:4124–9. doi:10.1200/JCO.2008.21.7752.
- Pace A, Mandoj C, Antenucci A, Villani V, Sperduti I, Casini B, Carosi M, Fabi A, Vidiri A, Koudriavtseva T, et al. A predictive value of von willebrand factor for early response to bevacizumab therapy in recurrent glioma. J Neurooncol. 2018;138:527–35. doi:10.1007/s11060-018-2820-x.
- Brockmann MA, Giese A, Mueller K, Kaba FJ, Lohr F, Weiss C, Gottschalk S, Nolte I, Leppert J, Tuettenberg J, et al. Preoperative thrombocytosis predicts poor survival in patients with glioblastoma. Neuro Oncol. 2007;9:335–42. doi:10.1215/15228517-2007-013.
- Gaist D, Garcia-Rodriguez LA, Sorensen HT, Hallas J, Friis S. Use of low-dose aspirin and non-aspirin nonsteroidal anti-inflammatory drugs and risk of glioma: a case-control study. Br J Cancer. 2013;108:1189–94. doi:10.1038/bjc.2013.87.
- Riedl J, Preusser M, Nazari PM, Posch F, Panzer S, Marosi C, Birner P, Thaler J, Brostjan C, Lötsch D, et al. Podoplanin expression in primary brain tumors induces platelet aggregation and increases risk of venous thromboembolism. Blood. 2017;129:1831–9. doi:10.1182/blood-2016-06-720714.
- Maegele M, Schöchl H, Menovsky T, Maréchal H, Marklund N, Buki A, Stanworth S. Coagulopathy and haemorrhagic progression in traumatic brain injury: advances in mechanisms, diagnosis, and management. Lancet Neurol. 2017;16:630–47. doi:10.1016/S1474-4422(17)30197-7.
- Kvint S, Schuster J, Kumar MA. Neurosurgical applications of viscoelastic hemostatic assays. Neurosurg Focus. 2017;43:E9. doi:10.3171/2017.8.FOCUS17447.
- Frontera JA, Provencio JJ, Sehba FA, McIntyre TM, Nowacki AS, Gordon E, Weimer JM, Aledort, Aledort L. The role of platelet activation and inflammation in early brain injury following subarachnoid hemorrhage. Neurocrit Care. 2017;26:48–57. doi:10.1007/s12028-016-0292-4.
- Tutwiler V, Peshkova AD, Andrianova IA, Khasanova DR, Weisel JW, Litvinov RI. Contraction of blood clots is impaired in acute ischemic stroke. Arterioscler Thromb Vasc Biol. 2017;37:271–9. doi:10.1161/ATVBAHA.116.308622.
- Jurk K, Jahn UR, Van Aken H, Schriek C, Droste DW, Ritter MA, Bernd Ringelstein E, Kehrel BE. Platelets in patients with acute ischemic stroke are exhausted and refractory to thrombin, due to cleavage of the seven-transmembrane thrombin receptor (PAR-1). Thromb Haemost. 2004;91:334–44. doi:10.1160/TH03-01-0044.
- Zhang J, Zhang F, Dong JF. Coagulopathy induced by traumatic brain injury: systemic manifestation of a localized injury. Blood. 2018;131:2001–6. doi:10.1182/blood-2017-11-784108.
- Wojtukiewicz MZ, Mysliwiec M, Matuszewska E, Sulkowski S, Zimnoch L, Politynska B, Wojtukiewicz AM, Tucker SC, Honn KV. Imbalance in coagulation/fibrinolysis inhibitors resulting in extravascular thrombin generation in gliomas of varying levels of malignancy. Biomolecules. 2021;11:663. doi:10.3390/biom11050663.
- Navone SE, Guarnaccia L, Locatelli M, Rampini P, Caroli M, La Verde N, Gaudino C, Bettinardi N, Riboni L, Marfia G, et al. Significance and prognostic value of the coagulation profile in patients with glioblastoma: implications for personalized therapy. World Neurosurg. 2019;121:e621–9. doi:10.1016/j.wneu.2018.09.177.
- Zhao L, Bi Y, Kou J, Shi J, Piao D. Phosphatidylserine exposing-platelets and microparticles promote procoagulant activity in colon cancer patients. J Exp Clin Cancer Res. 2016;35:54. doi:10.1186/s13046-016-0328-9.
- Leiva O, Connors JM, Al-Samkari H. Impact of tumor genomic mutations on thrombotic risk in cancer patients. Cancers (Basel). 2020;12:1958. doi:10.3390/cancers12071958.
- Yang SS, Yu CS, Yoon YS, Yoon SN, Lim SB, Kim JC. Symptomatic venous thromboembolism in Asian colorectal cancer surgery patients. World J Surg. 2011;35:881–7. doi:10.1007/s00268-011-0957-2.
- Petricevic M, Konosic S, Biocina B, Dirkmann D, White A, Mihaljevic MZ, Ivancan V, Konosic L, Svetina L, Görlinger K, et al. Bleeding risk assessment in patients undergoing elective cardiac surgery using ROTEM(®) platelet and Multiplate(®) impedance aggregometry. Anaesthesia. 2016;71:636–47. doi:10.1111/anae.13303.
- Curry NS, Davenport R, Pavord S, Mallett SV, Kitchen D, Klein AA, Maybury H, Collins PW, Laffan M. The use of viscoelastic haemostatic assays in the management of major bleeding: a British society for haematology guideline. Br J Haematol. 2018;182:789–806. doi:10.1111/bjh.15524.
- Görlinger K, Pérez-Ferrer A, Dirkmann D, Saner F, Maegele M, Calatayud ÁAP, Kim TY. The role of evidence-based algorithms for rotational thromboelastometry-guided bleeding management. Korean J Anesthesiol. 2019;72:297–322. doi:10.4097/kja.19169.
- Leal-Noval SR, Fernández-Pacheco J, Casado-Méndez M, Cancela P, Narros JL, Arellano-Orden V, Dusseck R, Díaz-Martín A, Muñoz-Gómez M. A prospective study on the correlation between thromboelastometry and standard laboratory tests - influence of type of surgery and perioperative sampling times. Scand J Clin Lab Invest. 2020;80:179–84. doi:10.1080/00365513.2019.1704051.
- Gomez-Builes JC, Acuna SA, Nascimento B, Madotto F, Rizoli SB. Harmful or physiologic: diagnosing fibrinolysis shutdown in a trauma cohort with rotational thromboelastometry. Anesth Analg. 2018;127:840–9. doi:10.1213/ANE.0000000000003341.
- Stettler GR, Moore EE, Moore HB, Nunns GR, Silliman CC, Banerjee A, Sauaia A. Redefining postinjury fibrinolysis phenotypes using two viscoelastic assays. J Trauma Acute Care Surg. 2019;86:679–85. doi:10.1097/TA.0000000000002165.
- Kruse JM, Magomedov A, Kurreck A, Münch FH, Koerner R, Kamhieh-Milz J, Kahl A, Gotthardt I, Piper SK, Eckardt KU, et al. Thromboembolic complications in critically ill COVID-19 patients are associated with impaired fibrinolysis. Crit Care. 2020;24:676. doi:10.1186/s13054-020-03401-8.
- Goh KY, Tsoi WC, Feng CS, Wickham N, Poon WS. Haemostatic changes during surgery for primary brain tumours. J Neurol Neurosurg Psychiatry. 1997;63:334–8. doi:10.1136/jnnp.63.3.334.
- Jansohn E, Bengzon J, Kander T, Schött U. A pilot study on the applicability of thromboelastometry in detecting brain tumour-induced hypercoagulation. Scand J Clin Lab Invest. 2017;77:289–94. doi:10.1080/00365513.2017.1306877.
- Kim OV, Nevzorova TA, Mordakhanova ER, Ponomareva AA, Andrianova IA, Le Minh G, Daminova AG, Peshkova AD, Alber MS, Vagin O, et al. Fatal dysfunction and disintegration of thrombin-stimulated platelets. Haematologica. 2019;104:1866–78. doi:10.3324/haematol.2018.202309.
- Nolte I, Przibylla H, Bostel T, Groden C, Brockmann MA. Tumor-platelet interactions: glioblastoma growth is accompanied by increasing platelet counts. Clin Neurol Neurosurg. 2008;110:339–42. doi:10.1016/j.clineuro.2007.12.008.
- Campanella R, Guarnaccia L, Cordiglieri C, Trombetta E, Caroli M, Carrabba G, La Verde N, Rampini P, Gaudino C, Costa A, et al. Tumor-educated platelets and angiogenesis in glioblastoma: another brick in the wall for novel prognostic and targetable biomarkers, changing the vision from a localized tumor to a systemic pathology. Cells. 2020;9:294. doi:10.3390/cells9020294.
- Llompart-Pou JA, Barea-Mendoza JA, Pérez-Bárcena J, Sánchez-Casado M, Ballesteros-Sanz MA, Chico-Fernández M. Survey of the neurocritical patient care in Spain. Part 1: trauma of the central nervous system. Med Intensiva (Engl Ed). 2021;45:250–2. doi:10.1016/j.medin.2019.09.001.
- Riedl J, Kaider A, Marosi C, Prager G, Eichelberger B, Koder S, Panzer S, Pabinger I, Ay C. Exhausted platelets in cancer patients with high risk of venous thromboembolism and poor prognosis. Thromb Res. 2016;140(Suppl 1):S199–200. doi:10.1016/S0049-3848(16)30196-7.
- Hoke M, Dieckmann K, Koppensteiner R, Schillinger M, Marosi C, Mlekusch W. Prognostic value of plasma d-dimer levels in patients with glioblastoma multiforme - results from a pilot study. Wien Klin Wochenschr. 2011;123:199–203. doi:10.1007/s00508-011-1556-9.
- Moreno G, Carbonell R, Bodí M, Rodríguez A. Revisión sistemática sobre la utilidad pronóstica del dímero-D, coagulación intravascular diseminada y tratamiento anticoagulante en pacientes graves con COVID-19. Med Intensiva (Engl Ed). 2021;45:42–55. doi:10.1016/j.medin.2020.06.006.
- Gabara C, Solarat B, Castro P, Fernández S, Badia JR, Toapanta D, Schulman S, Reverter JC, Soriano A, Moisés J, et al. Anticoagulation strategies and risk of bleeding events in critically ill COVID-19 patients. Med Intensiva (Engl Ed). 2021;30:S0210-5691(21)00178–9. English, Spanish. doi:10.1016/j.medin.2021.07.004.
