Abstract
Platelet transfusion refractoriness (PTR) is an intractable issue in hematological patients, which increases bleeding risks and hospitalization costs to a great extent. We reviewed 108 patients with hematological diseases including acute leukemia, myelodysplastic syndrome, aplastic anemia, and others who received allogeneic hematopoietic stem cell transplantation (HSCT) from January 2019 through December 2020. After multivariable logistic regression, we found that splenomegaly (odds ratio [OR] = 26.98, p < .001) and JAK mutation (OR = 17.32, p = .024) were independent risk factors for PTR. During the period of transplantation, patients in the PTR group had a significantly higher platelet transfusion demand, which was reflected in the increased number of platelet transfusions (10.23 ± 6.696 vs. 5.06 ± 1.904, p < .001). After multivariate adjustment, PTR turned out to be independently associated with worse overall survival (hazard ratio = 2.794, 95% confidence interval = 1.083–7.207, p = .034). In conclusion, we found that splenomegaly and JAK gene mutation were independent risk factors for PTR in patients with hematological diseases. A history of PTR prior to allo-HSCT indicates a poor prognosis.
Plain Language Summary
What is the context?
Platelet transfusion refractoriness is a critical issue, and it greatly increases bleeding risks and hospitalization costs.
Patients with hematological diseases tend to develop PTR.
PTR results from immune and nonimmune factors and the latter account for 80–90%.
At present, there are few studies focused on the inducing factors of PTR, and the specific mechanism is not clear.
What is new?
In this study, we investigated 108 patients with hematological disorders who received allogeneic HSCT from January 2019 to December 2020.
We found that splenomegaly and JAK gene mutation were independent risk factors for PTR in patients with hematological diseases.
PTR had a passive effect on the prognosis of patients after HSCT, as indicated by worse OS and a trend toward lower platelets after transplantation.
PTR might affect megakaryocyte reconstitution after transplantation.
What is the impact?
This study provides evidence that hematological patients with splenomegaly should be alert to the occurrence of PTR, which often indicates a worse prognosis of transplantation.
Spleen reduction and JAK inhibitors in the treatment of PTR are worth exploring.
Abbreviations
PLT: platelets; PTR: platelet transfusion refractoriness; HSCT: hematopoietic stem cell transplantation; OR: odds ratio; HR: hazard ratio; CI: confidence interval; IQR: interquartile range; SD: standard deviation; HLA: human leukocyte antigen; HPA: human platelet antigen; OS: overall survival; RFS: relapse free survival; PI: post-transfusion increment; PPR: percentage platelet recovery; CCI: corrected count increment; ICU: intensive care unit; AA: aplastic anemia; MDS: myelodysplastic syndrome; AML: acute myeloid leukemia; ALL: acute lymphocytic leukemia; CML: chronic myeloid leukemia; CMML: chronic myelomonocytic leukemia; MPN: myeloproliferative neoplasm; SI: splenic irradiation; Abs: antibodies; CR: complete remission; DAC: decitabine; GVHD: graft-versus-host disease; BM: bone marrow; PB: peripheral blood
Introduction
Doctors should be concerned about the occurrence of platelet transfusion refractoriness (PTR) if platelet counts fail to increase effectively in patients who have received at least two consecutive PLT (platelets) transfusions from a random donor in sufficient doses. A systematic analysis consisting of 310 studies suggested that more and more academic attention has been paid to PTR.Citation1 Currently, there are three mainstream indexes to calculate the increment of PLT transfusions: the post-transfusion increment (PI), the percentage platelet recovery (PPR), and the corrected count increment (CCI).Citation2,Citation3 The CCI is a commonly used index that takes body surface area into account and is more individual specific. Stanworth et al.Citation4 considered a CCI of >7.5 × 109/L at 1 h and >4.5 × 109/L at 20–24 h as an effective response; otherwise, PTR happened. PTR remains an intractable issue in clinical work and is commonly seen in patients with hematological diseases, especially those dependent on blood transfusion, with an overall incidence of 5–34%.Citation5–7 PTR results from immune and nonimmune factors,Citation8 with the latter accounting for 80–90%, as a consequence of platelet sequestration and consumption.Citation9 Previously, an observational study by Comont et al.Citation10 found that in adult patients with acute myeloid leukemia (AML), PTR tended to occur in parous women, patients with extra-medullary infiltration, leukopenia, infection, or hemophagocytic syndrome. A retrospective cohort conducted in the intensive care unit (ICU) found that patients admitted for hepatology/liver transplant had the highest rate of platelet refractoriness (69.6%) and larger spleen size was associated with platelet refractoriness.Citation11 Immune-mediated PTR is a minority but a non-negligible proportion, usually linked to antibodies against human leukocyte antigen (HLA) and human platelet antigen (HPA).Citation12–14 Through analysis of 533 patients in the famous TRAP study, Slichter et al. reckoned that lymphocytotoxic antibody positivity, use of heparin, fever, bleeding, increasing number of PLT transfusions, at least two pregnancies, and male gender were associated with PTR.Citation7 Zhao et al.Citation15 followed up on 55 patients with chronic myelomonocytic leukemia (CMML) after transplantation and diagnosed 14 patients with PTR after transplantation by PPR. Their analysis showed that CMML patients with PTR after allogeneic transplantation had an increased incidence of post-transplant bleeding events and decreased overall survival (OS). Tanoue et al.Citation16 retrospectively analyzed 185 patients with a single-unit cord blood transplant and found a significant effect of PTR on all-cause mortality.
Here, to better understand risk factors associated with PTR in patients diagnosed with hematological disorders and to explore the relationship between PTR and the prognosis of hematopoietic stem cell transplantation (HSCT), we presented this retrospective study.
Materials and methods
Patients enrollment
One hundred and eight patients diagnosed with hematological diseases and subsequently received allogeneic HSCT for the first time in our department during 2019–2020 were enrolled in this study. Clinical and laboratory features of eligible patients were retrospectively reviewed. Informed consent was obtained from each individual or from one’s direct relative. All protocols complied with the guidelines of the ethics committee of Soochow University and the principles expressed in the Declaration of Helsinki.
Definitions
If platelet counts did not increase effectively in patients who had received two or more consecutive transfusions of platelet from a random donor in sufficient doses, then platelet transfusion refractoriness occurred. We used CCI as the assessment index because it took individualization into account. The following equationCitation4 was used: CCI = [(post-transfusion count-pro-transfusion count) × body surface area (m2)]/(platelet dose transfused). We considered CCIs of ≤4.5 × 109/L at 20–24 h as the occurrence of PTR after two sequential transfusions, using ABO-compatible platelets.
The gold-standard definition of splenomegaly is splenic weight: the normal adult spleen weighs about 50–250 g, and this decreases with age. This is clearly only established at splenectomy or postmortem examination. Palpation is subject to a great deal of subjectivity and inaccuracy due to position and so on.Citation17 The accuracy, cost-effectiveness, and lack of radiation make abdominal ultrasonography a first-line step for confirmation of size. When attempting to evaluate the spleen and nearby organs in more detail, computed tomography is recommended.Citation18 Therefore, the diagnosis of splenomegaly was made based on imaging findings at presentation.
OS was defined from the time of transplant until death from any cause, or until the date of the last follow-up. Relapse-free survival (RFS) was defined from the time of transplant until disease relapse, or death from any cause, or until the date of the last follow-up.
Detection of antibodies and gene mutations
All included patients were preliminarily tested for antibodies against platelets using Galileo Neo Automated Platelet Antibody Screening, which in essence was a solid-phase antibody detection system. Furthermore, patients’ sera were high-throughput screened for HLA antibodies using Luminex 200 (LABScreenTM Single Antigen).
Gene mutations in the included patients were detected by DNA sequencing. The mean mutation detection sensitivity was 3%.
Classification of hemorrhage severity
There are many different scoring systems for bleeding events and severity of bleeding. We adopted the WHO grading system which has been widely used.Citation19 Bleeding events were categorized as grade 0 (no bleeding), grade 1 (mild), grade 2 (moderate; red-cell transfusion not needed immediately), grade 3 (severe; requiring red-cell transfusion within 24 h; no hemodynamic disturbance); grade 4 (debilitating or life-threatening).
Statistical analysis
Categorical variables were presented as percentages and compared using the χ2 test. When accorded to skew distribution, continuous variables are shown as medians with interquartile ranges and compared using Mann–Whitney U tests. If applied to a normal distribution, continuous variables were shown as means with standard deviations and compared using Student’s t tests. Cumulative incidence was visualized using Kaplan–Meier curves and compared using a log-rank test. Univariate and multivariate survival analyses for OS and RFS were undertaken using Cox proportional hazard models. The importance of individual variables was visualized using a forest plot. Analyses of risk factors were performed using multivariate logistic regression. P values less than 0.05 (two-tailed) were statistically significant. All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) version 26.0 (IBM) and GraphPad Prism 9.
Results
Patient characteristics
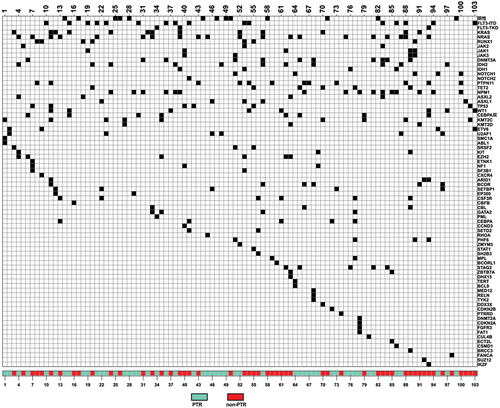
A total of 108 patients who underwent allo-HSCT at the First Affiliated Hospital of Soochow University between January 2019 and December 2020 were included in our study (one patient underwent second transplantation, but data were extracted from the primary transplant). Seventy were females (64.8%) and 38 were males (35.2%). The median age of the cohort was 38.50 years old (range,14–69). Nearly half of the patients were diagnosed with AML (55/108, 50.9%), followed by myelodysplastic syndrome (MDS) (19/108, 17.6%). Seven patients were diagnosed with aplastic anemia (AA) and 12 were classified as others, including various types of lymphoma and chronic myeloid leukemia (CML) in the accelerating phase and blast phase. During treatment, platelet transfusions were given to patients with platelet counts less than 20 × 109/L or patients with active hemorrhage. Fifty-six of those 108 patients had a history of PTR in induction/consolidation chemotherapies or in the conditioning regimens before the time of transplantation, whereas the remaining 52 patients never developed PTR during treatment. Pregnancy and ABO blood groups missed the edge of difference (p = .086, p = .068). Age, gender, diagnosis, and the PLT count onset were consistent in patients with or without PTR ().
Table I. Univariate analysis of PTR in patients.
Exploration between gene mutations and PTR
We further analyzed specific gene mutations of 103 patients (failure to achieve the results of five patients). There was no difference considering the number of mutations (The PTR group 3.25 ± 2.265 vs the non-PTR group 2.53 ± 1.689, p = .070). NRAS mutation was the most common (20/103), followed by FLT3 mutation (19/103) (). Regrettably, the distribution of mutations did not differ between the two groups. Among them, PTR might incline to manifest in patients with mutations of RUNX1 (p = .124), JAK (p = .172), DNMT (p = .128), and KMT2 (p = .118) (Table S1). Considering the genetic heterogeneity between myeloid and lymphoid lineages, we further screened patients with AML and MDS (74 in total). Through comparison (Table S2), we found that patients in the PTR group had a greater number of mutated genes (3.76 ± 2.191 vs 2.78 ± 1.669, p = .035) and the KMT2 mutation showed up on the edge of difference (p = .061).
Preliminary testing of antibody and high-throughput HLA antibody screening
When patients were initially screened for antibodies, the median value of the PTR group was 73.50 (inter-quartile range [IQR],47.50–98.75) and the median value of the non-PTR group was 40.50 (IQR,34.00–85.75). It showed a significant difference (p = .0008) ().
Figure 2. (a) Comparison of preliminary antibody screening values between the two groups. (b) Comparison of the frequency of HLA-I antibodies between the two groups. (c) Comparison of the frequency of HLA-II antibodies between the two groups. (d) Comparison of the number of patients with positive HLA-I and II antibodies between the two groups.
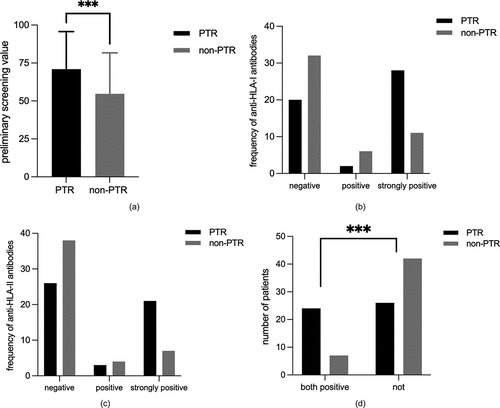
The distribution of HLA antibodies was shown in . Nine patients did not undergo this examination for financial or other unknown reasons. HLA-I antibodies were detected in 47.5% (47/99) and 39 patients tested strongly positive (p = .002, ). HLA-II antibodies were detected in 35.4% (35/99) and 28 patients tested strongly positive (p = .008, ). Notably, PTR occurred predominantly in patients with both positive antibodies against HLA-I and HLA-II (48.0% vs 14.3%, p = .0004, ). Also, 40.0% of patients in the PTR group had no antibodies against HLA compared to 57.14% in the non-PTR group (p = .1091), indicating the presence of other antibodies against platelets such as HPAs might not cause PTR.
Table II. The distribution of antibodies in patients.
Identification of risk factors for PTR
To further elucidate risk factors for PTR, we included splenomegaly, pregnancy, blood group, mutations of RUNX, JAK, DNMT, KMT2, anti-HLA-I Abs, anti-HLA-II Abs, and the benign or malignant nature of diseases into multivariable logistic regression (). It revealed that splenomegaly (odds ratio [OR] = 26.98, p < .001) and JAK (OR = 17.32, p = .024) mutation were independent risk factors for PTR.
Table III. Multivariate logistic analysis.
Bleeding events
Patients with extremely low platelet counts do have more or less mucocutaneous bleeding. Hence, we reported five patients with major bleeding events of grade 3 or higher,Citation19 all of whom were in the PTR group (9.6%), involving alimentary tract (n = 1), menometrorrhagia (n = 1), hematuria (n = 1), hemoptysis (n = 1), and diffuse alveolar hemorrhage (n = 1).
Adverse effect of PTR on prognosis
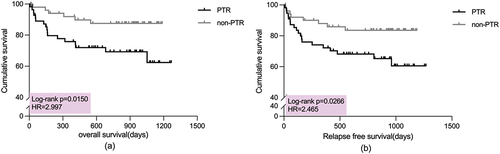
At follow-up, we found that the OS of patients with PTR was significantly worse than that of patients in the non-PTR group (hazard ratio [HR] = 2.997,95% confidence interval [CI] = 1.323–6.790, p = .0150) (). Seven patients in the PTR group experienced relapse of the disease after transplantation, while six in the non-PTR group. As with OS, patients with PTR had a markedly lower RFS than those without PTR (HR = 2.465,95%CI = 1.159–5.242, p = .0266) ().
Figure 3. (a) Kaplan–Meier curves for overall survival in the two groups. (b) Kaplan–Meier curves for relapse-free survival in the two groups.
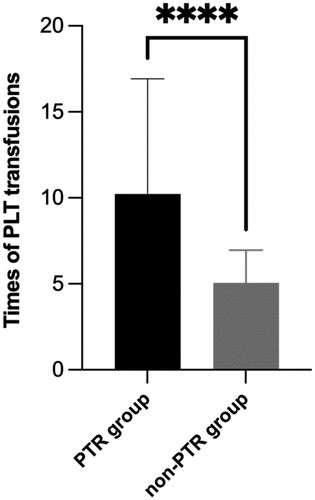
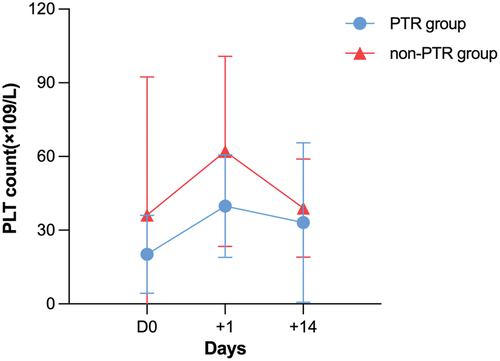
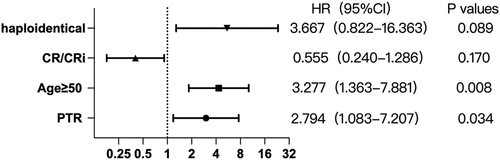
During transplantation, patients in the PTR group required observably more times of PLT transfusions (10.23 ± 6.696 vs 5.06 ± 1.904, p < .001) (). On the day of reinfusion of stem cells, the PLT count (×109/L) of patients in the PTR group was lower than that of patients in non-PTR group (18.5 vs 24.0, p = .016). Taking other transplant conditions such as donor, hematological diseases, etc. into consideration, PTR turned out to obviously affect the PLT level on the reinfusion day (Table S3). Similarly, the mean platelet levels in the PTR group were significantly different from those in the non-PTR group on the first day and 14 days after transplantation (p = .001, p = .015). The trend of platelet levels after transplantation in the PTR group was higher than that in the non-PTR group (). We performed a univariate analysis of risk factors affecting OS and RFS using COX regression (Table S4). After multivariate adjustment, PTR (HR = 2.794, 95%CI = 1.083–7.207, p = .034) and age ≥50 (HR = 3.277, 95%CI = 1.363–7.881, p = .008) turned out to be passively associated with OS ().
Discussion
In this paper, we included patients with different hematological disorders to explore the possible factors of PTR, not limited to a single disease. After a multivariate logistic analysis of potential risks, we confirmed that splenomegaly (OR = 26.98, p < .001) and JAK mutation (OR = 17.32, p = .024) were independent risk factors for PTR.
Aster et al.Citation20 estimated that 30% of total platelet mass resided in the spleen of normal subjects at any time and 50% to 90% of platelet mass in patients with expanded size. The spleen influences post-transfusion platelet counts via sequestration, which was considered reversible. In the rat sepsis model induced by lipopolysaccharide injected, Li et al.Citation21 observed that platelet aggregated in the splenic marginal zone in sepsis which could interact with infused platelets and thus could contribute to platelet infusion refractoriness in sepsis. This factor of splenomegaly warrants further investigation. Theoretically, splenomegaly and hypersplenism cannot be completely equated, and spleen enlargement in some patients may be physiologic. There is a distinction between primary and secondary hypersplenism. Primary hypersplenism is relatively rare, and most of them are congenital diseases. The main cause of secondary hypersplenism is splenic congestion, the most common one is liver cirrhosis and portal hypertension, in addition to splenic vein obstruction, Budd-Chiari syndrome, congestive heart failure, etc. Other infectious diseases, such as infectious mononucleosis, tuberculosis, etc.; immune diseases, such as systemic lupus erythematosus, Felty syndrome, etc.; metabolic diseases, such as Gaucher disease, hyperlipidemia, etc. can also cause hypersplenism. In terms of the blood system, hemolytic anemia such as autoimmune hemolytic anemia, thalassemia, and sickle cell anemia can lead to hypersplenism, and infiltrating splenomegaly, such as all kinds of acute and chronic leukemia, lymphoma, myeloproliferative neoplasms (MPNs), malignant histiocytosis, and amyloidosis, should be considered. Is there a difference in PTR between primary and secondary hypersplenism? Could the relevance of the factor splenomegaly be affected by the splenic infiltration of leukemic cells? In this study, we only explored the relationship between splenomegaly and PTR. Due to the lack of data, whether the degree of splenomegaly has different levels of influence on PTR deserves further exploration.
Among hematological diseases, splenomegaly has been closely connected to MPNs, such as polycythemia vera, essential thrombocythemia, or primary myelofibrosis. Notably, splenomegaly is a hallmark of myelofibrosis. Considerable splenic enlargement might be associated with significantly worse outcomes after HCT as compared with patients without enlarged splenomegaly.Citation22 There were studies that revealed that selective prophylactic splenectomy excluding relevant contraindications might reduce platelet destruction in the spleen, thereby improving PTR.Citation23,Citation24 A meta-analysis selected 27 articles including 766 courses of splenic irradiation (SI) for 486 patients from 1960 to 2016 and concluded that though 30% of patients were retreated within 6–12 months, SI is generally a safe and efficacious method for treating patients with symptomatic splenomegaly.Citation25 At present, there is a paucity of literature on the treatment of PTR with splenic irradiation. Irradiation can cause cytotoxicity and cytopenia. It may be worth considering whether PTR patients with platelets level in the recovery stage can instead induce PTR after irradiation.
Besides, frontline treatment for patients with MPNs with splenomegaly and JAK mutations are JAK inhibitors, and pacritinib has been approved by the Food and Drug Administration for patients with a platelet count of less than 50 × 109/L. Reductions in splenomegaly in patients with myelofibrosis can frequently be seen within the first few months, and the median duration of spleen response with ruxolitinib is approximately 3 years.Citation22 However, we did not include patients with typical MPNs, such as polycythemia vera, essential thrombocythemia, or primary myelofibrosis. Although we found JAK mutation was independently related to PTR, we did not make further distinctions between JAK1, JAK2, and JAK3 separately given the limited number. The association between JAK2 mutation and splenomegaly was also not evident in the patients included in this study. It is not clear whether JAK inhibitors can be used to shrink the spleen or treat PTR in patients with hematological diseases other than MPNs. It is also unknown how much refractoriness can be improved after spleen reduction therapy in patients with splenomegaly because there are many factors leading to PTR. How JAK mutations in patients with hematological malignancies affect the molecular pathways leading to PTR is still unknown. In future studies, we will expand the sample size and focus more on exploring the molecular etiology of PTR.
Through survival analysis, we found that the OS of patients in the PTR group was significantly worse than that in the non-PTR group. After multivariate adjustment, PTR turned out to be independently associated with worse OS (HR = 2.794, 95%CI = 1.083–7.207, p = .034). Though thrombopoietin receptor agonist therapy was initiated in all the patients within 5 days after hematopoietic stem-cell infusion, platelet counts were significantly lower in the PTR group. Of the 108 patients, five had adverse bleeding events, all of whom were in the PTR group, indicating PTR was predominantly relevant to increased bleeding risk. The decline in OS in the PTR group might be due to bleeding-related deaths and was not related to relapse. Besides, we considered that the difference in RFS between the two groups might be caused by the small sample size.
In conclusion, our study found that splenomegaly and JAK gene mutation were independent risk factors for PTR in patients with hematological diseases. Spleen reduction and JAK inhibitors in the treatment of PTR are worth exploring. In addition, PTR had a passive effect on the prognosis of patients after HSCT, as indicated by worse OS and a trend toward lower platelets after transplantation. As an intractable issue in hematological patients, PTR is still in urgent need of further research on its pathogenesis and resolution strategies.
Author contributions
XS, JQ, XL, MZ, JH: enrollment of patients, acquisition, analysis, and interpretation of data. YH, TC: design of study, acquisition of funding, supervision of the study and revision of the manuscript. XS, JQ, XL wrote the paper. All authors contributed to this article and approved the submitted version.
Ethics statement
This study was reviewed and approved by Ethics Committee of the First Affiliated Hospital of Soochow University. Informed consents were written by participants of their own accord.
Supplemental Material
Download PDF (326 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
If requested, the original data presented in this paper will be available through the corresponding author [email protected].
Supplementary data
Supplemental data for this article can be accessed online at https://doi.org/10.1080/09537104.2023.2229905.
Additional information
Funding
References
- Liu Y, Zhang Y, Chen D, Fu Y. Current status of and global trends in platelet transfusion refractoriness from 2004 to 2021: a bibliometric analysis. Front Med. 2022;9:873500. doi:10.3389/fmed.2022.873500. Epub 2022/05/24.
- Guidelines for the use of platelet transfusions. Br J Haematol. 2003;122(1):10–8. doi:10.1046/j.1365-2141.2003.04468.x. Epub 2003/06/26.
- Rebulla P. Formulae for the definition of refractoriness to platelet transfusion. Transfus Med. 1993;3:91–3. doi:10.1111/j.1365-3148.1993.tb00108.x. Epub 1993/03/01.
- Stanworth SJ, Navarrete C, Estcourt L, Marsh J. Platelet refractoriness–practical approaches and ongoing dilemmas in patient management. Br J Haematol. 2015;171:297–305. doi:10.1111/bjh.13597. Epub 2015/07/22.
- Forest SK, Hod EA. Management of the platelet refractory patient. Hematol Oncol Clin North Am. 2016;30(3):665–77. doi:10.1016/j.hoc.2016.01.008. Epub 2016/04/27.
- Saris A, Pavenski K. Human leukocyte antigen alloimmunization and alloimmune platelet refractoriness. Transfus Med Rev. 2020;34(4):250–7. doi:10.1016/j.tmrv.2020.09.010. Epub 2020/11/01.
- Slichter SJ, Davis K, Enright H, Braine H, Gernsheimer T, Kao KJ, Kickler T, Lee E, McFarland J, McCullough J, et al. Factors affecting posttransfusion platelet increments, platelet refractoriness, and platelet transfusion intervals in thrombocytopenic patients. Blood. 2005;105:4106–14. doi:10.1182/blood-2003-08-2724. Epub 2005/02/05.
- Hod E, Schwartz J. Platelet transfusion refractoriness. Br J Haematol. 2008;142(3):348–60. doi:10.1111/j.1365-2141.2008.07189.x. Epub 2008/05/31.
- Belizaire R, Makar RS. Non-alloimmune mechanisms of thrombocytopenia and refractoriness to platelet transfusion. Transfus Med Rev. 2020;34(4):242–9. doi:10.1016/j.tmrv.2020.09.002. Epub 2020/11/02.
- Comont T, Tavitian S, Bardiaux L, Fort M, Debiol B, Morère D, Bérard E, Delabesse E, Luquet I, Martinez S, et al. Platelet transfusion refractoriness in patients with acute myeloid leukemia treated by intensive chemotherapy. Leuk Res. 2017;61:62–7. doi:10.1016/j.leukres.2017.08.015. Epub 2017/09/15.
- Arabi S, Almahayni AO, Alomair AA, Masuadi EM, Damlaj M, Al-Dorzi HM. Prevalence, risk factors, and outcomes of platelet transfusion refractoriness in critically ill patients: a retrospective cohort study. Crit Care Res Pract. 2021;2021:1–11. doi:10.1155/2021/5589768. Epub 2021/10/05.
- Blandin L, Dougé A, Fayard A, Bay JO, Berlie G, Pereira B, Lemal R, Rouzaire P. Platelet transfusion refractoriness and anti-HLA immunization. Transfusion. 2021;61(6):1700–4. doi:10.1111/trf.16358. Epub 2021/03/13.
- Juskewitch JE, Norgan AP, De Goey SR, Duellman PM, Wakefield LL, Gandhi MJ, Stubbs JR, Kreuter JD. How do I … manage the platelet transfusion-refractory patient? Transfusion. 2017;57:2828–35. doi:10.1111/trf.14316. Epub 2017/09/30.
- Hayashi T, Hirayama F. Advances in alloimmune thrombocytopenia: perspectives on current concepts of human platelet antigens, antibody detection strategies, and genotyping. Blood Transfus. 2015;13:380–90. doi:10.2450/2015.0275-14. Epub 2015/06/10.
- Zhao C, Zhao XS, Wang Y, Yan CH, Xu LP, Zhang XH, Liu KY, Huang XJ, Sun YQ. Incidence and clinical significance of platelet transfusion refractoriness after allogeneic hematopoietic stem cell transplantation in patients with chronic myelomonocytic leukemia. Zhonghua Xue Ye Xue Za Zhi. 2022;43:738–44. Epub 2023/01/29.
- Tanoue S, Konuma T, Kato S, Oiwa-Monna M, Isobe M, Jimbo K, Takahashi S, Tojo A. Platelet transfusion refractoriness in single-unit cord blood transplantation for adults: risk factors and clinical outcomes. Biol Blood Marrow Transplant. 2018;24(9):1873–80. doi:10.1016/j.bbmt.2018.05.006. Epub 2018/05/14.
- Pozo AL, Godfrey EM, Bowles KM. Splenomegaly: investigation, diagnosis and management. Blood Rev. 2009;23(3):105–11. doi:10.1016/j.blre.2008.10.001. Epub 2008/12/09.
- Aldulaimi S, Mendez AM. Splenomegaly: diagnosis and management in adults. Am Fam Physician. 2021;104:271–6. Epub 2021/09/16.
- Stanworth SJ, Estcourt LJ, Powter G, Kahan BC, Dyer C, Choo L, Bakrania L, Llewelyn C, Littlewood T, Soutar R, et al. A no-prophylaxis platelet-transfusion strategy for hematologic cancers. N Engl J Med. 2013;368(19):1771–80. doi:10.1056/NEJMoa1212772. Epub 2013/05/10.
- Aster RH. Pooling of platelets in the spleen: role in the pathogenesis of “hypersplenic” thrombocytopenia. J Clin Invest. 1966;45(5):645–57. doi:10.1172/JCI105380. Epub 1966/05/01.
- Li Y, Ryan J, Xu F, Vostal JG. Macrophage depletion mitigates platelet aggregate formation in splenic marginal zone and alleviates LPS-Associated Thrombocytopenia in Rats. Front Med. 2019;6:300. doi:10.3389/fmed.2019.00300. Epub 2020/01/11.
- Polverelli N, Hernández-Boluda JC, Czerw T, Barbui T, D’Adda M, Deeg HJ, Ditschkowski M, Harrison C, Kröger NM, Mesa R, et al. Splenomegaly in patients with primary or secondary myelofibrosis who are candidates for allogeneic hematopoietic cell transplantation: a position paper on behalf of the chronic malignancies working party of the EBMT. Lancet Haematol. 2023;10:e59–e70. doi:10.1016/S2352-3026(22)00330-1. Epub 2022/12/10.
- Mauro M, Camoglio F, Piccoli P, De Bortoli M, Balter R, Pegoraro A, Cesaro S. The use of splenectomy to manage platelet transfusion refractoriness due to anti-human leukocyte antibodies in allogeneic stem cell transplantation. Pediatr Rep. 2016;8(1):6159. doi:10.4081/pr.2016.6159. Epub 2016/04/27.
- Machado NO, Grant CS, Alkindi S, Daar S, Al-Kindy N, Al Lamki Z, Ganguly SS. Splenectomy for haematological disorders: a single center study in 150 patients from Oman. Int J Surg. 2009;7(5):476–81. doi:10.1016/j.ijsu.2009.08.004. Epub 2009/08/22.
- Zaorsky NG, Williams GR, Barta SK, Esnaola NF, Kropf PL, Hayes SB, Meyer JE. Splenic irradiation for splenomegaly: a systematic review. Cancer Treat Rev. 2017;53:47–52. doi:10.1016/j.ctrv.2016.11.016. Epub 2017/01/08.