Abstract
Predicting the clinical course and allocating limited medical resources appropriately is crucial during the COVID-19 pandemic. Platelets are involved in microthrombosis, a critical pathogenesis of COVID-19; however, the role of soluble CLEC-2 (sCLEC-2), a novel platelet activation marker, in predicting the prognosis of COVID-19 remains unexplored. We enrolled 108 patients with COVID-19, hospitalized between January 2021 and May 2022, to evaluate the clinical use of sCLEC-2 as a predictive marker. sCLEC-2 levels were measured in plasma sampled on admission, as well as interleukin-6, cell-free DNA, von Willebrand factor, and thrombomodulin. We retrospectively classified the patients into two groups - those who required oxygenation during hospitalization (oxygenated group) and those who did not (unoxygenated group) - and compared their clinical and laboratory characteristics. The correlation between sCLEC-2 and the other parameters was validated. The sCLEC-2 level was significantly higher in the oxygenated group (188.8 pg/mL vs. 296.1 pg/mL). Multivariate analysis identified high sCLEC-2 levels (odds ratio per 10 pg/mL:1.25) as an independent predictor of oxygen therapy requirement. sCLEC-2 was positively correlated with cell-free DNA, supporting the association between platelet activation and neutrophil extracellular traps. In conclusion, sCLEC-2 is a clinically valuable marker in predicting oxygen therapy requirements for patients with COVID-19.
Plain Language Summary
What is the context?
During the COVID-19 epidemic with tremendous damage to healthcare systems worldwide, predicting the clinical course of patients and allocating limited medical resources appropriately is crucial.
Platelets are involved in microthrombosis - a critical pathogenesis of COVID-19. The role of soluble CLEC-2 (sCLEC-2), a novel in vivo platelet activation marker, in predicting the prognosis of COVID-19 remains unexplored.
What is new?
sCLEC-2 is an independent predictive marker of oxygen therapy requirement in COVID-19.
What is the impact?
In most cases, patients requiring oxygen therapy must be hospitalized. The ability to predict such cases during the COVID-19 epidemic, when medical recourses are depleted, may contribute to the appropriate allocation of medical resources.
Introduction
Since its first report in China in December 2019, the novel coronavirus disease (COVID-19) has rapidly spread globally, causing enormous difficulties in healthcare systems worldwide. The overload of healthcare systems has resulted in increased mortality, not only due to COVID-19Citation1 but also other diseases, such as cardiovascular diseases.Citation2 The clinical course and outcome of COVID-19 vary from asymptomatic to fatal.Citation3 Consequently, the treatment required varies from no treatment to intensive care. With limited medical resources, correctly identifying patients whose therapy should be prioritized is crucial.
Microthrombosis is a critical complication of severe COVID-19.Citation4 Therefore, identifying this pathological condition may help prioritize the most vulnerable individuals and appropriate allocation of medical resources. However, the mechanism of thrombosis in COVID-19 remains unclear; nonetheless, vascular endothelial cell dysfunction, inflammation, and hypercoagulability have been proposed as the three major pathways leading to thrombosis. Platelets are also involved in these pathwaysCitation5 and are reportedly activated in patients with COVID-19.Citation6
C-type lectin-like receptor-2 (CLEC-2) has been identified as a receptor for snake venom rhodocytin, which activates platelets.Citation7 Soluble CLEC-2 (sCLEC-2) is released upon platelet activation either as a free 25 kDa fragment or as a whole molecule bound to platelet microparticles, suggesting that it may serve as a novel biomarker for platelet activation.Citation8 Plasma sCLEC-2 is reportedly elevated in thrombotic microangiopathy and disseminated intravascular coagulation syndrome,Citation9 which are diseases associated with microthrombosis.
In this study, we measured sCLEC-2 levels in 108 patients with COVID-19 and compared them between patients who required oxygen administration and those who did not. Additionally, we validated the correlation between sCLEC-2 and other parameters. Collectively, we aimed to elucidate the role of sCLEC-2 as a biomarker for identifying patients who require immediate critical care.
Materials and methods
Study design and participants
This was a single-center, retrospective study. The study included patients with COVID-19 that were admitted to the University of Yamanashi Hospital between January 2021 and May 2022 (n = 108). COVID-19 was diagnosed based on a reverse transcription-polymerase chain reaction test for SARS-CoV-2.Citation10
Clinical information and laboratory tests
Clinical information, such as age, sex, comorbidities, medications, duration of hospitalization, outcomes, and oxygen requirement, was collected from medical records. We retrospectively classified 108 patients into two groups: those who required oxygenation at least once during the entire course of treatment (oxygenated group, n = 51) and those who remained in a mild disease state without oxygenation (unoxygenated group, n = 57). Blood tests were performed on all patients upon admission, as per routine medical procedures. Routine blood test results were collected from medical records. If the data were below or above the measurement limit, the value of the limit was adopted.
Measurement of sCLEC-2, interleukin-6 (IL-6), cell-free DNA (cfDNA), von Willebrand Factor (VWF) activity, thrombomodulin (TM), plasminogen activator inhibitor-1 (PAI-1), and soluble P-selectin (sP-sel)
After completing the requested clinical laboratory tests, the remaining plasma samples (with 3.2% citrate) were collected and stored at − 80°C. sCLEC-2 was measured using the chemiluminescent enzyme immunoassay (CLEIA) system on STACIA ® (LSI Medience, Tokyo, Japan), as previously described.Citation11 Briefly, the magnetic particles were coated with the anti-CLEC-2 monoclonal antibody 11D5. Plasma was then incubated with antibody-coated magnetic particles. After washing, the particles were further incubated with alkaline phosphatase-conjugated anti-CLEC-2 monoclonal antibody 11E6. After washing again, the magnetic particles were incubated with a chemiluminescent substrate solution (CDP-Star; Applied BioSystems, Thermo Fisher Scientific, Waltham, MA, USA), and luminescence was measured using a luminometer installed in STACIA®. IL-6 was measured using the CLEIA method installed in LUMIPULSE® G1200 (Fujirebio, Tokyo, Japan). cfDNA was measured using Sytox Green (Thermo Fisher Scientific), a DNA-binding dye. Briefly, 2 µM Sytox Green and plasma were mixed at a 5:3 ratio in a microplate. After incubation at 37°C for 1 h, the plate was scanned using a SpectraMAX GeminiEM fluorescent microplate reader (Molecular Devices, San Jose, CA, USA). LSI Medience measured VWF activity, TM, and PAI-1 using the ristocetin cofactor assay, the CLEIA method, and latex agglutination method respectively. sP-sel was measured by ELISA (Proteintech, Rosemont, IL, USA).
Statistical analysis
Statistical analyses were performed using JMP Pro 16 (SAS Institute, Cary, NC, USA). Categorical data are shown as numbers (%) and were analyzed using Fisher’s exact test. Age, duration of hospitalization, and the results of laboratory tests are presented as a median (range) and were analyzed using Wilcoxon’s rank-sum test. Univariate and multivariate logistic models were used to explore the risk factors associated with oxygen requirement. Considering the sample size in our study and to avoid overfitting in the model, we chose five variables that predicted the severity of illness in meta-analyses.Citation12,Citation13 These five variables included lymphocytes, C-reactive protein (CRP), D-dimer, lactate dehydrogenase (LDH), and glycated hemoglobin (HbA1c), which were widely measured in this study and were less confounded by each other. Therefore, a logistic regression analysis was performed for these five variables and sCLEC-2. Spearman’s correlation coefficient was calculated to evaluate the relationship between sCLEC-2 and other parameters. P-values <0.05 were considered significant.
Study approval
This study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Yamanashi University (approval number 2404). Informed consent was obtained from the patients through an opt-out process on the institutional webpage.
Results
Patient characteristics, clinical course, and outcomes
Patient characteristics, clinical course, and outcomes are listed in . Patients in the oxygenated group were significantly older (median age: unoxygenated group, 46 vs. oxygenated group, 60; P = .0451). No sex differences were observed between the two groups. The oxygenated group comprised more patients classified with diabetes; however, the difference compared to the unoxygenated group was not significant. Both groups included patients who were originally on antiplatelet or anticoagulant medications; however, the proportion of these patients was not significantly different. The number of intensive care unit admissions, macrothrombosis, and deaths during the clinical course was limited.
Table I. Patient characteristics, clinical course, and outcomes.
Laboratory findings
displays the laboratory findings on admission. Markers suggesting inflammation, such as CRP (median in unoxygenated group 0.8 µg/mL vs. in oxygenated group 4.51 µg/mL), fibrinogen (359.5 mg/dL vs. 492 mg/dL), and IL-6 (6.5 pg/mL vs. 21.1 pg/mL), as well as markers suggesting cell injury, such as LDH (208.5 U/L vs. 331 U/L), aspartate aminotransferase (24 U/L vs. 41 U/L), and alanine aminotransferase (18 U/L vs. 29 U/L), were significantly higher in the oxygenated group. Conversely, total protein (7.15 g/dL vs. 6.6 g/dL), albumin (4 g/dL vs. 3.4 g/dL), and cholinesterase (292 U/L vs. 259 U/L) levels were lower in the oxygenated group, suggesting that poor nutritional status was associated with an unfavorable clinical course. Moreover, sCLEC-2 levels were significantly higher in the oxygenated group (188.8 pg/mL vs. 296.1 pg/mL; P-value <.0001). Similarly, VWF (170% vs. 198%) and cfDNA (179.5 ng/mL vs. 254 ng/mL) levels were higher in the oxygenated group. These results may support previous findings that vascular endothelial damage and neutrophil extracellular traps (NETs) occur in severe COVID-19.Citation5
Table II. Blood test findings on admission.
Univariate and multivariate logistic regression analysis for oxygen requirement
To investigate the usefulness of sCLEC-2 as a prognostic predictor, logistic regression analysis was performed on gathered sCLEC-2 levels and five known variables associated with the adverse clinical course or outcome of COVID-19 (). Multivariate analysis revealed that low lymphocyte counts (odds ratio per 100/µL: 0.79, 95% CI: 0.63–0.98) and high sCLEC-2 levels (odds ratio per 10 pg/mL: 1.25, 95% CI: 1.05–1.49) were independent predictors of oxygen requirement during the course of the disease.
Table III. Predictive factors for the requirement of oxygen administration, according to univariate and multivariate logistic regression analysis.
Correlations between sCLEC-2 and other parameters
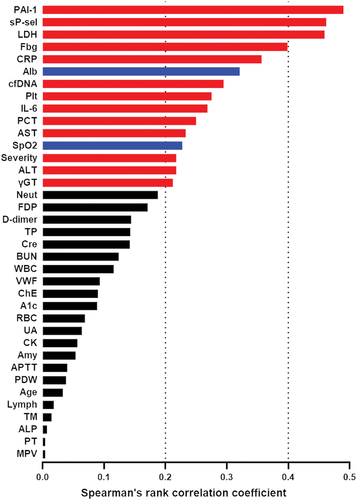
shows the correlation between sCLEC-2 and other parameters. sCLEC-2 had a relatively strong positive correlation with PAI-1 (ρ = 0.4901, P < .0001), sP-sel (ρ = 0.4616, P < .0001), and LDH (ρ = 0.4593, P < .0001). Notably, a positive correlation (ρ = 0.2948, P = .002) was observed between sCLEC-2 and cfDNA, a NET marker, suggesting a close relationship between platelet activation and NETs.
Figure 1. Correlations of sCLEC-2 with other clinical and laboratory parameters.
Discussion
Before the identification of platelet CLEC-2, platelet aggregation was known to be induced by two classes of receptors, G protein-coupled receptors and the immunoglobulin superfamily, including the GPVI/FcRγ-chain complex and FcγRIIA. CLEC-2 was identified as a new class of tyrosine kinase-dependent platelet activation receptor that belongs to the C-type lectin family. It induces platelet activation signals via the Src family kinase, as well as GPVI/FcRγ, and FcγRIIA. Unlike GPVI/FcRγ, which triggers platelet activation via the tandem YxxL motif immunoreceptor tyrosine-based activation motif, CLEC-2 initiates signaling via the single YxxL motif.Citation14 Platelet CLEC-2 plays various physiological and pathological roles by binding to its endogenous ligand, podoplanin.Citation15,Citation16 Recently, the clinical utility of CLEC-2 as a biomarker has gained interest.Citation8,Citation9
This study examined the usefulness of sCLEC-2 as a predictive marker in patients with COVID-19. Wada et al. reported that plasma sCLEC-2 levels were significantly higher in patients with COVID-19 than in those with non-COVID-19 infections. Moreover, plasma sCLEC-2 levels were significantly higher in patients with more severe COVID-19.Citation17 In the present study, we observed that sCLEC-2 additionally serves as a predictive biomarker for oxygen therapy requirements. Predicting the need for oxygenation is crucial to ensure optimal treatment for each patient and the appropriate allocation of medical resources, especially during the pandemic. Several attempts have been made to combine multiple factors to predict oxygen demand and hospitalization in patients with COVID-19.Citation18,Citation19 We addressed these concerns by establishing sCLEC-2 as a novel predictive marker for oxygen demand in patients with COVID-19.
Autopsy findings of patients with COVID-19 provide valuable insights into the relationship between platelet activation and hypoxemia. A systematic review of lung histopathology in COVID-19 revealed that 57% of the patients had microthrombi.Citation20 The increased dead space in the lungs caused by such microthrombi likely leads to hypoxemia in COVID-19.Citation21 A higher level of sCLEC-2 might predict hypoxemia by detecting the early stages of pulmonary microthrombosis; however, further investigation is required to confirm this notion. Additionally, autopsies of patients with COVID-19 revealed inflammatory microvascular thrombi containing neutrophil extracellular traps bound to platelets and fibrin in the lungs.Citation22 Although the underlying molecular mechanisms remain debatable, activated platelets are known to trigger NETs.Citation23 Conversely, histones are released extracellularly as a result of NETs and induce platelet aggregation via TLR2 and TLR4.Citation24 The positive correlation between sCLEC-2 and cfDNA might reflect a vicious process that occurs in patients with COVID-19, in which platelet activation induces NETs, further inducing platelet activation.
In our study, in contrast to sCLEC-2, sP-sel, one of the well-studied platelet activation markers, showed no difference between the two groups (). This discrepancy may be due to differences in the release mechanisms of the two molecules. The release mechanism of CLEC-2 is not fully understood.Citation25 However, CLEC-2 is a protein that is constantly expressed on the cell surface, whereas P-selectin is a membrane protein of α-granules,Citation26 indicating that the release mechanism would also be different. Moreover, the clinical utility of molecules released by different mechanisms might vary. We have previously reported that sCLEC-2 is superior in distinguishing acute coronary syndrome (ACS) from stable angina pectoris (SAP) and normal coronary arteries (NCA), whereas sGPVI is superior in distinguishing NCA from ACS and SAP.Citation25
This study had some limitations. First, there may have been a selection bias because the participants were inpatients at a single academic medical center. Moreover, owing to the rapidly changing circumstances of the epidemic, the criteria for hospitalization varied widely over time. Second, cases of macrothrombosis were limited. As sCLEC-2 is a platelet activation marker, it could potentially predict the development of macrothrombosis; however, this could not be validated due to the small sample size. This limitation may be overcome by increasing the sample size or by repeatedly measuring sCLEC-2 levels during the clinical course. Third, the relationship between platelet activation and NETs requires careful interpretation because cfDNA is a relatively less specific marker of NETs. This mechanism requires comprehensive validation with more specific NETs markers, such as citrullinated histone H3 or myeloperoxidase-DNA complexes.Citation27
In conclusion, sCLEC-2 is a potential predictive biomarker for the need for oxygenation during COVID-19 and may assist with the appropriate allocation of limited medical resources.
Author contributions
S. Oishi designed the study and analyzed and interpreted the data; S. Oishi, M. Ueda, H. Yamazaki, N. Tsukiji, T. Shirai, Y. Naito, M. Endo, R. Yokomori, and T. Sasaki performed the experiments. S. Oishi and K. Suzuki-Inoue assembled the figures and wrote the manuscript.
Acknowledgments
We thank Takako Otsuka, University of Yamanashi, for her technical assistance and Kyotaro Ozawa, a student at the University of Yamanashi, for data preparation.
Disclosure statement
K.S.-I. has received research grant funding from LSI Medience Corporation. The other authors report no conflicts of interest.
Additional information
Funding
References
- Rubino S, Kelvin N, Bermejo-Martin JF, Kelvin DJ. As COVID-19 cases, deaths and fatality rates surge in Italy, underlying causes require investigation. J Infect Dev Ctries. 2020;14(3):265–6. doi:10.3855/jidc.12734.
- Banerjee A, Chen S, Pasea L, Lai AG, Katsoulis M, Denaxas S, Nafilyan V, Williams B, Wong WK, Bakhai A, et al. Excess deaths in people with cardiovascular diseases during the COVID-19 pandemic. Eur J Prev Cardiol. 2021;28(14):1599–609. doi:10.1093/eurjpc/zwaa155.
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–62. doi:10.1016/S0140-6736(20)30566-3.
- Fahmy OH, Daas FM, Salunkhe V, Petrey JL, Cosar EF, Ramirez J, Akca O. Is microthrombosis the main pathology in coronavirus disease 2019 severity?—A systematic review of the postmortem pathologic findings. Crit Care Explor. 2021;3(5):e0427. doi:10.1097/cce.0000000000000427.
- Conway EM, Mackman N, Warren RQ, Wolberg AS, Mosnier LO, Campbell RA, Gralinski LE, Rondina MT, van de Veerdonk FL, Hoffmeister KM, et al. Understanding COVID-19-associated coagulopathy. Nat Rev Immunol. 2022;22(October):639–49. doi:10.1038/s41577-022-00762-9.
- Zaid Y, Puhm F, Allaeys I, Naya A, Oudghiri M, Khalki L, Limami Y, Zaid N, Sadki K, Ben El Haj R, et al. Platelets can associate with SARS-CoV-2 RNA and are hyperactivated in COVID-19. Circ Res. 2020;1404–1418. doi:10.1161/CIRCRESAHA.120.317703.
- Suzuki-Inoue K, Fuller GLJ, García Á, Eble JA, Pöhlmann S, Inoue O, Gartner TK, Hughan SC, Pearce AC, Laing GD, et al. Anovel Syk-dependent mechanism of platelet activation by the C-type lectin receptor CLEC-2. Blood. 2006;107(2):542–9. doi: 10.1182/blood-2005-05-1994.
- Kazama F, Nakamura J, Osada M, Inoue O, Oosawa M, Tamura S, Tsukiji N, Aida K, Kawaguchi A, Takizawa S, et al. Measurement of soluble C-type lectin-like receptor 2 in human plasma. Platelets. 2015;26(8):711–9. doi:10.3109/09537104.2015.1021319.
- Yamashita Y, Suzuki K, Mastumoto T, Ikejiri M, Ohishi K, Katayama N, Suzuki-Inoue K, Wada H. Elevated plasma levels of soluble C-type lectin-like receptor 2 (CLEC2) in patients with thrombotic microangiopathy. Thromb Res. 2019;178(November 2018):54–8. doi:10.1016/j.thromres.2019.03.018.
- Sasaki T, Inoue O, Ogihara S, Kubokawa K, Oishi S, Shirai T, Iwabuchi K, Suzuki-Inoue K. Detection of SARS-CoV-2 RNA using RT-Qpcr in nasopharyngeal swab, saliva, lingual, and buccal mucosal swab. Jpn J Infect Dis. 2021;75(1):102–4. doi:10.7883/yoken.JJID.2021.091.
- Yamamoto A, Wada H, Ichkawa Y, Tanaka M, Tashiro H, Shiraki K, Shimpo H, Yamashita Y, Mastumoto T, Shimaoka M, et al. Soluble C-type lectin-like receptor 2 is a biomarker for disseminated intravascular coagulation. J Clin Med. 2021;10(13):2860. doi: 10.3390/jcm10132860.
- Malik P, Patel U, Mehta D, Patel N, Kelkar R, Akrmah M, Gabrilove JL, Sacks H. Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ Evidence-Based Med. 2021;26(3):107–8. doi:10.1136/bmjebm-2020-111536.
- Prattichizzo F, de Candia P, Nicolucci A, Ceriello A. Elevated HbA1c levels in pre-Covid-19 infection increases the risk of mortality: A systematic review and meta-analysis. Diabetes Metab Res Rev. 2022;38(1):1–8. doi:10.1002/dmrr.3476.
- Suzuki‐Inoue K, Inoue O, Ozaki Y. Novel platelet activation receptor CLEC‐2: from discovery to prospects. J Thromb Haemost . 2011;9:44–55. doi:10.1111/j.1538-7836.2011.04335.x.
- Suzuki-Inoue K, Inoue O, Ding G, Nishimura S, Hokamura K, Eto K, Kashiwagi H, Tomiyama Y, Yatomi Y, Umemura K, et al. Essential in vivo roles of the C-type lectin receptor CLEC-2: embryonic/neonatal lethality of CLEC-2-deficient mice by blood/lymphatic misconnections and impaired thrombus formation of CLEC-2-deficient platelets. J Biol Chem . 2010;285(32):24494–507. doi:10.1074/jbc.M110.130575.
- Shirai T, Inoue O, Tamura S, Tsukiji N, Sasaki T, Endo H, Satoh K, Osada M, Sato-Uchida H, Fujii H, et al. C-type lectin-like receptor 2 promotes hematogenous tumor metastasis and prothrombotic state in tumor-bearing mice. J Thromb Haemost. 2017;15(3):513–25. doi:10.1111/jth.13604.
- Wada H, Ichikawa Y, Ezaki M, Yamamoto A, Tomida M, Yoshida M, Fukui S, Moritani I, Shiraki K, Shimaoka M, et al. Elevated plasma soluble C-Type lectin-like receptor 2 is associated with the worsening of coronavirus disease 2019. J Clin Med. 2022;11(4):3–11. doi:10.3390/jcm11040985.
- Paranjape N, Staples LL, Stradwick CY, Ray HG, Saldanha IJ. Development and validation of a predictive model for critical illness in adult patients requiring hospitalization for COVID-19. PLoS One . 2021;16(3 March):1–0. doi:10.1371/journal.pone.0248891.
- Lee EE, Hwang W, Song KH, Jung J, Kang CK, Kim JH, Oh HS, Kang YM, Lee EB, Chin BS, et al. Predication of oxygen requirement in COVID-19 patients using dynamic change of inflammatory markers: CRP, hypertension, age, neutrophil and lymphocyte (CHANeL). Sci Rep. 2021;11(1):1–8. doi:10.1038/s41598-021-92418-2.
- Hariri LP, North CM, Shih AR, Israel RA, Maley JH, Villalba JA, Vinarsky V, Rubin J, Okin DA, Sclafani A, et al. Lung histopathology in coronavirus disease 2019 as compared with severe acute respiratory syndrome and H1N1 influenza: a systematic review. Chest. 2021;159(1):73–84. doi:10.1016/j.chest.2020.09.259.
- Dhont S, Derom E, Van Braeckel E, Depuydt P, Lambrecht BN. Conceptions of the pathophysiology of happy hypoxemia in COVID-19. Respir Res. 2021;22(1):1–9. doi:10.1186/s12931-021-01614-1.
- Nicolai L, Leunig A, Brambs S, Kaiser R, Weinberger T, Weigand M, Muenchhoff M, Hellmuth JC, Ledderose S, Schulz H, et al. Immunothrombotic dysregulation in COVID-19 pneumonia is associated with respiratory failure and coagulopathy. Circulation. 2020;142(12):1176–89. doi:10.1161/CIRCULATIONAHA.120.048488.
- Martinod K, Deppermann C. Immunothrombosis and thromboinflammation in host defense and disease. Platelets. 2021;32(3):314–24. doi:10.1080/09537104.2020.1817360.
- Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL, Esmon CT. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood. 2011;118(7):1952–61. doi:10.1182/blood-2011-03-343061.
- Inoue O, Osada M, Nakamura J, Kazama F, Shirai T, Tsukiji N, Sasaki T, Yokomichi H, Dohi T, Kaneko M, et al. Soluble CLEC-2 is generated independently of ADAM10 and is increased in plasma in acute coronary syndrome: comparison with soluble GPVI. Int J Hematol. 2019;110(3):285–94. doi:10.1007/s12185-019-02680-4.
- Stenberg PE, McEver RP, Shuman MA, Jacques YV, Bainton DF. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J Cell Biol. 1985;101(3):880–6. doi:10.1083/jcb.101.3.880.
- Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, Blair CN, Weber A, Barnes BJ, Egeblad M, et al. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020; doi:10.1172/jci.insight.138999.
