Abstract
The older population represents a unique subset of patients due to a higher rate of comorbidities and risk factors, which can lead to a higher rate of ischemic and bleeding events. As a result, older adults are mainly underrepresented or excluded from randomized trials. Although the advancement in the percutaneous coronary intervention field with the development of new technologies, techniques, and potent antiplatelet therapy led to a reduction of ischemic risk, there is still a concern regarding bleeding hazards. Apart from the global utilization of less invasive trans-radial approach and proton pump inhibitors to reduce bleeding risk, proper tailoring of antiplatelet therapy in the older person is imperative. So far, several antiplatelet drugs have been introduced in different clinical scenarios, with dual antiplatelet therapy (combination of acetylsalicylic acid and P2Y12 inhibitor) recommended after percutaneous coronary intervention. The decision on the choice of antiplatelet drug and the DAPT duration is challenging and should be based on the relationship between ischemia and bleeding with the purpose of reducing ischemic events but not at the expense of increased bleeding complications. This is particularly important in the older population, where the evidence is obscure. The main objective of this review is to summarize the available evidence on contemporary antiplatelet therapy and different approaches of de-escalation strategies in older patients after percutaneous coronary intervention.
Plain Language Summary
What is the context?
The older population represents a unique subset of patients due to a higher rate of comorbidities, risk factors, and unfavorable prognostic features, which can lead to a higher rate of ischemic and bleeding events. They are either excluded or underrepresented in most randomized clinical trials, which is why guidelines recommendation should be taken cautiously. Thus, the decision on the choice of antiplatelet therapy and its duration after percutaneous coronary intervention in older adults is challenging and should be tailored to a particular patient to avoid bleeding complications but not at the expense of increased ischemic events.
What is new?
In this review, we summarize all available evidence on contemporary antiplatelet therapy and different approaches of de-escalation strategies in older patients after percutaneous coronary intervention. In particular, several recommended approaches in patients with high bleeding risk, are thoroughly discussed in this review:
De-escalation strategies with discontinuation of one antiplatelet drug
De-escalation strategy with switching between P2Y12 inhibitors
De-escalation strategy based on dose reduction
Finally, based on the current knowledge on factors contributing to high bleeding risk and the aforementioned antiplatelet modification approaches, in this review, we propose antiplatelet algorithm after percutaneous coronary intervention in older adults.
What is the impact?
The review provides comprehensive knowledge on antiplatelet therapy in older population and may help in tailoring antiplatelet therapy in this unique subset of patients.
Introduction
The older population (mainly defined as patients over 75 years of age) represents very specific subset of patients, with a higher prevalence of comorbidities, risk factors, and unfavorable prognostic features such as frailty that can impact the quality of life and survival.Citation1–6 Although advanced age initially was considered as a significant confounding factor influencing reduced adherence to guideline-directed therapies including percutaneous coronary intervention (PCI),Citation7 due to the advancement in medical therapy, introduction of drug-eluting stents (DES) and by favoring less invasive radial approach, PCI became highly recommended therapy in older adults, especially in acute coronary syndromes, leading to declined mortality rates.Citation8,Citation9
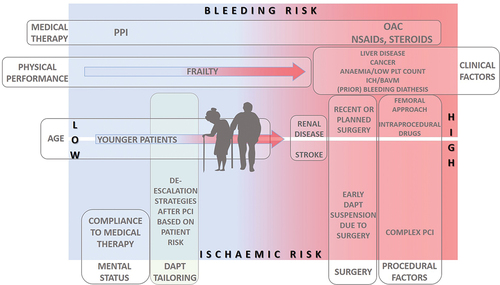
By reducing the ischemic risk in this population with PCI followed by potent dual antiplatelet therapy (DAPT), we expose them to higher bleeding risk and subsequently a higher mortality. It is well known that the more potent antithrombotic drug is, the higher the number of drugs are used and the longer duration of therapy is, the higher is the risk for bleeding. Furthermore, age itself is appraised as one of the criteria for high bleeding risk in several scoring systems.Citation10,Citation11 Both, high ischemic and bleeding risks in these patients cause them to be either excluded or underrepresented in most randomized clinical trials. Thus, when treating older patients, guidelines recommendation should be taken cautiously, and complex decision-making on the choice of antiplatelet therapy and its duration is necessary to avoid bleeding complications but not at the expense of increased ischemic events. Consequently, several strategies are tested to reduce bleeding risk, from early discontinuation of antithrombotic drug (either aspirin or P2Y12 inhibitor) to switching de-escalation strategies from more to less potent P2Y12 inhibitor or to dose reduction strategies. This review aims to summarize the available evidence on contemporary antiplatelet therapy in older patients after PCI ().
Current recommendation of antiplatelet therapy in older patients after PCI
According to the European Society of Cardiology guidelines for myocardial revascularization, dual antiplatelet therapy (DAPT) is recommended after PCI to reduce both stent-related events (stent thrombosis) in the early period and to reduce the risk for additional ischemic events in the long term.Citation12 DAPT with acetylsalicylic acid (ASA) and P2Y12 inhibitor is recommended for either 6 months in chronic coronary syndrome (CCS) or 12 months in acute coronary syndrome (ACS).Citation12 Regarding the type of P2Y12 inhibitors, clopidogrel is recommended in the chronic, while more potent ticagrelor and prasugrel are recommended in the acute coronary setting. However, the duration and the type of antiplatelet therapy are amenable to changes depending on the balance between ischemic and bleeding risk, thus leading to escalation or de-escalation of antithrombotic therapy. Furthermore, the benefit of DAPT can be hindered by the increased risk of bleeding and impact adverse prognosis. Therefore, assessing bleeding risk is crucial to guide decisions when tailoring antiplatelet therapy, particularly in the older person.
Role of bleeding risk scores
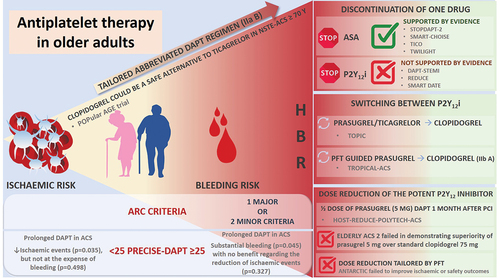
According to the most recent proposed criteria by Academic Research Consortium for high bleeding risk patients, age of ≥75 is considered as one of minor criteria for HBR patients (two minor or one major criteria is necessary to define HBR patient).Citation10 Furthermore, age is also one of the parameters of a five-item PRECISE-DAPT (PREdicting bleeding Complications In patients undergoing Stent implantation and subsEquent Dual Anti Platelet Therapy) scoreCitation11 that has been developed to predict out-of-hospital bleeding in patients treated with DAPT.Citation12 According to this score, a cutoff value of 25 is used to decide between standard/long DAPT duration (12/24 months if the score is <25) or short DAPT (3–6 months if the score is ≥25). It was observed that in patients with ACS and HBR (PRECISE-DAPT ≥25) prolongation of DAPT treatment was associated with substantial bleeding events (p = .045) with NNH (number needed to harm) of 38, but with no benefit regarding the reduction of ischemic events (p = .327). On the other hand, in patients with ACS and non-high bleeding risk (PRECISE DAPT < 25), prolonged DAPT treatment reduced ischemic events (p = .035), leading to NNT (number needed to treat) of 65, but not on the expense of bleeding events (p = .498). Thus, when the HBR is recognized, the abbreviated DAPT regimen should be considered (IIa B).Citation12,Citation13 Besides the proposed abbreviated regimen, several other approaches of DAPT modification are tested to reduce the bleeding hazard but to keep well-established ischemic benefit of DAPT.Citation14 Therefore, a personalized approach to each patient, particularly the older person is paramount.
De-escalation strategies with discontinuation of one antiplatelet drug
Discontinuation of P2Y12i
Given that acetylsalicylic acid (popularly known as aspirin) has been the mainstay of antiplatelet treatment for decades, one of the first de-escalation strategies was discontinuation of P2Y12i and de-escalation to ASA monotherapy. This relatively straightforward strategy was tested in 3 studies of ACS patients treated with a contemporary DES () mainly to define the optimal duration of DAPT in ACS.Citation15–17 However, neither one of these trials did not support a systematic, planned early de-escalation to ASA monotherapy in ACS patients. Furthermore, in SMART-DATE trial,Citation17 in 2712 patients with ACS, abbreviated 6-month DAPT duration was followed with a higher rate of MI (1.8% versus 0.8%; HR 2.41, 95% CI [1.15–5.05]) in comparison to 12 months DAPT. In the sub-analysis of REDUCE trial,Citation16 in patients ≥75 years, there was no difference in the primary endpoint between 3 and 12 months of DAPT (23% vs. 19.6%; HR 1.23 [0.67–2.26]). In addition, the abbreviated DAPT regimen did not reduce bleeding events across the trials. In a meta-analysis of 10 RCT conducted by Misumida et al.Citation18 comparing short-term (3–6 months) DAPT versus long-term DAPT (12–24 months) in 12 696 ACS patients undergoing PCI, both ischemic and bleeding events occurred with similar rates irrespective of DAPT duration. However, there was a trend in favor of short-term DAPT to reduce the risk of bleeding and on the contrary long-term regimen to prevent stent thrombosis. Of note, except DAPT-STEMICitation15 trial with a majority of patients (58.4%) treated with either ticagrelor or prasugrel, in all the rest 9 RCT, patients were receiving clopidogrel which is not according to the current recommendation.Citation12
Table I. P2Y12 discontinuation trials-ASA monotherapy.
Discontinuation of aspirin
Another approach of short DAPT or discontinuation de-escalation strategy is removing ASA and proceeding with P2Y12 inhibitor monotherapy – popularly called “ASA free” therapy. Several facts support this approach. First, the blockade of platelet P2Y12 receptors can inhibit thromboxane A2-dependent pathways of platelet activation independently of aspirin. In the presence of potent P2Y12 inhibitors, in vitro study showed that aspirin provides little additional inhibition of platelet aggregation.Citation19 This was followed by in vivo experimental findings that demonstrated no difference in the inhibition of platelet activation between P2Y12 monotherapy (clopidogrel or ticagrelor) and DAPT therapy in healthy subjects.Citation20
Furthermore, it is well known that although effective, DAPT with potent P2Y12 inhibitors takes a considerable risk of bleeding. In PLATO (PLATelet inhibition and patient Outcomes) trial, for instance, the rates of non–coronary artery bypass graft surgery (non-CABG) TIMI major bleeding were higher in the ticagrelor group (2.8 vs. 2.2, p = .025) in comparison to clopidogrel group.Citation21 Likewise, in TRITON-TIMI 38 (Trials to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel–Thrombolysis in Myocardial Infarction), non-CABG TIMI major bleeding was recorded in 2.4% of patients receiving prasugrel and 1.8% of patients receiving clopidogrel together with aspirin (HR 1.32; 95% CI [1.03 to 1.68]; p = .03).Citation22
Thus, supporting the concept that single antiplatelet therapy with a P2Y12 inhibitor alone inhibits hemostatic system activation to a similar extent, 5 RCT were conducted comparing standard DAPT regimen and short DAPT followed with P2Y12 monotherapy.Citation23–27 The main features of these trials are listed in . Although they differ with respect to the type of P2Y12 inhibitor monotherapy (STOP-DAPT 2Citation24 and SMART-CHOICECitation23 with clopidogrel and the rest with ticagrelor), length of DAPT (1 or 3 months) and the primary endpoint, there was no difference in the rate of ischemic events, while bleeding risk was significantly lower in the de-escalation arm in all trials except GLOBAL LEADERSCitation27 which was an overall neutral trial.
Table II. ASA discontinuation trials.
The GLOBAL LEADERS,Citation27 as the largest trial, failed to meet its primary objective, ticagrelor monotherapy was not superior to conventional DAPT (3.8 vs. 4.4%; RR 0.87%, 95% CI [0.75–1.01], p = .073) at 2 years follow-up. There was also no difference in the rates of stent thrombosis or major bleeding complications. Nevertheless, in a pre-specified subanalysis of GLOBAL LEADERS, among older patients (>75 years; n = 2565), the primary endpoint (two-year all-cause mortality or new Q-wave core lab-adjudicated MI) occurred in 7.2% and 9.4% of patients in the ticagrelor monotherapy and the reference group, respectively (HR 0.75, 95% CI [0.58–0.99], p = .041). At the same time, there was no difference in BARC 3/5 bleeding events (5.2 vs. 4.1%, p = .18).Citation28 These findings can be explained by the heterogenicity of the population, including ACS and CCS, however both were treated with ticagrelor.Citation12
On the other hand, in STOP-DAPT-2Citation24 trial, which included both ACS and CCS, patients were treated only with clopidogrel. Whether an abbreviated DAPT regimen (1 month with clopidogrel and ASA) followed with clopidogrel monotherapy can be safe in ACS patients was tested in STOP-DAPT-2 ACS trial.Citation29 Short DAPT was associated with a lower incidence of BARC 3/5 bleeding (0.54% vs. 1.31%), albeit at the cost of increasing the risk of MI (1.59% vs. 0.85%), highlighting the limits of abbreviated DAPT regimen followed with clopidogrel monotherapy in ACS patients.
In the TICO trial,Citation25 which included only ACS patients, the incidence of the primary net endpoint was significantly lower in the ticagrelor monotherapy arm than in the standard DAPT arm (3.9% vs 5.9%, HR 0.66; 95% CI [0.34–0.91]), largely driven by a 1.3% absolute reduction in the risk of major bleeding (HR 0.56; 95% CI [0.34–0.91]). As in SMART-CHOICECitation23 and STOP-DAPT,Citation24 the event rate in TICOCitation25 was low, perhaps in part reflecting the relatively lower risk usually observed in Asian PCI patients.Citation30
TWILIGHT (Ticagrelor With Aspirin or Alone in High-Risk Patients After Coronary Intervention), the second large trial studying ticagrelor monotherapy was designed as a double-blind study, randomized 7119 high-risk patients at 3 uneventful months on ticagrelor DAPT after PCI to either ticagrelor monotherapy or to ticagrelor DAPT. The primary endpoint- BARC type 2, 3, or 5 bleeding at 1 year was significantly lower in patients on ticagrelor monotherapy than in those on DAPT (4% vs. 7.1%, HR 0.56, 95% CI [0.45–0.68]). The rate of all-cause death, MI, or stroke was identical in both groups (p < .001 for non-inferiority).Citation26
The benefit of clopidogrel over aspirin is also observed in patients requiring indefinite single antiplatelet therapy after PCI with DES in the HOST-EXAMCitation31 and HOST-EXAM Extended study,Citation32 which demonstrate that clopidogrel monotherapy, compared with aspirin monotherapy during the chronic maintenance period, significantly reduced the risk of the composite of all-cause death, non-fatal myocardial infarction, stroke, readmission due to acute coronary syndrome, and BARC bleeding type 3 or greater.
Aspirin withdrawal in older adults
However, what would be an approach in the older population? It is well known that due to the higher rate of comorbidities and frailty, older age is a known predictor of bleeding risk, and it is included in several risk scores. Furthermore, postdischarge bleeding is strongly associated with higher mortality, substantially higher than postdischarge MI (HR 5.03, p < .0001 and HR 1.92, p = .009, respectively).Citation33 Thus, reducing bleeding risk after PCI, especially in older people, is a crucial issue.
In a prespecified analysis that included 1064 patients in the TWILIGHT HBR substudy,Citation34 ticagrelor monotherapy reduced the incidence of the primary endpoint (BARC 2, 3, or 5 bleeding) without increasing ischemic events in HBR patients (6.3% vs. 11.4%; HR 0.53, 95% CI [0.35–0.82]) and non-HBR patients as well (3.5% vs. 5.9%; HR 0.59, 95% CI [0.46–0.77]) but with the absolute risk reduction greater in HBR than non-HBR (−5.1% vs. −2.3%; 95% CI [−6.4% to 0.8%], p = .130). Another sub-analysis of TWILIGHTCitation35 that included 3113 patients equal to or older than 65 years of age demonstrated that ticagrelor monotherapy reduced the incidence of clinically relevant bleeding (BARC 2, 3 or 5) by 47% in comparison to DAPT (4.5 vs. 8.2%; HR 0.53, 95% CI [0.40–0.71]), with consistent risk reduction (p interaction = 0.09) across all age categories, and without increasing the rate of all-cause death, MI, or stroke.
Among patients from STOP-DAPT-2 trial,Citation24 1054 (35%) were classified as HBR and were included in the post hoc analysis in STOP-DAPT-2 HBR trial.Citation36 No difference in the primary endpoint between abbreviated and standard DAPT was noticed, while there were less TIMI major and minor bleedings (0.41 vs. 2.71%; HR 0.15, 95% CI [0.03–0.65]; p = .01) and less BARC 3/5 bleeding (0.61 vs. 3.26%; HR 0.19, 95% CI [0.05–0.63]; p = .007) in short DAPT arm. However, the effects of 1-month DAPT for the primary and major secondary endpoints were consistent in HBR and non-HBR patients without any significant interactions.
The presence of HBR may be a crucial factor in shortening DAPT duration at some time point beyond the acute phase was recognized and investigated in a large randomized MASTER-DAPTCitation37 trial that included 4579 HBR patients. Regarding the distribution of HBR criteria, almost 70% of patients were ≥75 years old, more than 50% with PRECISE-DAPT score ≥25 and one-third of patients requiring oral anticoagulation, on average more than 2 HBR criteria were present in each patient. One month after successful PCI with a biodegradable-polymer sirolimus-eluting coronary stent in ACS or CCS, patients were randomized to abbreviated therapy (1 month of DAPT) or standard therapy (at least 2 additional months of DAPT). The choice to stop either ASA or P2Y12 inhibitor was left to the investigator’s discretion, leading to ASA-free therapy in 70% of patients (55.6% of them taking clopidogrel), while only 30% of patients continued with ASA monotherapy. The trial concluded that one month of DAPT was non-inferior to the standard DAPT regarding the occurrence of NACE (net adverse clinical events) (7.5% vs. 7.7%, p < .001 for noninferiority) and MACCE (6.1 vs. 5.9%, p < .001 for noninferiority). However, it was superior in reducing major or clinically relevant non-major bleeding (6.5 vs. 9.4%, p < .001 for superiority). These findings encourage discontinuation of one antiplatelet drug (preferably ASA) 1 month after PCI in older patients with HBR.
Meta-analysis of aspirin withdrawal studies
A recent meta-analysis on 5 randomized trialsCitation38 including over 32 000 patients (56.1% with ACS, and only 16.5% on clopidogrel), concluded that discontinuation of aspirin 1–3 months after PCI significantly reduces the risk of major bleeding (BARC 3 or 5) by 40% compared to DAPT (1.97% versus 3.13%; HR 0.60, 95% CI [0.45–0.79]), with no excesses in adverse cardiovascular events (2.73% versus 3.11%; HR 0.88, 95% CI [0.77–1.02]), myocardial infarction (1.08% versus 1.27%; HR 0.85, 95% CI [0.69–1.06]), or death (1.25% versus 1.47%; HR 0.85, 95% CI [0.70–1.03]). Findings were consistent among patients who underwent PCI for an acute coronary syndrome, in whom discontinuation of aspirin after 1 to 3 months reduced bleeding by 50% (1.78% versus 3.58%; HR 0.50, 95% CI [0.41–0.61]) and did not appear to increase the risk of MACE (2.51% versus 2.98%; HR 0.85, 95% CI [0.70–1.03]).
However, a meta-analysis of four RCT,Citation39 including 8,961 older patients, showed that compared with standard duration, short-duration DAPT was associated with similar rates of major bleeding (relative risk, RR 0.70 [0.47–1.05]) and the composite efficacy endpoint (RR 0.85 [0.63–1.14]). There was a high level of heterogeneity between the studies (ICitation2 = 68%) regarding major bleeding.Citation39 This meta-analysis suggests that in older patients short DAPT may be a valid option after PCI. Regarding all these findings it seems that in ACS, abbreviated regimen of DAPT followed with P2Y12 monotherapy (either clopidogrel or ticagrelor), reduces the risk of bleeding, however in clopidogrel monotherapy at the cost of increased risk for ischemic events (particularly MI).
De-escalation strategy with switching between P2Y12 inhibitors
Switch from prasugrel or ticagrelor to clopidogrel
Another way to de-escalate antithrombotic therapy in patients with ACS but to remain on dual antiplatelet therapy is to switch from more potent to less potent P2Y12 inhibitor (from prasugrel and ticagrelor to clopidogrel). This strategy is supported by a specific time-dependent relation between ischemic and bleeding risk after ACS, taking into account that the highest ischemic risk is present in the first month, then decreases exponentially, with bleeding risk which is generally lower than ischemic risk but tends to remain unchanged in the long term. This interplay between ischemia and bleeding may be particularly relevant in HBR population, such as the older patients in whom this switch de-escalation strategy could offer a favorable equilibrium of prevention of both ischemia and bleeding (Central illustration).
The first switch de-escalation study was the open-label single center Timing Of Platelet Inhibition after acute Coronary syndrome (TOPIC) trial,Citation40 which included 646 ACS patients and examined the impact of a planned, unguided switch from prasugrel or ticagrelor to clopidogrel after uneventful 1 month of DAPT. The primary endpoint, a net composite of CV death, urgent revascularization, stroke and BARC bleeding ≥2 at 1 year, was significantly lower in the switched DAPT than in the standard, potent DAPT (13.4% vs. 26.3%; HR 0.48, 95% CI [0.34–0.68]). This primary endpoint was driven by a reduction in BARC ≥ 2 bleedings in the switched DAPT arm (HR 0.30; 95% CI [0.18–0.50]) but with no difference in ischemic events between arms (p = .36).
Platelet function guided switch from potent prasugrel to clopidogrel
The second, most extensive switch de-escalation study was the open-label TROPICAL-ACS (Testing Responsiveness To Platelet Inhibition On Chronic Antiplatelet Treatment For Acute Coronary Syndromes) study,Citation41 that included 2610 patients with ACS and tested guided switch from potent prasugrel to clopidogrel, based on platelet functional testing (PFT). After 1 week of DAPT with prasugrel (10 or 5 mg), patients were randomized to prasugrel or clopidogrel arm, but only patients with sufficient platelet inhibition (61% of them) were kept in clopidogrel arm, whereas non-responders were switched back to prasugrel. However, with this guided strategy, there was no difference between arms in the net primary endpoint of CV death, MI, stroke or BARC ≥ 2 (p = .004 for noninferiority), ischemic (p = .0115 for noninferiority) and bleeding endpoints (p = .2257). Thus, it is a question who would benefit from this type of guided de-escalation strategy, would it be suitable for the older population with higher bleeding risk and would it be enough in patients with very high ischemic risk?
Based on these findings, de-escalation of P2Y12 inhibitor, guided by PFT may be considered an alternative to potent DAPT strategy, especially for patients deemed unsuitable for 12 months of potent platelet inhibition (IIb A recommendation).Citation12,Citation13 Less potent clopidogrel can be a satisfactory alternative to ticagrelor in patients aged 70 years or older presenting with NSTE-ACS was demonstrated in POPular AGE trialCitation42 since it led to less bleeding events (18% vs. 24%, p = .02 for superiority) without an increase in the combined endpoint of all-cause death, myocardial infarction, stroke, and bleeding (p = .03 for noninferiority).
De-escalation strategy based on dose reduction
In addition to early antiplatelet (either ASA or P2Y12i) discontinuation or switch to a less potent P2Y12 inhibitor (from ticagrelor and prasugrel to clopidogrel), another practical option is to decrease the dose of the potent P2Y12 inhibitor. This strategy was tested in HOST-REDUCE-POLYTECH-ACS trial,Citation43 which randomized 2338 ACS patients to standard DAPT with 10 mg prasugrel and half dose of prasugrel (5 mg) DAPT 1 month after PCI. At 1 year, the rate of the primary endpoint (all-cause death, MI, ST, repeat revascularization, stroke, and BARC 2–3 bleeding) was lower in the reduced dose group (7.2% vs. 10.1%; HR 0.70, 95% CI [0.52–0.92]), mainly driven by reduced bleeding complications in reduced dose arm (HR 0.48; 95% CI [0.32–0.73]), predominantly by reduction in minor BARC 2 bleedings. However, this study was conducted in the South Korean population, less prone to ischemic events than the Western population.
Whether dose reduction, tailored by platelet functional testing, may influence ischemic and bleeding events was investigated in the ANTARCTIC (Tailored Antiplatelet Therapy Versus Recommended Dose of Prasugrel) trial.Citation44 The trial included 877 patients from France, aged >74 years, randomized to conventional prasugrel dose reduction and the monitored reduction (adjusted by the results of PFT). This sophisticated approach, guided by PFT, failed to improve ischemic or safety outcomes in older patients treated with coronary stenting for ACS.
The strategy of reduced prasugrel dose (to 5 mg) was investigated in the multicenter, randomized, open-label, blinded end point trial, ELDERLY ACS 2 trial,Citation45 including 1443 older ACS patients treated with PCI (40% of women, mean age of 80 years). The trial was designed to demonstrate the superiority of prasugrel 5 mg over standard clopidogrel 75 mg. However, the trial was prematurely terminated due to the futility of efficacy. There was no difference in the primary endpoint (composite of mortality, myocardial infarction, disabling stroke, and rehospitalization for cardiovascular causes or bleeding within 1 year) between prasugrel and clopidogrel arms (HR 1.007; 95% CI [0.78–1.30], p = .955), although a trend for a lower stent thrombosis (ST) rate was observed in the prasugrel group (OR 0.36; 95% CI [0.13–1.00], p = .06). There was no difference in BARC ≥ 2 bleeding events (4.1% in prasugrel vs. 2.7% in clopidogrel group, p = .18). Accordingly, in this de-escalation strategy with a reduced dose of prasugrel, it seems that in older patients with prevailing bleeding risk low-dose prasugrel might have an advantage over full dose, however, with no advantage over clopidogrel in this subset of patients, which sets clopidogrel as an alternative to P2Y12 inhibitor in older patients.
DAPT strategy based on the type of stents used in PCI
Older patients and patients with HBR used to receive bare-metal stents (BMS) instead of DES to shorten the duration of DAPT and to minimize the risk of bleeding complications associated with prolonged antiplatelet therapy. Due to the advantages of contemporary DES (thin stent struts of 50–100 µm, rapid endothelialization) over BMS in terms of reduced target vessel revascularization (TVR) and stent thrombosis (ST), the trials started to compare these two stent technologies, particularly in patients with HBR undergoing short DAPT regimen. In the first trials that compared BMS and DES in HBR patients, age was one of the major criteria for HBR, with 51% of patients older than 80 years in ZEUS,Citation46 64% of patients ≥75 years in LEADERS FREECitation47 and 100% of patients ≥75 years in SENIORCitation48 trial.
In the ZEUS trial, 1606 patients were randomized to the second-generation zotarolimus-eluting stent (ZES) versus bare-metal stents (BMS) with abbreviated DAPT regimen (median DAPT duration was 32 days). The primary endpoint (death, myocardial infarction, and TVR) was lower in the ZES group (17.5 vs. 22.1%; HR 0.76, 95% CI [0.61–0.95], p = .011) in comparison to the BMS group. Definite or probable ST was also significantly reduced in ZES patients (2.0% vs. 4.1%; p = .019). Bleeding complications did not differ between these two stent platforms.
Similarly, in the SENIOR trial, which enrolled 1200 patients ≥75 years, patients treated with the bioabsorbable polymer DES and a short DAPT duration (1 month for patients with stable and 6 months for patients with acute coronary syndrome) demonstrated superiority over BMS regarding the occurrence of all-cause mortality, myocardial infarction, and target lesion revascularisation (12 vs. 16%; RR 0.71, 95% CI [0·52–0·94]; p = .02). Bleeding complications and the rate of ST did not differ between DES and BMS groups.
In the LEADERS FREE trial,Citation47 2466 patients at HBR were randomized to receive polymer-free DES with biolimus A9 and BMS followed by 1 month of DAPT. Polymer-free DES was superior to a BMS with respect to the primary safety endpoint of cardiac death, myocardial infarction, or stent thrombosis (9.4% vs. 12.9%, p = .005 for superiority) and efficacy endpoint of clinically driven target-lesion revascularization (5.1 vs. 9.8%, p < .001). As expected, in this HBR population, despite the short course of DAPT, the rate of bleeding (BARC types 3 to 5) was high but similar in the two groups (7.2 vs. 7.3%, p = .96).
The ONYX ONECitation49 trial with a population of 1996 patients, was designed to compare polymer-based zotarolimus-eluting stent (ZES) with polymer-free DES followed by 1 month of DAPT. The primary outcome (composite of all-cause death, MI or ST) was observed with a similar rate (17.1% in ZES and 16.9% in polymer-free DES, p = .01 for noninferiority) at 1-year follow-up. No difference in target vessel failure or bleeding complications was noticed as well.
All these trials have demonstrated that current DES are preferred over BMS for HBR patients. However, to establish the optimal duration of DAPT, XIENCE Short DAPTCitation50 and EVOLVE short DAPTCitation51 trials were followed. The XIENCE Short DAPTCitation50 program included three prospective, multicenter, single-arm studies enrolling 1487 HBR patients (more than 2/3 of them were ≥75 of age) who underwent successful PCI (STEMI patients and complex lesions were excluded) with a cobalt-chromium everolimus-eluting stent. The program compared a short DAPT regimen of 1 month (XIENCE 28 USA and XIENCE 28 Global studies) or 3 months (XIENCE 90 study) with the recommended 6 or 12 months of DAPT duration. Abbreviated DAPT regimen of 1 or 3 months compared with DAPT for 6 or 12 months resulted in non-inferior ischemic outcomes and a low incidence of ST and was associated with significantly lower major bleeding (BARC 3–5). Similarly, EVOLVE short DAPT trialCitation51 enrolling 2009 patients (stable patients, no complex lesions), evaluated the safety of 3 month-DAPT in patients with HBR treated with platinum-chromium everolimus-eluting stent. Again, abbreviated DAPT in patients with HBR treated with contemporary DES was not inferior to the standard DAPT duration in terms of death, myocardial infarction, and stent thrombosis, supporting the safety of abbreviated DAPT with the abovementioned stent platforms.
Therefore, when deciding between two stent platforms and DAPT duration in HBR patients, with older adults representing the majority of them, DES has an advantage over BMS irrespective of age, clinical presentation, and lesion type, particularly with the proven possibility of concomitant short, BMS-like DAPT therapy.
Conclusion
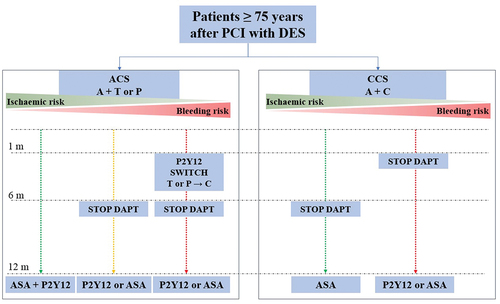
Older patients represent population with a higher rate of comorbidities and risk factors predisposing them to ischemic and bleeding complications. The development of PCI technology and techniques, the introduction of DES, potent antiplatelet therapy, and high dose of statins led to the reduction of ischemic risk. On the other hand, despite the prevalent use of a trans-radial approach and the extensive use of proton pump inhibitors, potent antiplatelet therapy exposes them to a higher rate of bleeding complications and subsequently to higher mortality. Therefore, when tailoring antiplatelet therapy in older patients, meticulous periprocedural planning and postprocedural follow-up are paramount. Different DAPT modifications are introduced and investigated to reduce bleeding complications, from early discontinuation of antithrombotic drugs, to de-escalation strategies from more to less potent P2Y12 inhibitors or to dose reduction strategies. A personalized approach to a single patient, guided by current evidence and recommendations, is advisable ().
Figure 2. Proposed antiplatelet algorithm in older adults after PCI with DES.
Author contributions
MK wrote the first draft. VK underlook mutliple revisions and invited author. GP undertook critical review and created the figures.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Damluji AA, Huang J, Bandeen‐Roche K, Forman DE, Gerstenblith G, Moscucci M, Resar JR, Varadhan R, Walston JD, Segal JB, et al. Frailty among older adults with acute myocardial infarction and outcomes from percutaneous coronary interventions. J Am Heart Assoc Cardiovasc Cerebrovasc Dis. 2019 Aug 31;8(17):e013686. doi:10.1161/JAHA.119.013686.
- Damluji AA, Forman DE, Wang TY, Chikwe J, Kunadian V, Rich MW, Young BA, Page RL, DeVon HA, Alexander KP, et al. Management of acute coronary syndrome in the older adult population: a scientific statement from the American Heart association. Circulation. 2023 Jan 17;147(3):e32–10. doi:10.1161/CIR.0000000000001112.
- Ratcovich H, Beska B, Mills G, Holmvang L, Adams-Hall J, Stevenson H, Veerasamy M, Wilkinson C, Kunadian V, Back M, et al. Five-year clinical outcomes in patients with frailty aged ≥75 years with non-ST elevation acute coronary syndrome undergoing invasive management. Eur Heart J Open. 2022 May;2(3):oeac035. doi:10.1093/ehjopen/oeac035.
- Beska B, Mills GB, Ratcovich H, Wilkinson C, Damluji AA, Kunadian V. Impact of multimorbidity on long-term outcomes in older adults with non-ST elevation acute coronary syndrome in the North East of England: a multi-centre cohort study of patients undergoing invasive care. BMJ Open. 2022 Jul 26;12(7):e061830. doi:10.1136/bmjopen-2022-061830.
- Beska B, Coakley D, MacGowan G, Adams-Hall J, Wilkinson C, Kunadian V. Frailty and quality of life after invasive management for non-ST elevation acute coronary syndrome. Heart Br Card Soc. 2022 Feb;108(3):203–11. doi:10.1136/heartjnl-2021-319064.
- Chung KJNC, Wilkinson C, Veerasamy M, Kunadian V. Frailty scores and their utility in older patients with cardiovascular disease. Interv Cardiol Lond Engl. 2021 Apr;16:e05. doi:10.15420/icr.2020.18.
- Schoenenberger AW, Radovanovic D, Stauffer JC, Windecker S, Urban P, Eberli FR, Stuck AE, Gutzwiller F, Erne P. Age-related differences in the use of guideline-recommended medical and interventional therapies for acute coronary syndromes: a cohort study. J Am Geriatr Soc. 2008 Mar;56(3):510–6. doi:10.1111/j.1532-5415.2007.01589.x.
- Damluji AA, Resar JR, Gerstenblith G, Gross AL, Forman DE, Moscucci M. Temporal trends of percutaneous coronary interventions in older adults with acute myocardial infarction. Circ Cardiovasc Interv. 2019 May;12(5):e007812. doi:10.1161/CIRCINTERVENTIONS.119.007812.
- Elbadawi A, Elgendy IY, Ha LD, Mahmoud K, Lenka J, Olorunfemi O, Reyes A, Ogunbayo GO, Saad M, Abbott JD, et al. National trends and outcomes of percutaneous coronary intervention in patients ≥70 years of age with acute coronary syndrome (from the national inpatient sample database). Am J Cardiol. 2019 Jan 1;123(1):25–32. doi:10.1016/j.amjcard.2018.09.030.
- Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, Cuisset T, Cutlip D, Eerdmans P, Eikelboom J, et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention. Circulation. 2019 Jul 16;140(3):240–61. doi:10.1161/CIRCULATIONAHA.119.040167.
- Costa F, van Klaveren D, James S, Heg D, Räber L, Feres F, Pilgrim T, Hong M-K, Kim H-S, Colombo A, et al. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet Lond Engl. 2017 Mar 11;389(10073):1025–34. doi:10.1016/S0140-6736(17)30397-5.
- Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet J-P, Falk V, Head SJ, et al., 2018 ESC/EACTS Guidelines on myocardial revascularization, EuroIntervention J Eur Collab Work Group Interv Cardiol Eur Soc Cardiol. 2019;14(14):1435–534. 2019 Feb 20. doi:10.4244/EIJY19M01_01.
- Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, et al., ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation, Eur Heart J. 2020;42(14):1289–367. 2021 Apr 7. doi:10.1093/eurheartj/ehaa575.
- Sinnaeve PR, Adriaenssens T. Dual antiplatelet therapy De-escalation strategies. Am J Cardiol. 2021 Apr 1;144(Suppl 1):S23–31. doi:10.1016/j.amjcard.2020.12.020.
- Kedhi E, Fabris E, van der Ent M, Buszman P, von Birgelen C, Roolvink V, Zurakowski A, Schotborgh CE, Hoorntje JCA, Eek CH, et al. Six months versus 12 months dual antiplatelet therapy after drug-eluting stent implantation in ST-elevation myocardial infarction (DAPT-STEMI): randomised, multicentre, non-inferiority trial. BMJ. 2018 Oct 2;363:k3793. doi:10.1136/bmj.k3793.
- De Luca G, Damen SA, Camaro C, Benit E, Verdoia M, Rasoul S, Liew HB, Polad J, Ahmad WA, Zambahari R, et al. Final results of the randomised evaluation of short-term dual antiplatelet therapy in patients with acute coronary syndrome treated with a new-generation stent (REDUCE trial). EuroIntervention J Eur Collab Work Group Interv Cardiol Eur Soc Cardiol. 2019 Dec 6;15(11):e990–8. doi:10.4244/EIJ-D-19-00539.
- Hahn JY, Song YB, Oh JH, Cho DK, Lee JB, Doh JH, Kim S-H, Jeong J-O, Bae J-H, Kim B-O, et al. 6-month versus 12-month or longer dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (SMART-DATE): a randomised, open-label, non-inferiority trial. Lancet Lond Engl. 2018 Mar 31;391(10127):1274–84. doi:10.1016/S0140-6736(18)30493-8.
- Misumida N, Abo-Aly M, Kim SM, Ogunbayo GO, Abdel-Latif A, Ziada KM. Efficacy and safety of short-term dual antiplatelet therapy (≤6 months) after percutaneous coronary intervention for acute coronary syndrome: a systematic review and meta-analysis of randomized controlled trials. Clin Cardiol. 2018 Nov;41(11):1455–62. doi:10.1002/clc.23075.
- Armstrong PCJ, Leadbeater PD, Chan MV, Kirkby NS, Jakubowski JA, Mitchell JA, Warner TD. In the presence of strong P2Y12 receptor blockade, aspirin provides little additional inhibition of platelet aggregation. J Thromb Haemost JTH. 2011 Mar;9(3):552–61. doi:10.1111/j.1538-7836.2010.04160.x.
- Traby L, Kollars M, Kaider A, Eichinger S, Wolzt M, Kyrle PA. Effects of P2Y12 receptor inhibition with or without aspirin on hemostatic system activation: a randomized trial in healthy subjects. J Thromb Haemost JTH. 2016 Feb;14(2):273–81. doi:10.1111/jth.13216.
- Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009 Sep 10;361(11):1045–57. doi:10.1056/NEJMoa0904327.
- Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann F-J, Ardissino D, De Servi S, Murphy SA, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007 Nov 15;357(20):2001–15. doi:10.1056/NEJMoa0706482.
- Hahn JY, Song YB, Oh JH, Chun WJ, Park YH, Jang WJ, Im E-S, Jeong J-O, Cho BR, Oh SK, et al. Effect of P2Y12 inhibitor monotherapy vs dual antiplatelet therapy on cardiovascular events in patients undergoing percutaneous coronary intervention: the SMART-CHOICE randomized clinical trial. JAMA. 2019 Jun 25;321(24):2428–37. doi:10.1001/jama.2019.8146.
- Watanabe H, Domei T, Morimoto T, Natsuaki M, Shiomi H, Toyota T, Ohya M, Suwa S, Takagi K, Nanasato M, et al. Effect of 1-month dual antiplatelet therapy followed by clopidogrel vs 12-month dual antiplatelet therapy on cardiovascular and bleeding events in patients receiving PCI: the STOPDAPT-2 randomized clinical trial. JAMA. 2019 Jun 25;321(24):2414–27. doi:10.1001/jama.2019.8145.
- Kim BK, Hong SJ, Cho YH, Yun KH, Kim YH, Suh Y, Cho JY, Her A-Y, Cho S, Jeon DW, et al. Effect of ticagrelor monotherapy vs ticagrelor with aspirin on major bleeding and cardiovascular events in patients with acute coronary syndrome: the TICO randomized clinical trial. JAMA. 2020 Jun 16;323(23):2407–16. doi:10.1001/jama.2020.7580.
- Mehran R, Baber U, Sharma SK, Cohen DJ, Angiolillo DJ, Briguori C, Cha JY, Collier T, Dangas G, Dudek D, et al. Ticagrelor with or without aspirin in high-risk patients after PCI. N Engl J Med. 2019 Nov 21;381(21):2032–42. doi:10.1056/NEJMoa1908419.
- Vranckx P, Valgimigli M, Jüni P, Hamm C, Steg PG, Heg D, van Es, G.A., McFadden, E.P., Onuma, Y., van Meijeren, C., et al. Ticagrelor plus aspirin for 1 month, followed by ticagrelor monotherapy for 23 months vs aspirin plus clopidogrel or ticagrelor for 12 months, followed by aspirin monotherapy for 12 months after implantation of a drug-eluting stent: a multicentre, open-label, randomised superiority trial. Lancet Lond Engl. 2018 Sep 15;392(10151):940–9.
- Tomaniak M, Chichareon P, Modolo R, Takahashi K, Chang CC, Kogame N, Spitzer E, Buszman PE, van Geuns RJM, Valkov V, et al. Ticagrelor monotherapy beyond one month after PCI in ACS or stable CAD in elderly patients: a pre-specified analysis of the GLOBAL LEADERS trial. EuroIntervention J Eur Collab Work Group Interv Cardiol Eur Soc Cardiol. 2020 Apr 3;15(18):e1605–14. doi:10.4244/EIJ-D-19-00699.
- Watanabe H, Morimoto T, Natsuaki M, Yamamoto K, Obayashi Y, Ogita M, Suwa S, Isawa T, Domei T, Yamaji K, et al. Comparison of clopidogrel monotherapy after 1 to 2 months of dual antiplatelet therapy with 12 months of dual antiplatelet therapy in patients with acute coronary syndrome: the STOPDAPT-2 ACS randomized clinical trial. JAMA Cardiol. 2022 Apr 1;7(4):407–17. doi:10.1001/jamacardio.2021.5244.
- Onuma Y, Kimura T, Räber L, Magro M, Girasis C, van Domburg R, Windecker, S., Mitsudo, K., Serruys, P.W., et al. Differences in coronary risk factors, procedural characteristics, mortality and stent thrombosis between two all-comers percutaneous coronary intervention registries from Europe and Japan: a patient-level data analysis of the bern-rotterdam and j-cypher registries. EuroIntervention J Eur Collab Work Group Interv Cardiol Eur Soc Cardiol. 2015 Sep;11(5):533–40.
- Koo BK, Kang J, Park KW, Rhee TM, Yang HM, Won KB, Rha S-W, Bae J-W, Lee NH, Hur S-H, et al. Aspirin versus clopidogrel for chronic maintenance monotherapy after percutaneous coronary intervention (HOST-EXAM): an investigator-initiated, prospective, randomised, open-label, multicentre trial. Lancet Lond Engl. 2021 Jun 26;397(10293):2487–96. doi:10.1016/S0140-6736(21)01063-1.
- Kang J, Park KW, Lee H, Hwang D, Yang HM, Rha SW, Bae J-W, Lee NH, Hur S-H, Han J-K, et al. Aspirin versus clopidogrel for long-term maintenance monotherapy after percutaneous coronary intervention: the HOST-EXAM extended study. Circulation. 2023 Jan 10;147(2):108–17. doi:10.1161/CIRCULATIONAHA.122.062770.
- Généreux P, Giustino G, Witzenbichler B, Weisz G, Stuckey TD, Rinaldi MJ, Neumann F-J, Metzger DC, Henry TD, Cox DA, et al. Incidence, predictors, and impact of post-discharge bleeding after percutaneous coronary intervention. J Am Coll Cardiol. 2015 Sep 1;66(9):1036–45. doi:10.1016/j.jacc.2015.06.1323.
- Escaned J, Cao D, Baber U, Nicolas J, Sartori S, Zhang Z, Dangas G, Angiolillo DJ, Briguori C, Cohen DJ, et al. Ticagrelor monotherapy in patients at high bleeding risk undergoing percutaneous coronary intervention: TWILIGHT-HBR. Eur Heart J. 2021 Dec 1;42(45):4624–34. doi:10.1093/eurheartj/ehab702.
- Angiolillo DJ, Cao D, Baber U, Sartori S, Zhang Z, Dangas G, Mehta S, Briguori C, Cohen DJ, Collier T, et al. Impact of age on the safety and efficacy of ticagrelor monotherapy in patients undergoing PCI. JACC Cardiovasc Interv. 2021 Jul 12;14(13):1434–46. doi:10.1016/j.jcin.2021.04.043.
- Watanabe H, Domei T, Morimoto T, Natsuaki M, Shiomi H, Toyota T, Ohya M, Suwa S, Takagi K, Nanasato M, et al. Details on the effect of very short dual antiplatelet therapy after drug-eluting stent implantation in patients with high bleeding risk: insight from the STOPDAPT-2 trial. Cardiovasc Interv And Ther. 2021 Jan;36(1):91–103. doi:10.1007/s12928-020-00651-9.
- Valgimigli M, Frigoli E, Heg D, Tijssen J, Jüni P, Vranckx P, Ozaki Y, Morice M-C, Chevalier B, Onuma Y, et al. Dual antiplatelet therapy after PCI in patients at high bleeding risk. N Engl J Med. 2021 Oct 28;385(18):1643–55. doi:10.1056/NEJMoa2108749.
- O’Donoghue ML, Murphy SA, Sabatine MS. The safety and efficacy of aspirin discontinuation on a background of a P2Y12 inhibitor in patients after percutaneous coronary intervention: a systematic review and meta-analysis. Circulation. 2020 Aug 11;142(6):538–45. doi:10.1161/CIRCULATIONAHA.120.046251.
- Roule V, Lemaitre A, Pommier W, Bignon M, Sabatier R, Blanchart K, Beygui F. Safety and efficacy of very short dual antiplatelet therapy followed by P2Y12 inhibitor monotherapy in older patients undergoing percutaneous coronary intervention: meta-analysis of randomised controlled trials. Age Ageing. 2021 Jun 28;50(4):1102–7. doi:10.1093/ageing/afab047.
- Cuisset T, Deharo P, Quilici J, Johnson TW, Deffarges S, Bassez C, Bonnet G, Fourcade L, Mouret JP, Lambert M, et al. Benefit of switching dual antiplatelet therapy after acute coronary syndrome: the TOPIC (timing of platelet inhibition after acute coronary syndrome) randomized study. Eur Heart J. 2017 Nov 1;38(41):3070–8. doi:10.1093/eurheartj/ehx175.
- Sibbing D, Aradi D, Jacobshagen C, Gross L, Trenk D, Geisler T, Orban M, Hadamitzky M, Merkely B, Kiss RG, et al. Guided de-escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL-ACS): a randomised, open-label, multicentre trial. Lancet Lond Engl. 2017 Oct 14;390(10104):1747–57. doi:10.1016/S0140-6736(17)32155-4.
- Gimbel M, Qaderdan K, Willemsen L, Hermanides R, Bergmeijer T, de Vrey E, Heestermans T, Tjon Joe Gin M, Waalewijn R, Hofma S, et al. Clopidogrel versus ticagrelor or prasugrel in patients aged 70 years or older with non-ST-elevation acute coronary syndrome (POPular AGE): the randomised, open-label, non-inferiority trial. Lancet Lond Engl. 2020 Apr 25;395(10233):1374–81. doi:10.1016/S0140-6736(20)30325-1.
- Kim HS, Kang J, Hwang D, Han JK, Yang HM, Kang HJ, Koo B-K, Rhew JY, Chun K-J, Lim Y-H, et al. Prasugrel-based de-escalation of dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (HOST-REDUCE-POLYTECH-ACS): an open-label, multicentre, non-inferiority randomised trial. Lancet Lond Engl. 2020 Oct 10;396(10257):1079–89. doi:10.1016/S0140-6736(20)31791-8.
- Cayla G, Cuisset T, Silvain J, Leclercq F, Manzo-Silberman S, Saint-Etienne C, Delarche N, Bellemain-Appaix A, Range G, El Mahmoud R, et al. Platelet function monitoring to adjust antiplatelet therapy in elderly patients stented for an acute coronary syndrome (ANTARCTIC): an open-label, blinded-endpoint, randomised controlled superiority trial. Lancet Lond Engl. 2016 Oct 22;388(10055):2015–22. doi:10.1016/S0140-6736(16)31323-X.
- Savonitto S, Ferri LA, Piatti L, Grosseto D, Piovaccari G, Morici N, Bossi I, Sganzerla P, Tortorella G, Cacucci M, et al. Comparison of reduced-dose prasugrel and standard-dose clopidogrel in elderly patients with acute coronary syndromes undergoing early percutaneous revascularization. Circulation. 2018 Jun 5;137(23):2435–45. doi:10.1161/CIRCULATIONAHA.117.032180.
- Valgimigli M, Patialiakas A, Thury A, McFadden E, Colangelo S, Campo G, Tebaldi M, Ungi I, Tondi S, Roffi M, et al. Zotarolimus-eluting versus bare-metal stents in uncertain drug-eluting stent candidates. J Am Coll Cardiol. 2015 Mar 3;65(8):805–15. doi:10.1016/j.jacc.2014.11.053.
- Urban P, Meredith IT, Abizaid A, Pocock SJ, Carrié D, Naber C, Lipiecki J, Richardt G, Iñiguez A, Brunel P, et al. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med. 2015 Nov 19;373(21):2038–47. doi:10.1056/NEJMoa1503943.
- Varenne O, Cook S, Sideris G, Kedev S, Cuisset T, Carrié D, Hovasse T, Garot P, El Mahmoud R, Spaulding C, et al. Drug-eluting stents in elderly patients with coronary artery disease (SENIOR): a randomised single-blind trial. Lancet Lond Engl. 2018 Jan 6;391(10115):41–50. doi:10.1016/S0140-6736(17)32713-7.
- Windecker S, Latib A, Kedhi E, Kirtane AJ, Kandzari DE, Mehran R, Price MJ, Abizaid A, Simon DI, Worthley SG, et al. Polymer-based or polymer-free stents in patients at high bleeding risk. N Engl J Med. 2020 Mar 26;382(13):1208–18. doi:10.1056/NEJMoa1910021.
- Mehran R, Cao D, Angiolillo DJ, Bangalore S, Bhatt DL, Ge J, Hermiller J, Makkar RR, Neumann F-J, Saito S, et al. 3- or 1-month DAPT in patients at high bleeding risk undergoing everolimus-eluting stent implantation. JACC Cardiovasc Interv. 2021 Sep 13;14(17):1870–83. doi:10.1016/j.jcin.2021.07.016.
- Kirtane AJ, Stoler R, Feldman R, Neumann FJ, Boutis L, Tahirkheli N, Toelg R, Othman I, Stein B, Choi JW, et al. Primary results of the EVOLVE short DAPT study: evaluation of 3-month dual antiplatelet therapy in high bleeding risk patients treated with a bioabsorbable polymer-coated everolimus-eluting stent. Circ Cardiovasc Interv. 2021 Mar;14(3):e010144. doi:10.1161/CIRCINTERVENTIONS.120.010144.