Abstract
The conventional dose of recombinant human thrombopoietin (rhTPO) in the treatment of immune thrombocytopenia (ITP) is 300 U/kg per day, but the clinical reaction rate is not satisfactory. Accordingly, we explored the efficacy and safety of increasing rhTPO dose in the treatment of ITP. A retrospective study was conducted to collect the clinical data of 105 ITP patients who were divided into two groups, a low-dose group (15 000 U/day) and a high-dose group (30 000 U/day) according to the dose of rhTPO. The total effective rate of the low-dose group and the high-dose group was 31/44 (70.45%) vs. 56/61 (91.80%) (P = .049), and the average time of using rhTPO in the high-dose group was shorter than that in the low-dose group (7 days vs. 10 days, P = .001). On the 7th and 14th day of treatment, the efficacy of the high-dose group was better than that of the low-dose group [45/61 (73.77%) vs. 17/44 (38.64%), P < .001; 55/60 (91.67%) vs. 30/44 (68.18%), P < .05)]. The incidence of treatment related adverse events in the low-dose group and the high-dose group was 6/44 (13.64%) vs. 6/61 (9.84%) (P > .05), which were mild and transient in nature. In our study, high-dose rhTPO had good efficacy and high safety in the treatment of ITP with the efficacy better than low-dose rhTPO especially at day 7.
Plain Language Summary
What is the context?
Immune thrombocytopenia (ITP) is an acquired autoimmune disease characterized by low platelet counts due to increased platelet destruction and impaired platelet production.
The therapy direction of ITP involves promoting platelet generation, reducing excessive platelet destruction, immune regulation and so on.
Recombinant human thrombopoietin (rhTPO), a promote platelet production drug, has pharmacological action similar to that of endogenous TPO. It can increase platelet count rapidly and effectively and has immunological regulation effect as well.
It is found that rhTPO can rapidly and effectively increase platelet count, which has potential clinical application value in emergency situations.
What is new?
Traditionally, rhTPO has been recommended at 300 U/kg per day. Although it can greatly improve the treatment effect of ITP, the effect is not very satisfactory. In clinical practice, it has been observed that rhTPO dosage is often relatively insufficient and the therapeutic effect is poor. Therefore, we explored the efficacy and safety of increasing rhTPO dose in the treatment of ITP.
Within the efficacy and safety of rhTPO 15 000 U/day and 30 000 U/day in the treatment of ITP, our analyses suggest that:
The early response rate of the high-dose group was better than that of the low-dose group.
In the high-dose group, the effective rate of rhTPO alone or combined with glucocorticoids was more than 90%.
Treatment related adverse events occurred at a low rate and remained mild and transient.
What is the impact?
Comparing with conventional dose rhTPO, high-dose rhTPO may have better efficacy and safety in the treatment of immune thrombocytopenia and shorter administration time.
Introduction
Immune thrombocytopenia (ITP), a common hemorrhagic disorder characterized by bleeding of the mucous membranes, skin, brain or other vital organs, is an acquired autoimmune disease characterized by low platelet counts due to increased platelet destruction and impaired platelet production. Its incidence is estimated to be 2 to 5 per 100 000 persons in the general population.Citation1
Its therapy involves promoting platelet generation, reducing excessive platelet destruction, immune regulation and so on.Citation2 First-line treatments for ITP include glucocorticoids (dexamethasone, prednisone) and intravenous immunoglobulin (IVIg) which generally produce a swift and transient response.Citation3 Unfortunately, long-term use of glucocorticoids is susceptible to a variety of adverse reactions, such as osteoporosis, osteonecrosis of the femoral head, acute gastric mucosal lesions, etc.Citation4 Second-line therapy principally include drugs that promote platelet production, rituximab, splenectomy, danazol, vincristine, azathioprine, cyclosporine and mycophenolate mofetil, etc.Citation3 Although these therapies are usually efficient, the low long-term response rates and high recurrence rates in many patients are often a great challenge. In many cases, adverse drug reactions may outweigh the benefits of therapy. Up to now, most treatments have failed to modify the natural course of ITP.
The goal of ITP therapy is to increase platelet counts to safe levels rapidly in patients at high risk of bleeding or active bleeding, with durable response and long-term safety.Citation5 Due to shortage of large randomized clinical trials on ITP management, therapy decisions commonly rely on clinician experience. Thus, there are still significant controversies and differences in management strategies for patients with ITP. Accordingly, it is vital to explore a platelet raising drug with high long-term reaction rate and safety. A commonly used drug to raise platelet level is recombinant human thrombopoietin (rhTPO).
RhTPO, a full-length glycosylated recombinant human thrombopoietin expressed by Chinese hamster ovarian cells by gene recombination technology and purified,Citation6 is a platelet stimulating drug independently developed and marketed in China. It has the same amino acid sequence and physiological activity as endogenous TPO. The level of plasma TPO in ITP patients did not increase or only increased slightly and the production of endogenous TPO was not increased correspondingly, which provided a basis for the treatment of ITP patients with rhTPO.Citation7,Citation8 By binding with TPO-specific receptor c-Mpl and activating JAK/STAT and RAS/MAPK, rhTPO can exert biological effects in megakaryocyte lines. It is an important regulator of megakaryocyte proliferation, differentiation and maturation, and forms platelets with complete physiological functions after cell division. It can strengthen platelet count promptly and successfully.Citation6
The efficacy and safety of rhTPO in the treatment of ITP has been confirmed by Zhao et al in a multi-center clinical experiment.Citation9,Citation10 According to their study, after multiple subcutaneous injections of rhTPO, only transient low titer anti-TPO antibody was detected in the subjects, suggesting that the antibody did not neutralize TPO. Admittedly, PEG-rHuMGDF, a drug similar to rhTPO, was once developed in the United States but withdrawn after it was found to be associated with persistent thrombocytopenia during clinical trials. The thrombocytopenia was later revealed to be caused by the production of anti-TPO antibody neutralization in patients during treatment.Citation11,Citation12 Unlike PEG-rHuMGDF, rhTPO contains a C-terminal domain which can reduce the immunogenicity of TPO, maintain the stability of rhTPO, improve the half-life of rhTPO in vivo, and enhance the efficacy of rhTPO.Citation11 Due to its higher efficacy and safety than PEG-rHuMGDF, rhTPO has been used in China for nearly two decades.
RhTPO has been widely used in the treatment of ITP, chemotherapy related thrombocytopenia, aplastic anemia and other secondary thrombocytopenia, with definite efficacy and few adverse reactions.Citation13–16 The 2020 ITP guidelines for adults in ChinaCitation17 recommend rhTPO as a second-line therapy with high clinical evidence support (Grade A recommendation, grade l b evidence).
A number of clinical studies conducted in China have found that rhTPO can promptly and successfully increase platelet count, which has potential clinical application value in emergency situations.Citation14,Citation18,Citation19 Currently, 300 U/kg per day rhTPO is recommended in clinical practice. Although it can improve the treatment effect of ITP considerably, the effect is not very satisfactory since the onset time is generally prolonged, the effective maintenance time is short, and the recurrence rate is high.Citation20 In clinical practice, the dose of rhTPO is commonly comparatively insufficient and the therapeutic effect is poor. Accordingly, we conducted this retrospective study to report whether increasing the therapeutic dose of rhTPO could lead to better efficacy.
Patients and methods
Patients
A total of 105 ITP patients were included in this study, all hospitalized in the Department of Hematology, the Second Affiliated Hospital of Kunming Medical University from January 2016 to October 2021. The diagnosis of ITP was consistent with established criteria from International Consensus Report.Citation2 The exclusion criteria include patients with (1) severe heart, lung, liver and renal insufficiency, (2) severe or difficult to control infection, (3) a virus, such as HIV, hepatitis B or C virus infection, (4) secondary thrombocytopenia caused by malignant tumors and rheumatic immune system diseases; hereditary thrombocytopenia. The study was approved by the institutional ethical review board of the hospital.
Study design
RhTPO was purchased from Shenyang Sansheng Pharmaceutical Co., LTD. with specifications for 15 000 U/1 ml. It is recommended in the instructions to be administered subcutaneously at 300 U/kg once daily for 14 days. In this study, the patients were divided into two groups according to the actual dose of rhTPO used, the low-dose group (15 000 U/day) and the high-dose group (30 000 U/day). RhTPO was injected subcutaneously once a day for 14 consecutive days.
The patients treated before April, 2020 were all given the dosage of 15 000 U/day (1 ml in one bottle). However, since the therapeutic effect was not very satisfactory, the researchers, also the hematologists treating the patients in this study, decided to double the dosage by adding bottle. The dosage was not adjusted by weight to avoid unnecessary waste as rhTPO is expensive. Due to shortage of related literature, the researchers only used 30 000 U/day for 15 patients and 15 000 U/day for 8 patients treated from April to October, 2020. By November of the same year, the researchers decided to use the high dose to all patients as they developed the impression that it was more effective than the low dose and did not result in more adverse effect. There were 44 ITP patients in the low-dose group (26 males and 18 females), with an average age of 55 years ranging from 23 to 82 years and 61 ITP patients in the high-dose group (25 males and 36 females), with an average age of 52 years, ranging from 17 to 84 years (see for details).
Table I. ITP patients’ clinical and laboratory baseline characteristics.
All ITP patients treated with rhTPO had platelet counts lower than 20 × 109/L or were prone to aggravated bleeding. RhTPO was discontinued if platelet count increased to ≥ 100 × 109/L within 14 days of treatment. All patients were given symptomatic treatment for hemostasis. Platelet transfusion was given if bleeding tended to worsen. Data were collected from the patients’ medical records in a retrospective study.
Assessments and outcome measures
Platelet (PLT) counts in ITP patients were monitored during rhTPO treatment. Response to rhTPO therapy was used as the primary evaluation measure. Response to rhTPO was evaluated according to the following criteria: complete response (CR): PLT count ≥ 100 × 109/L and absence of bleeding; partial response (PR): PLT count ≥ 30 × 109/L and absence of bleeding, and at least two times more than the base PLT; no response (NR): PLT count < 30 × 109/L or the PLT increased less than twice the baseline value or there was bleeding. Overall response (OR) = CR + PR. Other evaluation indicators include: (1) initial response time; (2) natural course; (3) blood routine; (4) drug-related adverse reactions; (5) hemorrhage symptom; (6) minimal follow-up of 1 month. Since some patients did not regularly review their blood routine after discharge, complete data could not be collected from them. Bleeding severity was evaluated according to the Chinese Guidelines for Diagnosis and treatment of Adult ITP in 2020.Citation17
Statistical analysis
Data were analyzed using SPSS 23.0 for Windows (SPSS Inc., Chicago, IL). When the numerical data conformed to normality, the mean (standard deviation) was used to describe the homogeneity of variances was compared with the T-test, and the heterogeneity of variance used was corrected T-test. Otherwise, the data is described by the median with the maximum and minimum and the non-parametric test is used. Chi-squared test was used for enumeration data. The effectiveness of rhTPO treatment was evaluated using the Mann-Whitney U-test. For all statistical analysis, P < .05 after correction was considered significant difference.
Results
Baseline data according to rhTPO dosages
summarizes the main clinical and laboratory baseline characteristics of all the 105 ITP patients according to rhTPO dosages. Bleeding was assessed in all patients. Within the low-dose group, while 17 ITP patients did not have any bleeding, others suffered from bleeding of various levels, 16 with mucocutaneous hemorrhage, four with epistaxis, one with gastrointestinal hemorrhage, two with urinary tract hemorrhage, three with menorrhagia and one with intracranial hemorrhage. Similarly, in the high-dose group, while 22 patients did not have any bleeding, others presented different degrees of bleeding, 27 with mucocutaneous hemorrhage, four with epistaxis, two with gastrointestinal hemorrhage, one with urinary tract hemorrhage, four with menorrhagia and one with intracranial hemorrhage. All patients with bleeding were relieved after receiving symptomatic supportive treatment such as platelet elevation, platelet infusion and bleeding prevention. No statistically significant difference was observed in the distribution of gender, age, weight, recommended dose of rhTPO based on body weight, bleeding score and baseline of platelet count among the groups of different rhTPO dosages. The baseline data of ITP patients in both groups, including duration of disease, corticodependence, follow-up, and whether being treated with glucocorticoid simultaneously or not, were also consistent among the two groups.
Therapeutic response
In this study, 105 ITP patients were treated with rhTPO 29 cases achieved PR (27.62%), 58 cases CR (55.24%) and 18 cases NR (17.14%). The overall response rate was 87/105 (82.86%), with the high-dose group, significantly better than that the low-dose group [56/61 (91.80%) vs. 31/44 (70.45%), P = .049]. In the high-dose group, there were 19 cases of PR (31.14%) and 37 cases of CR (60.66%). In low-dose group, 10 patients had PR (22.73%) and 21 patients had CR (47.72%). On the 7th and 14th day of treatment, the efficacy of the high-dose group was better [45/61 (73.77%) vs. 17/44 (38.64%), P < .001; 55/60 (91.67%) vs. 30/44 (68.18%), P = .028]. No significant difference in treatment response rate between the low-dose group and the high-dose group on day 21 and day 28 was observed [27/42 (64.29%) vs. 39/50 (78.00%), P > .05; 19/42 (45.24%) vs. 28/48 (58.33%), P > .05] (see for details). While there were significant differences in platelet count between ITP groups on day 7 of treatment (P < .001), no statistical significance on day 0, day 14, day 21 and day 28 was detected. The variation tendency of platelet count (mean and standard deviation) among the ITP groups of rhTPO dosages is shown in .
Figure 1. The variation tendency of platelet count (mean and standard deviation) among the ITP groups of rhTPO dosages.
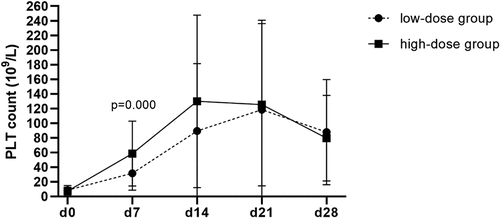
Table II. Efficacy evaluation of rhTPO in treatment of ITP patients.
RhTPO administration time of the high-dose group is shorter than that of the low-dose group 7 (3–14) day vs. 10 (3–14) day (P = .001). The median drug onset time was seven days in the low-dose group and six days in the high-dose group (P = .123). The median maintenance time of efficacy was three and four weeks in the low-dose and high-dose groups respectively (P = .260). 14 patients re-used rhTPO in the low-dose group due to ITP relapse, with an effective rate of 8/14 (57.14%) and 13 patients in the high-dose group did so, with an effective rate of 11/13 (84.62%) (P = .209) (see for details).
Comparison of efficacy of single drug and combination drug
The effective rate of rhTPO monotherapy in the low-dose and high-dose groups was 22/27 (81.48%) and 19/21 (90.48%) respectively (P > .05); the effective rate of combined glucocorticoid treatment was 9/16 (56.25%) for the low-dose group and 30/32 (93.75%) for the high-dose group (P = .038). In the low-dose group, rhTPO monotherapy and combined with glucocorticoid showed no statistical significance [22/27 (81.48%) vs. 9/16 (56.25%), P > .05]. In the high-dose group, the effective rate of rhTPO monotherapy and combined with glucocorticoids was more than 90% [19/21 (90.48%) vs. 30/32 (93.75%), P > .05]. There was no difference in the drug response time of rhTPO monotherapy between the two groups [7.5 (3–13) days vs. 6 (3–14) days, P > .05]. The response time of the low-dose and high-dose groups combined with glucocorticoid therapy was [5 (3–13) days vs. 6 (3–13) days, P > .05]. There was no significant difference in the response time between rhTPO monotherapy and combined glucocorticoid therapy in the low-dose and high-dose groups [7.5 (3–13) days vs. 5 (3–13) days, P > .05; 6 (3–14) days vs. 6 (3–13) days, P > .05] (see for details).
Table III. Analysis of efficacy of rhTPO single drug and combined drug.
Comparison of effective rate of different disease stages
The statistical method used for this part of the research results was the Mann-Whitney U-test. There was no difference in the efficacy of the newly diagnosed ITP and persistent ITP between both groups [5/8 (62.50%) vs. 13/13 (100.00%), P > .05; 5/6 (83.33%) vs. 8/9 (88.88%), P > .05]. The effective rate of chronic ITP in the high-dose group was higher than that in the low-dose group [35/39 (89.74%) vs. 21/30 (70.00%), P = .029]. In the high-dose group, the efficacy of newly diagnosed, persistent and chronic ITP was 88% or above (see for details).
Table IV. Response of different doses of rhTPO to disease staging.
Comparison of blood routine before and after treatment
Before treatment, there were no significant differences in white blood cell, hemoglobin and platelet counts between the low-dose and high-dose groups [(8.77 ± 3.94) × 109/L vs. (8.43 ± 3.76) × 109/L, P > .05; (125.55 ± 27.19) g/L vs. (127.63 ± 30.31) g/L, P > .05; (8.59 ± 6.36) × 109/L vs. (7.27 ± 5.30) × 109/L, P > .05)]. There were no significant differences in white blood cell, hemoglobin and platelet counts between the two groups after treatment, that is, between the 14th and 21st day since the first day of using rhTPO [(8.95 ± 4.85) × 109/L vs. (9.67 ± 3.63) × 109/L, P > .05; (124.00 ± 27.08) g/L vs. (126.79 ± 23.06) g/L, P > .05; (125.61 ± 119.63) × 109/L vs. (153.35 ± 136.49) × 109/L, P > .05)]. No significant changes were observed in white blood cell and hemoglobin counts before and after treatment in ITP patients of the low-dose and high-dose groups. After treatment, the platelet counts of the two groups were significantly increased, with the low-dose group being (8.59 ± 6.36) × 109/L vs. (125.61 ± 119.63) × 109/L (P < .001) and the high-dose group being (7.27 ± 5.30) × 109/L vs. (153.35 ± 136.49) × 109/L (P < .001) (see ).
Table V. Comparison of blood routine between the two groups before and after treatment.
Bleeding assessment
One patient in each group had intracranial hemorrhage (CT plain scan + Magnetic resonance diffusion weighted of another hospital) before admission, that is, an elderly (67 years old) male patient with a 3-year course of disease and a history of hypertension in the low-dose group and a young female (29 years old) with 1-year disease without underlying diseases and intracranial hemorrhage in the high-dose group. After admission, the patient in the low-dose group received rhTPO monotherapy and the patient in the high-dose group was given rhTPO combined with glucocorticoid to increase platelets, as well as symptomatic supportive treatment such as platelet transfusion and bleeding prevention. The platelet level of the elderly male patient rose to 68 × 109/L on the 9th day of rhTPO treatment while that of the young female was raised to 113 × 109/L on the 6th day of rhTPO treatment. No further bleeding was observed in either patient. The hematoma was absorbed without fresh intracranial hemorrhage by head CT plain scan during treatment on day 9 and day 8 respectively. The platelet count of the patient in the low-dose group was maintained at 115 × 109/L at 21st of treatment. For the patient in the high-dose group, platelets decreased again to 2 × 109/L after 28th, but she did not come to our hospital for further treatment.
Adverse reaction
In the low-dose group, three patients had fever (6.82%), one hypokalemia (2.27%), and tow diarrhea (4.55%), with a total incidence of 6/44 (13.64%). In the high-dose group, three patients had fever (4.92%) and three hypokalemia (4.92%), with a total incidence of 6/61 (9.84%). There was no significant difference in the incidence of adverse reactions between the two groups (P = .546). All adverse reactions, mild and transient in nature, were relieved after symptomatic treatment.
Discussion
In this study, we compared the effects of different dosages of rhTPO in the treatment of immune thrombocytopenia patients. A total of 105 ITP patients were treated with rhTPO, of which 44 patients were treated with a dose of 15 000 U/day and 61 patients were treated with a dose of 30 000 U/day. We found that the total effective rate and early response rate of the high-dose group were better than those of the low-dose group. Moreover, in the high-dose group, the response rate of rhTPO treatment alone and rhTPO combined with glucocorticoid treatment was similar, both as high as 90%. In addition, treatment related adverse events occurred at a low rate and were mild and transient in nature. In our study, high-dose rhTPO had good efficacy and high safety in the treatment of ITP with the efficacy better than low-dose rhTPO especially at day 7. Moreover, we also found that high-dose rhTPO had a good effect as a single drug, which may further reduce the adverse reactions caused by combination therapy with glucocorticoid and other drugs.
The most important finding of our study seems to be that the overall response rate of ITP patients treated with high-dose rhTPO was more than 90%, especially on the 7th and 14th days of treatment, which was significantly better than that of the low-dose group. High-dose rhTPO outperforming low-dose of the same drug has been found in both in vitro and animal studies before. Angchaisuksiri et alCitation21 demonstrated that rhTPO induced the formation of bone marrow megakaryocyte colonies in vitro in a dose-dependent manner by promoting the proliferation and differentiation of megakaryocyte progenitors into platelets, thereby increasing peripheral blood plate count. Liu et al.Citation22 in an animal study, found that after 14 days of continuous subcutaneous injection of different doses of rhTPO into ITP pregnant mouse models, platelet count increased in a dose-dependent manner on the 7th, 10th and 14th days of treatment. Further experiments showed that the frequency of Treg and serum TGF-β1 were increased in spleen cells of mice treated with rhTPO, which may induce immune tolerance without significant side effects.
Our finding that high-dose rhTPO appeared to be more efficacious in the treatment of ITP than low-dose rhTPO seems to differ from the observations of Yang et al .Citation23 In a multicenter, randomized controlled trial, Yang and his colleagues showed that there was no difference between patients receiving 15 000-U QD & 30 000-U QOD respectively. However, they administered 30000-U to one group every other day and 15 000-U to another group every day. Overall, the total amount of rhTPO received by each patient in both groups was identical. In contrast, in our study, patients in the high dose group received 30 000 U/day, twice as much as those in the low-dose group. RhTPO was discontinued if platelet count increased to ≥ 100 × 109/L within 14 days of treatment. Very likely, the increased dose in our study led to better treatment outcomes.
We did not observe significant difference in terms of response rate identified between the two groups on the 21st and 28th days of treatment. We believe this might have resulted from incomplete data as 11 and 13 patients in the high-dose group were lost to follow-up on the 21st and 28th days of treatment and some were lost in the low-dose group.
Furthermore, we also found that the duration of rhTPO administration was shorter in the high-dose group than in the low-dose group (7 days vs. 10 days, P = .001) (see ). It seems that high-dose rhTPO raised platelet to safe level much more quickly than low-dose rhTPO, which may significantly shorten the patients’ hospitalization period. The therapeutic effect of rhTPO has been reported elsewhere. In a multicenter clinical trial in China, Zhao et al.Citation9 evaluated the efficacy and safety of rhTPO evaluated in patients with persistent primary ITP who had failed glucocorticoid therapy, following the recommended dosage. They reported that rhTPO temporarily increased platelet counts and achieved an 85% remission rate. However, due to the lack of appropriate controls in their study, it is difficult to evaluate the efficacy of rhTPO objectively. Therefore, Zhao et al.Citation14 conducted a multicenter, open-label, randomized controlled study to determine the efficacy and safety of rhTPO in patients with persistent ITP who failed glucocorticoid therapy. They found that rhTPO response rates in ITP patients with glucocorticoid resistance or recurrence ranged from 60.3% to 66.7%. They concluded that rhTPO is well tolerated and can significantly increase platelet count in patients with chronic ITP, which could be used to salvage treatment of chronic ITP.
Obviously, the overall response rate in Zhao et al (2012) was lower than that of both groups in this study. One possible reason was that the patients they treated were all those with persistent ITP and having failed glucocorticoid therapy. However, since the OR rate in the high-dose group of our study was significantly higher than that of the low-dose group on the 7th and 14th day, Zhao et al might have achieved higher OR rate if they had administered higher dosage.
Meanwhile, we also found that there was no significant difference in the efficacy and time of drug onset between high-dose rhTPO monotherapy and combined glucocorticoid therapy [19/21 (90.48%) vs. 30/32 (93.75%), P > .05; 6 (3–14) days vs. 6 (3–13) days, P > .05] (see ). The high-dose rhTPO monotherapy in our study was similar in effect to the standard-dose rhTPO combined with glucocorticoids in the treatment of newly diagnosed ITP patients in a previous respective study.Citation24 In that study, standard dose rhTPO (300 U/kg) combined with glucocorticoids had a high remission rate of 14/15 (93.3%) and was found to improve the OR rate while glucocorticoids alone had a poor effect 16/26 (61.5%). Therefore, high-dose rhTPO monotherapy can be used in lieu of glucocorticoids monotherapy or the standard-dose rhTPO combined with glucocorticoids when ITP patients cannot endure the adverse effects of glucocorticoids. Admittedly, since rhTPO is much more expensive than glucocorticoids, we must take the patient’s affordability into consideration.
Somewhat surprisingly, the effect of rhTPO combined with glucocorticoid was not satisfactory in the low-dose group. A possible reason might be that three of the seven patients who failed to respond to treatment gave up due to financial difficulties within 14 days (day 6 and day 11) of treatment. In addition, we speculated that some patients’ lack of response to low-dose rhTPO combined with glucocorticoid treatment may have resulted from the possibility that platelet autoantibodies specificity affected responsiveness to rhTPO treatment. A previous study found that patients with anti-GPIB/IX autoantibodies had a lower response to rhTPO treatment and the presence of anti-GPIB/IX autoantibodies may be a predictor of poor response to rhTPO treatment in ITP patients.Citation25
Meanwhile, in our study, high-dose rhTPO did not cause increase in adverse effects. Only six of the patients in the high-dose group (9.84%) had adverse reactions, the incidence lower than 6/44 (13.64%) in the low-dose group while the difference being statistically significant. Overall, the adverse events were mild and transient in nature. All of them resolved after symptomatic management. Impressively, no thromboembolic events were noted during the study.
In conclusion, our study shows that high-dose rhTPO (30 000 U/day) may give a better chance than low-dose rhTPO (15 000 U/day) for ITP patients for considerations of effectiveness, time of hospitalization, the possibility of avoiding side effects of glucocorticoids. Meanwhile, the increased dosage does not cause extra side effects.
Admittedly, this study suffers from some limitations. Firstly, data collection was incomplete due to practical reasons, particularly the loss to follow up 11 patients in the high dose group by the third week of initial treatment. Secondly, there is no strict standard for the dosage and duration of combined glucocorticoid treatment. Some patients had been treated with glucocorticoid before hospitalization and rhTPO was used in combination due to poor treatment effect of prior medication. Although glucocorticoids were tapered, it was a slow process. Therefore, the combined glucocorticoid dose varied from patient to patient. Meanwhile, since glucocorticoids are still the first-line treatment for ITP patients, they were used in combination when rhTPO alone was not effective in this study. Therefore, the results of the current study need verification by a rigorously designed multicenter, prospective, randomized controlled clinical study.
Authorship contributions
Wang Xiuli designed the research plan, statistically analyzed the data, and wrote the paper. Bi Hui and Lin Liu et al. were responsible for collecting medical record information. Zeping Zhou participated in the study design, interpretation and discussion of data. All the authors wrote the paper and approved the final version.Footnote1
Ethics approval
The study protocol was reviewed and approved by the Medical Ethics Committee of the Second Affiliated Hospital of Kunming Medical University in accordance with the 1964 Declaration of Helsinki and its later amendments.
Acknowledgments
We thank Professor Luo Na of Lanzhou University for her extensive writing support on this manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes
1. Xiuli Wang and Hui Bi were Co-listed as first author.
References
- Neunert C, Terrell DR, Arnold DM, Buchanan G, Cines DB, Cooper N, Cuker A, Despotovic JM, George JN, Grace RF, et al. American society of hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829–7. doi:10.1182/bloodadvances.2019000966.
- Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, Chong BH, Cines DB, Gernsheimer TB, Godeau B, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010;115(2):168–86. doi:10.1182/blood-2009-06-225565.
- Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Crowther MA. The American society of hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190–207. doi:10.1182/blood-2010-08-302984.
- Matschke J, Müller-Beissenhirtz H, Novotny J, Vester I, Hertenstein B, Eisele L, Lax H, Ose C, Dührsen U. A randomized trial of daily prednisone versus pulsed dexamethasone in treatment-naïve adult patients with immune thrombocytopenia: EIS 2002 study. Acta Haematol. 2016;136(2):101–7. doi:10.1159/000445420.
- Karpatkin S. Autoimmune thrombocytopenic purpura. Blood. 1980;56(3):329–43. doi:10.1182/blood.V56.3.329.329.
- Sabath DF, Kaushansky K, Broudy VC. Deletion of the extracellular membrane-distal cytokine receptor homology module of Mpl results in constitutive cell growth and loss of thrombopoietin binding. Blood. 1999;94(1):365–7. doi:10.1182/blood.V94.1.365.413a46_365_367. PMID: 10381535.
- Bussel JB, Lakkaraja M. Thrombopoietic agents: there is still much to learn. Presse Med. 2014;43(4 Pt 2):e69–78. doi:10.1016/j.lpm.2014.02.008.
- Wörmann B. Clinical indications for thrombopoietin and thrombopoietin-receptor agonists. Transfus Med Hemother. 2013;40(5):319–25. doi:10.1159/000355006.
- Zhao YQ, Wang QY, Zhai M, Xu J, Chen X-Q, Liu W-L, Zhang M, Song S-J, Wang J-M, Meng F-Y, et al. A multi-center clinical trial of recombinant human thrombopoietin in chronic refractory idiopathic thrombocytopenic purpura. Zhonghua Nei Ke Za Zhi. 2004;43(8):608–10. PMID: 15355668.
- Zhou H, Xu M, Qin P, Zhang H-Y, Yuan C-L, Zhao H-G, Cui Z-G, Meng Y-S, Wang L, Zhou F, et al. A multicenter randomized open-label study of rituximab plus rhTPO vs rituximab in corticosteroid-resistant or relapsed ITP. Blood. 2015;125(10):1541–7. doi:10.1182/blood-2014-06-581868.
- Li J, Yang C, Xia Y, Bertino A, Glaspy J, Roberts M, Kuter DJ. Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood. 2001;98(12):3241–8. doi:10.1182/blood.v98.12.3241.
- Kuter DJ, Begley CG. Recombinant human thrombopoietin: basic biology and evaluation of clinical studies. Blood. 2002;100(10):3457–69. doi:10.1182/blood.V100.10.3457.
- Townsley DM, Desmond R, Dunbar CE, Young NS. Pathophysiology and management of thrombocytopenia in bone marrow failure: possible clinical applications of TPO receptor agonists in aplastic anemia and myelodysplastic syndromes. Int J Hematol. 2013;98(1):48–55. doi:10.1007/s12185-013-1352-6.
- Wang S, Yang R, Zou P, Hou M, Wu D, Shen Z, Lu X, Li Y, Chen X, Niu T, et al. A multicenter randomized controlled trial of recombinant human thrombopoietin treatment in patients with primary immune thrombocytopenia. Int J Hematol. 2012;96(2):222–8. doi:10.1007/s12185-012-1124-8.
- Kuter DJ. Managing thrombocytopenia associated with cancer chemotherapy. Oncology (Williston Park). 2015;29(4):282–94. PMID: 25952492.
- Wang H, Dong Q, Fu R, Qu W, Ruan E, Wang G, Liu H, Wu Y, Song J, Xing L, et al. Recombinant human thrombopoietin treatment promotes hematopoiesis recovery in patients with severe aplastic anemia receiving immunosuppressive therapy. Biomed Res Int. 2015;2015(1):2314–6133. doi:10.1155/2015/597293.
- Hou M, Hu Y. Chinese guideline on the diagnosis and management of adult primary immune thrombocytopenia (version 2020). Zhonghua Xue Ye Xue Za Zhi. 2020;41(8):617–23. doi:10.3760/cma.j.issn.0253-2727.2020.08.001.
- Wen B, Zhang X, Chen S, Fan J, Yang S, Cai Y, Wang P, Zhang Q, Gu Q, Du X, et al. Oral eltrombopag versus subcutaneous recombinant human thrombopoietin for promoting platelet engraftment after allogeneic stem cell transplantation: a prospective, non-inferiority, randomized controlled trial. Hematol Oncol. 2022;40(4):777–86. doi:10.1002/hon.3017.
- Mei H, Xu M, Yuan G, Zhu F, Guo J, Huang R, Qin J, Lv T, Qin F, Cai H, et al. A multicentre double-blind, double-dummy, randomised study of recombinant human thrombopoietin versus eltrombopag in the treatment of immune thrombocytopenia in Chinese adult patients. Br J Haematol. 2021;195(5):781–9. doi:10.1111/bjh.17808.
- Liu X, Huang Y, Liu W, Chen Y, Xue F, Zhang L, Yang R. Clinical analysis of recombinant human thrombopoietin for 92 adults with severe primary immune thrombocytopenia. Zhonghua Xue Ye Xue Za Zhi. 2015;36(4):312–5. doi:10.3760/cma.j.issn.0253-2727.2015.04.011.
- Angchaisuksiri P, Carlson PL, Dessypris EN. Effects of recombinant human thrombopoietin on megakaryocyte colony formation and megakaryocyte ploidy by human CD34+ cells in a serum-free system. Br J Haematol. 1996;93(1):13–17. doi:10.1046/j.1365-2141.1996.4761013.x.
- Liu Y, Wang R, Han P, Zhao Y, Li G, Li G, Nie M, Wang L, Chen J, Liu X, et al. Effect of recombinant human thrombopoietin on immune thrombocytopenia in pregnancy in a murine model. Int Immunopharmacol. 2019;67(2019):287–93. doi:10.1016/j.intimp.2018.12.032.
- Liu X, Bai Y, Wang T, Song Y, Sun F, Xia R, Zhu F, Ma J, Lu Q, Ye X, et al. Recombinant human thrombopoietin (rhTPO) of different dosing regimens for refractory/relapsed primary immune thrombocytopenia: a multicenter, randomized controlled trial and pharmacokinetics study. Platelets. 2023 Dec 1;34(1):1–8. doi:10.1080/09537104.2022.2157806.
- Wang X, Xu Y, Gui W, Hui F, Liao H. Retrospective analysis of different regimens for Chinese adults with severe newly diagnosed immune thrombocytopenia. Clin Exp Med. 2020;20(3):381–5. doi:10.1007/s10238-020-00630-7.
- Yu Y, Hou Y, Zhao Y, Zhou H, Jing F, Liu Y, Peng J, Liu X, Hou M. Platelet autoantibody specificity and response to rhTPO treatment in patients with primary immune thrombocytopenia. Br J Haematol. 2021;194(1):191–4. doi:10.1111/bjh.17510.
