Abstract
Previous research suggests that individuals with 22q11.2 deletion syndrome (DS) have an increased risk of bleeding following cardiac surgery. However, current guidelines for management of patients with 22q11.2DS do not provide specific recommendations for perioperative management. This study sought to identify specific risk factors for bleeding in this patient population. Examine the factors determining bleeding and transfusion requirements in patients with 22q11.2DS undergoing cardiac surgery. This was a single center review of patients who underwent cardiac surgery at the Children’s Hospital of Philadelphia from 2000 to 2016. Data was extracted from the medical record. Frequency of bleeding events, laboratory values, and transfusion requirements were compared. We included 226 patients with 22q11.2DS and 506 controls. Bleeding events were identified in 13 patients with 22q11.2DS (5.8%) and 27 controls (5.3%). Platelet counts were lower among patients with 22q11.2DS than in control patients, but not statistically different comparing bleeding to not bleeding. Patients with 22q11.2DS received more transfusions (regardless of bleeding status). However, multivariate analysis showed only procedure type was associated with increased risk of bleeding (p = .012). The overall risk of bleeding when undergoing cardiac surgery is not different in patients with 22q11.2DS compared to non-deleted patients. Though platelet counts were lower in patients with 22q11.2DS, only procedure type was significantly associated with an increased risk of bleeding.
Introduction
The 22q11.2 Deletion Syndrome (22q11.2DS) is the most frequent chromosomal microdeletion syndrome with an estimated prevalence between 1 per 2,000 and 6,000 live births.Citation1–3 The majority of patients have de novo deletions, mostly the classical LCR22A-LCR22D deletion.Citation4 Key features include palatal anomalies, hypocalcemia, immune deficiency, gastrointestinal problems, renal abnormalities, developmental delay/behavioral differences, and congenital cardiac disease. In fact, 22q11.2DS is the second most common chromosomal cause of congenital heart disease (after Down syndrome).Citation5
While the estimates of prevalence of cardiac disease in 22q11.2DS of 75–80% likely overestimates the true prevalence (due to missed diagnosis in patients without cardiac involvement), many patients do have abnormalities and approximately 20–45% of patients present with tetralogy of Fallot (TOF).Citation5 Approximately 45% of patients with 22q11.2DS require cardiac surgery during infancy or childhood. One study showed increased rates of transfusion following cardiac surgery in these patients,Citation6 but there is limited data on the overall risk of bleeding and contributing factors. This study sought to compare bleeding risk in patients with and without 22q11.2DS undergoing cardiac surgery and identify potential risk factors for bleeding.
Methods
This protocol was approved by the Children’s Hospital of Philadelphia (CHOP) Institutional Review Board. Patients with 22q11.2 DS who underwent cardiac surgery at CHOP between 1 January 2000 and 12/30/2016 were identified from the institutional 22q11.2DS registry and cross referenced with the institutional cardiothoracic surgery database. Subjects were eligible if they were less than 18 years old when enrolled in the registry and had available surgical records available. Controls from within the same time period were chosen from the cardiothoracic surgery database, matching for procedure type. Up to three controls were obtained for each case where possible and cases/controls were matched for dates and procedures. Overall, there were no differences in weight or age at procedure except in the group undergoing valve and conduit placement/replacement (). Procedural risk was categorized on a 1 to 5 scale using the Society of Thoracic Surgeons European Association for Cardio-Thoracic Surgery (STAT) Congenital Heart Surgery Mortality Categories.Citation7
Table I. Demographics and procedures.
Patient data were extracted from the medical record, and included demographics, anticoagulation, time on pump, or antiplatelet medications at the time of procedure (yes or no), blood bank data, laboratory data, and medical history.
The total number of units and volume of whole blood (WB), packed red blood cells (PRBC), fresh frozen plasma (FFP), platelets (plts), and cryoprecipitate (CP) transfused after leaving the operating room, but within 48 h of the surgical procedure were collected. Transfusion volumes were calculated in terms of units, ml/kg, and donor exposures. Bleeding events were defined a priori as intraoperative administration of a hemostatic agent (recombinant activated factor seven (rFVIIa), or procoagulant concentrate (PCC)), reoperation for surgical bleeding, documentation of unexpected surgical bleeding, unplanned post-operative open chest, or post-operative bleeding of a non-surgical site (i.e. subdural, intracranial). Events were combined into a dichotomous endpoint (yes or no).
Statistical analysis was performed using the R statistical programming language. Median and interquartile range (IQR) were used to describe patient characteristics. Two-way ANOVA with Tukey HS multiple comparisons correction was used to analyze continuous variables. Wilcox signed rank tests with false discovery rate multiple comparison corrections were used for dosages and donor exposures. Fisher’s exact or chi-squared tests were used for count data (conservatively measured using pairwise T-tests without correction for multiple testing). Multivariate mixed effects analysis (with the individual patient being a random effect because they underwent multiple procedures) was performed using the GLMM adaptive package.
Results
There were 226 patients with 22q11.2DS eligible for inclusion and 506 controls matched by procedure. There was no statistical difference in age and weight at the time of surgery for most procedures (). However, for valve replacement surgeries, the control cohort was older and for conduit replacements, the control cohort had slightly higher weight.
Overall bleeding
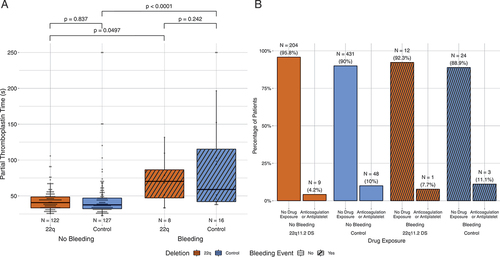
The overall bleeding risk in the cohort was not different between patients with and without the 22q11.2DS (p = .86, ). The procedure type had a greater influence on the risk of bleeding than the presence of the 22q11.2DS () (). Platelet counts were lower in patients with 22q11.2DS than controls for all surgery types (). There were no statistically significant differences between patients who experienced bleeding with and without 22q11.2DS (p > .05, ). Mean platelet volumes were higher in patients with 22q11.2DS versus control patients, regardless of bleeding status, and were highest in patients with 22q11.2DS and bleeding events (p < .05, ). Irrespective of deletion status, patients with bleeding events were likely to have a longer PTT than those without bleeding (p < .05, ). Only a few patients were on anticoagulant or antiplatelet therapies prior to the procedure, but this did not appear to impact procedural bleeding (p = .23, ).
Figure 1. Bleeding risk and platelet counts for patients with and without 22q11.2DS. Orange denotes patients with the deletion and blue denotes patients without the deletion, cross-hatch denotes bleeding. Boxes demonstrate mean with min/max. (A) Overall incidence of bleeding (p = .86). (B) incidence of bleeding by STAT procedure risk category. (C) Platelet counts by surgical procedure.
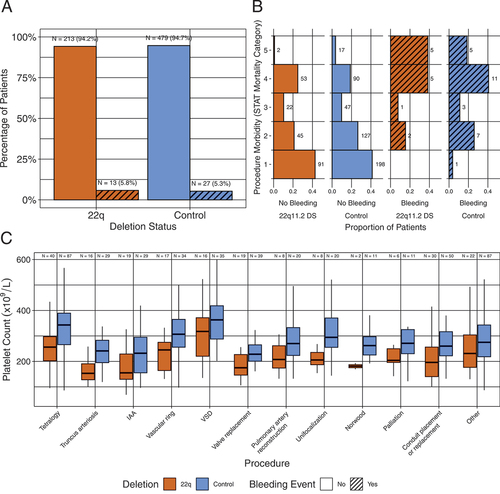
Figure 2. Platelet counts and volumes. Boxes show median and IQR, overlying circles represent individual patient data points. (A) Platelet counts by cohort. (B) Mean platelet volume by cohort. Bars show p values from two-way ANOVA with Tukey HSD correction for multiple comparisons.
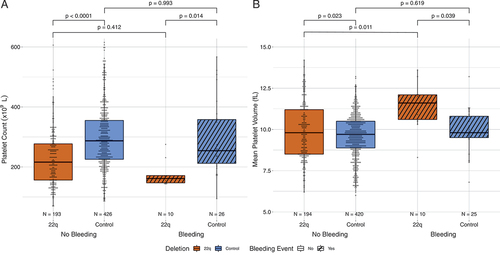
Table II. Risk of bleeding by surgical procedure.
Transfusion requirements
Deleted patients who had bleeding required more PRBC transfusions and had more donor exposures than control patients with bleeding (). Patients with 22q11.2DS who did not have a clinically significant bleeding episode also required more PRBC transfusions and had more donor exposures than those patients without 22q11.2DS (p < .001, ). Despite disparate platelet counts, platelet transfusion volumes were similar in patients with and without bleeding, irrespective of deletion status (p < .001, ) while volumes of transfused CP and FFP were increased in bleeding patients (data not shown).
Figure 3. Transfusion requirements. Orange denotes patients with the deletion and blue denotes patients without the deletion, cross-hatch denotes bleeding. (A) Histogram of PRBC transfusion events in the patient cohorts with and without bleeding. (B) Number of transfusion donor exposures. (C) Platelet transfusion volume in mL/kg. Bars show p values from pairwise Wilcoxon signed rank tests corrected for multiple comparisons.
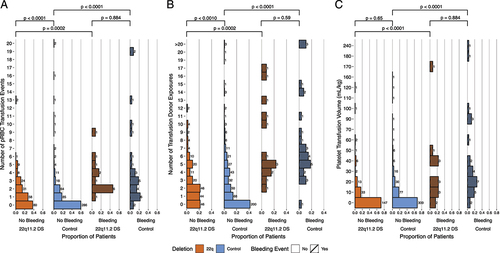
Patients with bleeding were likely to have a longer PTT than those without bleeding (irrespective of deletion status) (, p < .05). There were not many patients on anticoagulation or antiplatelet therapies prior to the procedure, but with the limited number of patients, this did not appear to impact procedural bleeding (, p = .23).
Figure 4. Additional factors influencing bleeding risk. Orange denotes patients with the deletion and blue denotes patients without the deletion, cross-hatch denotes bleeding. (A) Relationship of PTT (seconds) and bleeding. Bars show p values calculated by two-way ANOVA with Tukey HSD correction for multiple comparisons. (B) Number of patients with bleeding who received pre-operative anticoagulation or antiplatelet therapy was not different compared to those without bleeding (p = .213, chi-squared test).
Multivariate analysis
When controlling for age, biological sex, procedure risk category, platelet count, platelet transfusions, anticoagulation/antiplatelet therapy, and deletion status, only procedure category was associated with an increased risk of bleeding (, ).
Figure 5. Multivariate analysis of factors influencing bleeding in patients undergoing cardiac surgery. Dotted line represents neutral. Diamond represents odds ratio (OR) with line representing 95% confidence interval.
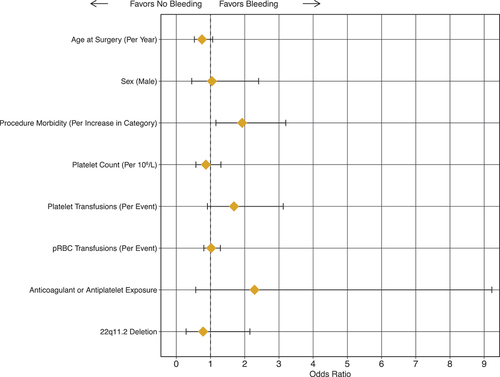
Table III. Multivariate analysis.
Discussion
Bleeding risk in cardiac surgery is highly dependent on the surgical procedureCitation8 and congenital heart lesion.Citation9 Blood product transfusion in the perioperative period, especially in neonates and infants undergoing high-risk operations, has been previously associated with increased risk of thrombosis developmentCitation10 especially when no intraoperative monitoring of coagulation is performed.Citation11 In addition, excessive PRBC transfusion in the during surgery is also associated with excess post-operative bleeding and worse post-operative outcomes.Citation12 Therefore, efforts to minimize bleeding risk and/or identify high-risk patients a priori to develop preventative strategies will impact patient outcomes, in addition to making blood products more available by optimizing use.
Prior studies have suggested that patients with 22q11.2DS have an increased risk of bleeding when undergoing cardiac surgery.Citation6 Other studies have suggested that patients with 22q11.2DS have an underlying bleeding diathesis,Citation13,Citation14 but the nature of underlying reasons for increased bleeding have not been elucidated as patients typically have normal platelet function and coagulation studies.Citation15 Understanding the factors that influence the intra-operative coagulation balance is important in order to reduce post-operative bleeding and transfusions and thus improve patient outcomes.
Our data is consistent with previously published reports, demonstrating an increase in PRBC transfusions. However, after controlling for multiple factors, procedure risk category and not deletion status was associated with an increased bleeding risk.
In previous reports, immediate post-operative fibrinogen and PTT were associated with increased risk of bleeding.Citation16 We found that pre-procedurally neither PT nor PTT values correlated with transfusion requirements and an elevated pre-procedural PTT was associated with increased risk of bleeding, independent of deletion status. Although the coagulation testing data was incomplete for the cohort (i.e. not all patients had pre-operative PT/PTT), this suggests that in patients undergoing cardiac surgery, one could consider pre-operative PTT elevation among risk factors for bleeding and patients may benefit from assessment in the appropriate clinical scenarios.
Immunodeficiency affects up to 75% of 22q11.2DS patients,Citation17,Citation18 which may predispose to development of autoimmune cytopenias,Citation18,Citation19 and some reports have suggested lower baseline platelet countsCitation18 and Bernard Soulier Syndrome, a severe autosomal recessive platelet disorder due to heterozygous mutations/CNVs involving the GPIBB gene located within the 22q11.2 region, in patients with 22q11.2DS.Citation18,Citation20 Given the involvement of the critical platelet gene GPIBB in the classical deletion,Citation4 a predisposition to bleeding would not be surprising and there have been reports of increased transfusion needs in the setting of cardiac surgeryCitation6 and report of cerebral microbleeds in individuals with 22q11.2DS.Citation14 Evidence suggests that while deletion status is associated with lower platelet counts,Citation21 most patients do not have an identified bleeding diathesis and recent reports examining bleeding risk in other surgical settings have suggested patients with 22q11.2DS do not have elevated risk compared to non-deleted counterparts.Citation22
Limitations of this study include that it is a single center study which may limit generalizability. However, it describes a relatively large cohort of patients with 22q11.2DS and our control population includes many patients with complex congenital heart disease of various etiologies. Additionally, pre-operative PT/PTT were not available for all patients, which may confound interpretation of other factors influencing bleeding risk. However, both cohorts were affected by the missing data; thus, it is unlikely to have significantly impacted the conclusions. Finally, while the cause of thrombocytopenia in 22q11.2DS appears to be primarily related to deletional status and loss of the LCR22A-LCR22B segment (PMID: 30675864), acquisition of additional bleeding factors such as acquired von Willebrand disease or concomitantly inherited platelet disorders were not systematically evaluated for each patient.
As with all retrospective studies, some data is not available for all patients, and these data and the conclusions suggesting that procedure more than deletion status determines bleeding risk for cardiac surgery in patient patients with the 22q11.2DS will need to be confirmed with additional studies. However, in this large cohort of patients with 22q11.2DS, deletion status is not an independent driver of bleeding outcomes in cardiac surgery, thus these patients should be screened for standard risk factors for bleeding. Further study is needed to understand the impact of 22q11.2DS on cardiac surgical outcomes and inform guidelines for perioperative transfusion.
Acknowledgments
The authors gratefully acknowledge funding from the following sources: The Children’s Hospital of Philadelphia. We would also like to thank the patients and families for their participation and the CHOP 22q and You Center and the Cardiac Center for their support to collect and analyze the data.
Disclosure statement
MPL is an advisory board member for Octapharma, Dova, Principia, Rigel and Shionogi, a consultant for Novartis, Shionogi, Dova, Principia, Argenx, Rigel, Sobi, Sanofi and Janssen, and has received research funding from Sysmex, Novartis, Rigel, Principia, Argenx, Dova, Octapharma.
All other authors report no relevant or potential conflict of interest.
Additional information
Funding
References
- Botto LD, May K, Fernhoff PM, Correa A, Coleman K, Rasmussen SA, Merritt RK, O’Leary LA, Wong L-Y, Elixson EM, et al. A population-based study of the 22q11.2 deletion: phenotype, incidence, and contribution to major birth defects in the population. Pediatrics. 2003 Jul;112(1):101–7. doi:10.1542/peds.112.1.101.
- Tezenas Du Montcel S, Mendizabai H, Ayme S, Levy A, Philip N. Prevalence of 22q11 microdeletion. J Med Genet. 1996 Aug;33(8):719. doi:10.1136/jmg.33.8.719.
- Blagojevic C, Heung T, Theriault M, Tomita-Mitchell A, Chakraborty P, Kernohan K, Bulman DE, Bassett AS. Estimate of the contemporary live-birth prevalence of recurrent 22q11.2 deletions: a cross-sectional analysis from population-based newborn screening. CMAJ Open. 2021 Jul-Sep;9(3):E802–E809. doi:10.9778/cmajo.20200294.
- McDonald-McGinn DM, Sullivan KE, Marino B, Philip N, Swillen A, Vorstman JAS, Zackai EH, Emanuel BS, Vermeesch JR, Morrow BE, et al. 22q11.2 deletion syndrome. Nat Rev Dis Primers. Nov 19 2015;1(1):15071. doi:10.1038/nrdp.2015.71.
- Cirillo A, Lioncino M, Maratea A, Passariello A, Fusco A, Fratta F, Monda E, Caiazza M, Signore G, Esposito A, et al. Clinical manifestations of 22q11.2 deletion syndrome. Heart Fail Clin. 2022 Jan;18(1):155–64. doi:10.1016/j.hfc.2021.07.009.
- Brenner MK, Clarke S, Mahnke DK, Simpson P, Bercovitz RS, Tomita-Mitchell A, Mitchell ME, Newman DK. Effect of 22q11.2 deletion on bleeding and transfusion utilization in children with congenital heart disease undergoing cardiac surgery. Pediatr Res. 2016 Feb;79(2):318–24. doi:10.1038/pr.2015.216.
- O’Brien SM, Jacobs JP, Pasquali SK, Gaynor JW, Karamlou T, Welke KF, Filardo G, Han JM, Kim S, Shahian DM, et al. The society of thoracic surgeons congenital heart surgery database mortality risk model: part 1—statistical methodology. Ann Thorac Surg. 2015 Sep;100(3):1054–62. doi:10.1016/j.athoracsur.2015.07.014.
- Butt W. Bleeding after cardiac surgery: multiple strategies and teamwork are essential! Perfusion. 2019 Nov;34(8):637–9. doi:10.1177/0267659119867196.
- Willems A, Patte P, De Groote F, Van der Linden P. Cyanotic heart disease is an independent predicting factor for fresh frozen plasma and platelet transfusion after cardiac surgery. Transfus Apher Sci. 2019 Jun;58(3):304–9. doi:10.1016/j.transci.2019.03.014.
- Ali U, Goldenberg N, Foreman C, Lynn LC, Honjo O., O’Leary J, Faraoni D. Association between cyanosis, transfusion, and thrombotic complications in neonates and children undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2020 Feb;34(2):349–355. doi:10.1053/j.jvca.2019.07.123.
- Faraoni D, Emani S, Halpin E, Bernier R, Emani SM, DiNardo JA, Ibla JC. Relationship between transfusion of blood products and the incidence of thrombotic complications in neonates and infants undergoing cardiac surgery. J Cardiothorac Vasc Anesth. 2017 Dec;31(6):1943–1948. doi:10.1053/j.jvca.2017.04.039.
- Agarwal HS, Barrett SS, Barry K, Xu M, Saville BR, Donahue BS, Harris ZL, Bichell DP. Association of blood products administration during cardiopulmonary bypass and excessive post-operative bleeding in pediatric cardiac surgery. Pediatr Cardiol. 2015 Mar;36(3):459–67. doi:10.1007/s00246-014-1034-z.
- Patel PO, Baylis AL, Hickey SE, Stanek J, Kirschner RE, Rand ML, Kumar R. Bleeding severity and phenotype in 22q11.2 deletion syndrome—a cross-sectional investigation. J Pediatr. 2021 Aug;235:220–225. doi:10.1016/j.jpeds.2021.03.071.
- Bonati MT, Vanelli C, Sangalli D, Sina C, Giardino D, Sassone J, Girotti F, Silani V, Ciammola A. Cerebral microbleeds: a new presenting feature of chromosome 22q11.2 deletion syndrome. J Neurol Sci. 2016 Sep 15; 368:300–3. doi:10.1016/j.jns.2016.07.044.
- Rosa RF, Rosa RC, Dos Santos PP, Zen PR, Paskulin GA. Hematological abnormalities and 22q11.2 deletion syndrome. Rev Bras Hematol Hemoter. 2011;33(2):151–4. doi:10.5581/1516-8484.20110037.
- Pekelharing J, Furck A, Banya W, Macrae D, Davidson SJ. Comparison between thromboelastography and conventional coagulation tests after cardiopulmonary bypass surgery in the paediatric intensive care unit. Int J Lab Hematol. 2014 Aug;36(4):465–71. doi:10.1111/ijlh.12171.
- Jyonouchi S, McDonald-McGinn DM, Bale S, Zackai EH, Sullivan KE. CHARGE (coloboma, heart defect, atresia choanae, retarded growth and development, genital hypoplasia, ear anomalies/deafness) syndrome and chromosome 22q11.2 deletion syndrome: a comparison of immunologic and nonimmunologic phenotypic features. Pediatrics. 2009 May;123(5):e871–7. doi:10.1542/peds.2008-3400.
- Lawrence S, McDonald-McGinn DM, Zackai E, Sullivan KE. Thrombocytopenia in patients with chromosome 22q11.2 deletion syndrome. J Pediatr. 2003 Aug;143(2):277–8. doi:10.1067/S0022-3476(03)00248-8.
- Kratz CP, Niehues T, Lyding S, Heusch A, Janssen G, Gobel U. Evans syndrome in a patient with chromosome 22q11.2 deletion syndrome: a case report. Pediatr Hematol Oncol. 2003 Mar;20(2):167–72. doi:10.1080/0880010390158685.
- Budarf ML, Konkle BA, Ludlow LB, Michaud D, Li M, Yamashiro DJ, McDonald-McGinn D, Zackai EH, Driscoll DA. Identification of a patient with Bernard-Soulier syndrome and a deletion in the DiGeorge/velo-cardio-facial chromosomal region in 22q11.2. Hum Mol Genet. 1995 Apr;4(4):763–6. doi:10.1093/hmg/4.4.763.
- Campbell IM, Crowley TB, Jobaliya C, Bailey A, McGinn DE, Gaiser K, Bassett A, Gur RE, Morrow B, Emanuel BS, et al. Platelet findings in 22q11.2 deletion syndrome correlate with disease manifestations but do not correlate with GPIb surface expression. Clin Genet. 2023 Jan;103(1):109–13. doi:10.1111/cge.14227.
- Arganbright JM, Hankey PB, Tracy M, Narayanan S, Noel-MacDonnell J, Ingram D. Tonsillectomy in children with 22q11.2 deletion syndrome. Genes (Basel). 2022 Nov 23; 13(12):2187. doi:10.3390/genes13122187.
