Abstract
Background
Platelet-rich plasma (PRP) is a therapeutic approach that is gaining attention for its potential in the treatment of poor ovarian response. This meta-analysis aimed to systematically review and analyze clinical studies to evaluate the impact of PRP on poor responders undergoing ovarian stimulation for IVF.
Methods
A comprehensive search was conducted in electronic databases, including PubMed, Embase, Scopus, Web of Science, and the Cochrane Library to identify relevant studies published in English. The pooled data, such as pregnancy outcome, number of MII oocytes, number of transferable embryos, and ovarian reserve markers were analyzed using R version 4.2.3.
Results
A total of 10 trials were enrolled in the present meta-analysis. Following PRP treatment, live birth rate was found to be 16.6% (95% CI 8.8%-26.1%), while clinical pregnancy rate was observed to be 25.4% (95% CI 13.1%-39.9%). PRP pretreatment resulted in a higher number of MII oocytes (MD 1.073, 95% CI 0.720 to 1.427), a higher number of embryos (MD 0.946, 95% CI 0.569 to 1.323), a higher antral follicle count (MD 1.117; 95% CI 0.689 to 1.544), and the change of hormone levels.
Conclusions
Among the studies evaluated in this review, PRP showed promising results in poor responder. Further research is required to clarify the potential role of PRP in female reproductive health.
Plain Language Summary
What is the context?
The incidence of poor ovarian response following ovarian stimulation ranges globally from 5.6% to 35.1%.
Although various interventions have been implemented in patients with POR, there is a lack of empirical evidence demonstrating the superiority of any of these therapies over one another.
Platelet-rich plasma, which is rich in growth factors that have been implicated in cellular growth, differentiation, angiogenesis, and tissue repair, is emerging as a promising therapeutic modality.
Limited data determines the viability of PRP as an alternative therapy for POR patients, but further evidence is needed to quantify this effect.
What is new?
To the best of our knowledge, this is the first systematic review and meta-analysis that investigated the efficacy of PRP on women with POR, including ten trials and 876 patients.
This review provides a comprehensive overview of the existing evidence on the utilization of PRP in poor responders, while also emphasizing the primary limitations in the literature and the necessity for future research based on evidence.
What is the impact?
Among the studies evaluated in this review, PRP showed a potential positive impact on the regulation of sex hormone levels, ovarian response, and pregnancy outcomes.
Introduction
Controlled ovarian stimulation (COS) is a crucial component of in vitro fertilization (IVF) therapy, which seeks to generate an adequate number of follicles and oocytes to achieve clinical pregnancy.Citation1,Citation2 Despite the successful resolution of numerous cases of infertility through assisted reproductive technology (ART), reproductive clinicians continue to face the daunting challenge of poor ovarian response (POR) during COS.Citation3
The incidence of POR following ovarian stimulation exhibits a global range of 5.6% to 35.1%.Citation4 In individuals experiencing POR, there is a notable decrease in ovulation and fertility outcomes, which can be attributed to factors such as increased age or diminished ovarian reserve.Citation5 Ovarian reserve, serving as an indicator of ovarian function, primarily reflects the quantity and quality of eggs available.Citation6 It is worth noting that the presence of low-quality eggs can significantly contribute to female infertility and pose a significant challenge in the context of in vitro fertilization.Citation7 Different interventions have been implemented in patients with POR, such as various stimulation protocols and adjuvant therapies to enhance rates of ovarian response and achieve clinical pregnancy.Citation8,Citation9 Regrettably, there is a lack of empirical evidence demonstrating the superiority of any of these therapeutics over one another.
Platelet-rich plasma (PRP) is emerging as a promising therapeutic modality that shows promise in the treatment of refractory conditions.Citation10,Citation11 PRP is rich in growth factors that have been implicated in cellular growth, differentiation, angiogenesis, and tissue repair.Citation12 The administration of PRP into the ovaries is thought to stimulate the activation of potential ovarian stem cells, resulting in the secretion of factors that facilitate follicular growth and development.Citation13 Furthermore, PRP may augment ovarian blood flow through the promotion of angiogenesis, thereby improving the delivery of oxygen and nutrients to developing follicles.Citation12,Citation14
Although there have been reviews of PRP in patients with POR,Citation15 the effectiveness of PRP has been questioned due to a broad range of study subjects, a small number of studies, and few outcome measures. Therefore, a systematic assessment is necessary to determine the viability of PRP as an alternative therapy for POR patients. This meta-analysis aimed to systematically review and analyze clinical studies to evaluate the impact of PRP on poor responders undergoing ovarian stimulation for IVF.
Methods
Search strategy and selection criteria
The present systematic review and meta-analysis was registered in the PROSPERO International Prospective Register of Systematic Reviews (CRD42023424117).
This systematic review adhered to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines and the PRISMA-IPD Statement.Citation16 A comprehensive search of PubMed, EMBASE, Scopus, Web of Science, and the Cochrane Library was conducted in May 2023. No time constraints and any filters were used for the search. The search terms used were “Ovulation Induction OR Ovarian Stimulation OR Poor Ovarian Response OR Poor Responder OR POR” AND “Platelet Rich Plasma OR Plasma, Platelet-Rich OR PRP.”
Two authors (LLW; FFS) independently performed the database search, and after removing duplicates, all records were screened based on titles and abstracts. Full texts were subsequently accessed for records of interest. Disagreements between the reviewers were solved by a third author (PXL).
Eligibility criteria
The study selection criteria were as follows:
Studies published in the English language;
Administration of PRP in the intervention;
Inclusion of women who were classified as poor ovarian responders;
Inclusion of women undergoing IVF or ICSI, with any ovarian stimulation protocol;
The studies needed to report at least one of the following outcomes: a. Primary outcomes: live birth rate, clinical pregnancy rate; b. Secondary outcomes: number of MII oocytes formed, number of transferable embryos, ovarian reserve markers.
Secondary studies such as systematic reviews and meta-analyses were excluded from the analysis, and studies that involved the administration of an additional drug in conjunction with PRP were also excluded.
Data extraction and quality assessment
After a comprehensive assessment of the eligibility of all identified citations and data extraction from original trial reports, we used a specifically designed form that captured information on first author, publication year, country, participants, interventions, time of PRP infusion and outcome. Two reviewers (LLW and FFS) selected the studies independently and extracted data for each study; disagreements between the two reviewers were resolved by a third reviewer (PXL) to reach consensus.
Articles were assessed for the risk of bias using the ROBINS-I of the Cochrane Collaboration.Citation17 This tool assigns a rating of low, moderate, serious, or critical risk of bias to each item assessed.
Statistical analysis
The statistical analysis was performed using R version 4.2.3 to create forest plots and risk of bias summaries. Dichotomous outcomes (clinical pregnancy) and continuous data (MII oocyte yield, embryos yield, and ovarian reserve markers) were reported as proportion and mean difference (MD), respectively, with 95% confidence intervals (CI). Heterogeneity was assessed using the Q test, tau-squared, and I2 index values. Random effect models were used due to significant clinical heterogeneity between studies. Publication bias was evaluated using funnel plots.
Result
Search result
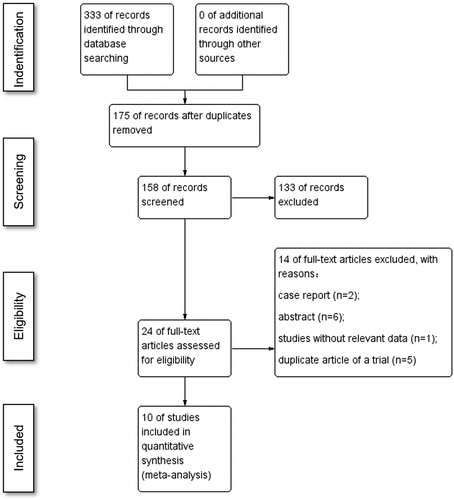
A total of 333 records were initially identified for review. Out of these, 175 studies were excluded due to duplication, and 133 records were excluded based on a preliminary assessment of their titles and abstracts. This left 24 studies for a more thorough evaluation. After applying the inclusion criteria, 14 studies were further removed from the analysis. These included two case reports, six conference abstracts, one study with irrelevant outcomes, and five duplicate articles of a trial. Ultimately, 10 cohort studiesCitation18–27 were deemed suitable for inclusion in the analysis. Among these 10 trials, one study compared a group treated with PRP to an untreated control group, while the remaining nine trials were before-after studies. The process of selecting and extracting the relevant studies is depicted in .
Characteristics and quality of the included studies
The ten included studies enrolled 876 participants. The sample size varied across the trials and ranged from 12 to 510 participants. provides an overview of the characteristics of the included trials, including information such as the first author, publication year, country of origin, number of participants, interventions used, timing of platelet-rich plasma (PRP) infusion, and all reported outcomes.
Table I. Characteristics of included studies.
Risk of bias in the included trials is represented in Supplementary Table S1. In general, the quality of the included studies was considered to have low to moderate risk of bias. One study did not adhere to the Bologna or POSEIDON criteria when selecting participants. Additionally, two studies had a small amount of missing data in their outcome assessment, which could introduce bias. Furthermore, three studies were found to have a moderate risk of bias due to potential confounding in their study design.
Results of the meta-analysis
Live birth rate
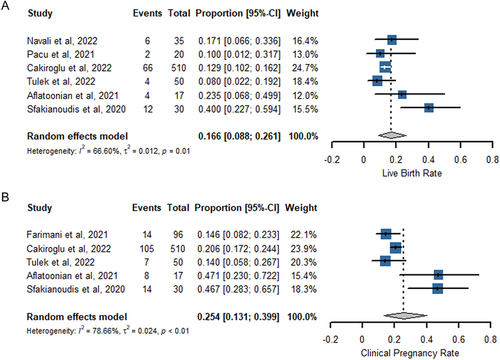
Six studies provided data on the rate of clinical pregnancy, as shown in . The studies exhibited significant heterogeneity (I2 = 66.60%; P = .01), necessitating the use of a random effects model to combine the findings. By pooling the results of five studies involving a total of 662 participants, it was determined that the live birth rate was 16.6% (95% CI 8.8–26.1%).
Additionally, a subgroup analysis (Supplementary Figure S1A,B) was conducted to evaluate the impact of activators of PRP and the quantity of PRP injection. The live birth rate was 33.4% in the platelet-activated group and 12.0% in the non-activated group. The analysis revealed significant differences between the two groups, with and without activators in the PRP preparation process (P < .01). However, the results of subgroup analysis based on PRP injection volume were not statistically significant (P = .59).
Clinical pregnancy rate
Five studies reported data on the rate of clinical pregnancy (). There was significant heterogeneity between studies (I2 = 78.66%; P < .01), thus a random effects model was employed to combine the results. Pooling the results of the five studies (n = 703) showed clinical pregnancy rate was achieved at 25.4% (95% CI 13.1–39.9%).
Subgroup analysis of clinical pregnancy rate was shown in supplemental materials . Marked declines in heterogeneity were observed when we conducted subgroup analysis for platelet activation or non-activation. The clinical pregnancy rate was 46.8% in the platelet-activated group and 17.9% in the non-activated group (P < .01). However, the results of subgroup analysis by PRP injection volume were not statistically significant (P = .56).
Number of MII oocytes
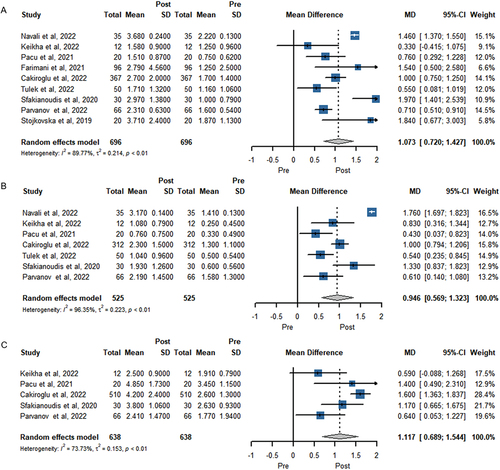
With regard to the number of MII stage oocytes as a secondary outcome, 9 studies with 696 women were included in the meta-analysis (). PRP treatments led to the highest number of MII oocytes (MD 1.073, 95% CI 0.720 to 1.427, P < .001; n = 9).
Number of embryos formed
In seven trials involving a total of 525 women, the number of embryos formed was assessed (). The meta-analysis conducted using a random-effects model revealed a statistically significant difference in post-PRP treatment (MD 0.946, 95% CI 0.569 to 1.323, P < .001; n = 7).
Antral follicle count
Pooling of results of 638 women from 5 studies that reported antral follicle count before and after PRP treatment show a significant difference in (MD 1.117; 95% CI 0.689 to 1.544, P < .001; n = 5).
Antimüllerian hormone
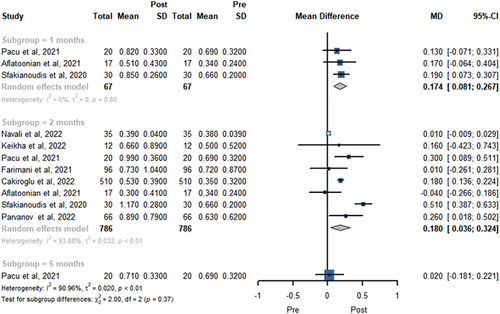
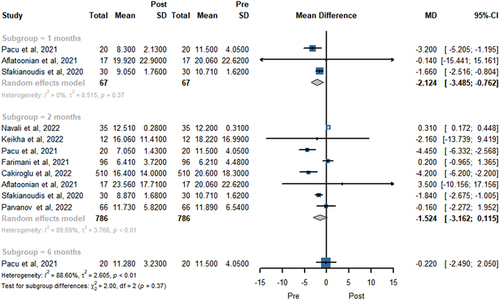
AMH level was reported in 8 trials involving 786 patients in . A total of three, eight, and one study showed the FSH level at 1, 2, and 6 months, respectively. The pooled results revealed PRP treatment provided a higher AMH level at 1 (MD = 0.174, 95% CI 0.081 to 0.267, P < .01) and 2 months (MD 0.180; 95% CI 0.036 to 0.324, P = .014). There was no statistically significant difference in AMH level change at 6 months (MD 0.020; 95% CI −0.181 to 0.221, P = 0.846).
Follicle-stimulating hormone
The meta-analysis results of FSH level at 1, 2, and 6 months after PRP treatment were showed in . A total of three, eight, and one study showed the FSH level at 1, 2, and 6 months, respectively. The pooled data indicated that PRP treatment was associated with a decrease of the FSH level at 1 month (MD −2.124; 95% CI −3.485 to −0.762, P < .01). Nevertheless, there was no statistically significant difference in FSH level change at 2 months (MD −1.524; 95% CI −3.162 to 0.115, P = .068) and 6 months (MD = −0.220, 95% CI − 2.490 to 2.050, P = .849).
Luteinizing hormone
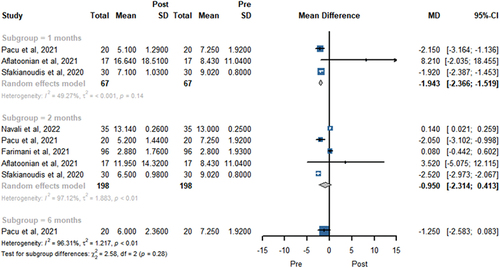
presents the LH level in the random-effects model before and after PRP treatment. A total of three, five, and one study showed the LH level at 1, 2, and 6 months, respectively. PRP injection showed lower LH level at 1 months (MD −1.943; 95% CI −2.366 to −1.519, P < .001). However, there was no significant difference in LH level change at 2 months (MD −0.950; 95% CI −2.314 to 0.413, P = .1718) and 6 months (MD −1.250; 95% CI −2.583 to 0.083, P = .066).
Discussion
Main findings
While assisted reproduction techniques have made significant advancements in achieving successful pregnancies and live births, poor responders constitute an ongoing challenge for assisted reproduction experts. To the best of our knowledge, this is the first systematic review and meta-analysis that investigated the efficacy of PRP on women with POR. Applying strict inclusion criteria, studies with co-intervention or those available only in abstract form were excluded, including ten trials and 876 patients. In this review, we evaluated the effects of autologous PRP on live birth rate, Clinical pregnancy rate, number of MII oocytes, number of embryos, AFC, AMH, FSH, and LH levels. This valuable information provides a reference for clinical applications, suggesting that PRP may be considered as a potential treatment option for women with POR.
Comparison with existing literature
The real effectiveness of PRP for poor responder still represents a popular topic for a debate. In a case series study of 19 women, Farimani et al. first reported live births following intrauterine PRP injections in poor responder.Citation28 The study was later expanded to include more cases, which were strictly classified according to POSEIDON Group 1–4 criteria.Citation21 Significant differences were found in the number of fetuses and clinical pregnancies among the groups, with POSEIDON Group 1 showing the highest number. Another study by Sfakianoudis et al. reported 12 live births out of 30 women with POR, all of whom had experienced at least one failed IVF cycle.Citation25 Additionally, a large case series study by Cakiroglu et al. reported that 66 out of 496 patients with POR achieved continuous implantation or live birth.Citation22 These trials were all included in our meta-analysis, which aimed to analyze pregnancy outcomes. The meta-analysis showed that patients with POR who underwent treatment with PRP achieved a live birth rate of 16.6% and a clinical pregnancy rate of 25.4%. While this result is encouraging, it is important to note that these studies have limitations in terms of their ability to identify improvements in pregnancy rates without the presence of rigorous control groups.
There is no consensus currently regarding the inclusion or exclusion of activators in the preparation of PRP.Citation29,Citation30 To address this issue, subgroup analyses were conducted comparing PRP activation and non-activation. The results revealed statistically significant differences in clinical pregnancy rates and live birth rates between the PRP-activated subgroup and the PRP-unactivated subgroup. This discrepancy may be attributed to the significant increase in the release of growth factors associated with PRP activation, which are believed to contribute to follicular development and the restoration of reproductive ability, as compared to PRP-unactivated subgroup.Citation31,Citation32 Despite the observed differences, it is not possible to make a definitive recommendation regarding the optimal type of PRP due to the limited availability of high-quality randomized controlled trials comparing PRP activation versus PRP non-activation.
PRP can differ based on the specific kit and regimen.Citation33 None of the included studies provided platelet concentrations, white blood cell counts, and growth factor concentrations. Consequently, it was not possible to standardize the PRP and make comparisons across the studies. Furthermore, in addition to the controlled variability between studies, the intrinsic variability of PRP itself cannot be disregarded. Some degree of heterogeneity may ultimately be unavoidable, although larger trials can help mitigate this limitation by offering a more comprehensive dataset.
Optimal timing for initiating IVF cycles after PRP injections is another subject of extensive research, as it is likely to impact the efficacy of PRP. Sills et al. conducted a study in which they measured serum AMH at two-week intervals for up to three months following intraovarian PRP injection.Citation34 They observed an improvement in serum AMH levels in 51 patients (28%), with a median increase of 167% after treatment [95% CI 91 to 280]. The peak improvement occurred four weeks after PRP treatment. One study reported that hormone levels undergo more noticeable changes during the second menstrual cycle following PRP treatment.Citation20 Tulek et al. observed that the highest levels of hormones were recorded between 60 and 90 days following the administration of ovarian PRP injection.Citation23 In this meta-analysis, statistically significant changes were observed in the levels of AMH (MD 0.174; 95% CI 0.081 to 0.267, p < .01), FSH (MD −2.124; 95% CI −3.485 to −0.762, p < .01), and LH (MD −1.943; 95% CI −2.366 to −1.519, p < .01) one month after the administration of PRP injection. Furthermore, two months after the PRP injection, AMH levels(MD 0.180; 95% CI 0.036 to 0.324, p < .05) remained at a high level. However, the hormone(MD 0.020; 95% CI −0.181 to 0.221) returned to pre-treatment levels by the sixth month. A more comprehensive monitoring of hormonal changes will aid in determining the optimal timing for PRP administration.
Potential mechanisms
The mechanism of autologous PRP treatment is complex and has not been fully elucidated.Citation14,Citation35 A vitro studies conducted by Hosseini et al. have demonstrated that the addition of PRP to culture media can enhance the survival and growth of isolated human precaval follicles. PRP contains a variety of growth factors that play a role in the recruitment, proliferation, and differentiation of stem cells, as well as the remodeling of the extracellular matrix.Citation36 Some studies propose that PRP may potentially activate dormant follicles, leading to their development and maturation, thereby augmenting the number of ovulating follicles.Citation14,Citation37 Additionally, PRP may stimulate ovarian stem cells to differentiate into new oocytes, potentially resulting in the production of high-quality oocytes, which may eventually produce high-quality oocytes.
PRP has the potential to enhance tissue functionality through the stimulation of angiogenesis. Callejo et al. were the pioneers in utilizing platelet-rich plasma (PRP) in the context of ovarian autologous transplantation.Citation38 In this application, PRP serves as an agent that promotes angiogenesis and cell proliferation, ultimately leading to successful live births. A study by Ahmadian et al. further highlighted the pro-angiogenic role of PRP.Citation39 In a rat model of premature ovarian failure, intraovarian injection of PRP resulted in enhanced expression of genes associated with angiogenesis and increased microvascular density. By identifying the sites of major angiogenesis following PRP treatment, it may be possible to target injections in these areas in the future to optimize treatment outcomes.
Limitations
Our study has several limitations that should be acknowledged: (1) there were variations in the diagnostic criteria for POR among the included studies, with three studies using the Bologna Consensus and six studies using the POSEIDON diagnostic, which may affect high heterogeneity. Therefore, it is recommended that future studies adopt uniform diagnostic criteria to minimize discrepancies between studies. (2) the preparation of PRP is not standardized and can vary in terms of pre-treatment testing, preparation method, injection technique, the quantity of PRP injected, and postoperative treatment and follow-up. This lack of standardization introduces potential sources of variability in the treatment outcomes.Citation40 (3) the application of PRP in the field of assisted reproductive technology is relatively new, resulting in a limited number of included studies. Therefore, due to the limitations of the existing literature, it is challenging to conduct a comprehensive analysis of the relationship between individual factors of concern and treatment outcomes. For example, the age variable in the majority of the selected articles was confined to a narrow range of 37–40 years, thereby posing minimal difficulty in conducting an in-depth analysis. Furthermore, most of the studies available are before-after trials, which may have inherent limitations in terms of study design and potential biases. Based on available data, any impact of publication bias on the final estimates could not be excluded.
Conclusion
This review provides a comprehensive overview of the existing evidence on the utilization of PRP in poor responders, while also emphasizing the primary limitations in the literature and the necessity for future research based on evidence. Although our findings suggest a potential positive impact of PRP on the regulation of sex hormone levels, ovarian response, and pregnancy outcomes, it is important to interpret these results cautiously due to the relatively low to moderate quality of the available evidence. Further research is required, specifically well-designed randomized controlled trials RCTs with sufficient sample sizes, that also incorporate safety data, to elucidate the role of PRP in female reproduction.
Supplemental Material
Download PDF (549 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/09537104.2023.2292612.
Additional information
Funding
References
- Racca A, Drakopoulos P, Neves AR, Polyzos NP. Current therapeutic options for controlled ovarian stimulation in assisted reproductive technology. Drugs. 2020;80(10):973–10. doi:10.1007/s40265-020-01324-w.
- Quaas AM, Legro RS. Pharmacology of medications used for ovarian stimulation. Best Pract Res Clin Endocrinol Metab. 2019;33(1):21–33. doi:10.1016/j.beem.2018.10.002.
- Zhang Y, Zhang C, Shu J, Guo J, Chang H-M, Leung PCK, Sheng J-Z, Huang H. Adjuvant treatment strategies in ovarian stimulation for poor responders undergoing IVF: a systematic review and network meta-analysis. Hum Reprod Update. 2020;26(2):247–263. doi:10.1093/humupd/dmz046.
- Giannelou P, Simopoulou M, Grigoriadis S, Makrakis E, Kontogeorgi A, Pantou A, Galatis D, Kalampokas T, Bakas P, Bolaris S, et al. The conundrum of poor ovarian response: from diagnosis to treatment. Diagnostics (Basel, Switzerland). 2020;10(9):687. doi:10.3390/diagnostics10090687.
- Devine K, Mumford SL, Wu M, DeCherney AH, Hill MJ, Propst A. Diminished ovarian reserve in the United States assisted reproductive technology population: diagnostic trends among 181,536 cycles from the society for assisted reproductive technology clinic outcomes reporting System. Fertil Steril. 2015;104(3):612–619.e613. doi:10.1016/j.fertnstert.2015.05.017.
- Pirtea P, Ayoubi JM. Diminished ovarian reserve and poor response to stimulation are not reliable markers for oocyte quality in young patients. Fertil Steril. 2020;114(1):67–8. doi:10.1016/j.fertnstert.2020.03.040.
- Glujovsky D, Quinteiro Retamar AM, Alvarez Sedo CR, Ciapponi A, Cornelisse S, Blake D. Cleavage-stage versus blastocyst-stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. 2022;2022(6):Cd002118. doi:10.1002/14651858.CD002118.pub6.
- Pantou A, Giannelou P, Grigoriadis S, Maziotis E, Tzonis P, Koutsouni A, Pappa C, Philippou A, Koutsilieris M, Pantos K, et al. Evaluating different strategies for poor ovarian response management: a retrospective cohort study and literature review. Ann NY Acad Sci. 2021;1500(1):93–111. doi:10.1111/nyas.14614.
- Labarta E. DuoStim: a new strategy proposed for women with poor ovarian response. Fertil Steril. 2020;113(1):76–7. doi:10.1016/j.fertnstert.2019.10.024.
- Everts P, Onishi K, Jayaram P, Lana JF, Mautner K. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020;21(20):7794. doi:10.3390/ijms21207794.
- Streit-Ciećkiewicz D, Kołodyńska A, Futyma-Gąbka K, Grzybowska ME, Gołacki J, Futyma K. Platelet rich plasma in gynecology—discovering undiscovered—review. Int J Environ Res Public Health. 2022;19(9):5284. doi:10.3390/ijerph19095284.
- Sills ES, Wood SH. Autologous activated platelet-rich plasma injection into adult human ovary tissue: molecular mechanism, analysis, and discussion of reproductive response. Biosci Rep. 2019;39(6). doi:10.1042/BSR20190805.
- Marchante M, Buigues A, Ramirez-Martin N, Martinez J, Pellicer N, Pellicer A, Herraiz S. Single intraovarian dose of stem cell– and platelet-secreted factors mitigates age-related ovarian infertility in a murine model. Am J Obstet Gynecol. 2023;228(5):561.e561–561.e517. doi:10.1016/j.ajog.2023.01.018.
- Seckin S, Ramadan H, Mouanness M, Kohansieh M, Merhi Z. Ovarian response to intraovarian platelet-rich plasma (PRP) administration: hypotheses and potential mechanisms of action. J Assist Reprod Genet. 2022;39(1):37–61. doi:10.1007/s10815-021-02385-w.
- Herlihy NS, Seli E. The use of intraovarian injection of autologous platelet rich plasma (PRP) in patients with poor ovarian response and premature ovarian insufficiency. Curr Opin Obstet Gynecol. 2022;34(3):133–7. doi:10.1097/GCO.0000000000000784.
- Stewart LA, Clarke M, Rovers M, Riley RD, Simmonds M, Stewart G, Tierney JF. Preferred reporting items for systematic review and meta-analyses of individual participant data: the PRISMA-IPD statement. JAMA. 2015;313(16):1657–1665. doi:10.1001/jama.2015.3656.
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Clinical Research Ed). 2016;355:i4919. doi:10.1136/bmj.i4919.
- Navali N, Sadeghi L, Farzadi L, Ghasemzadeh A, Hamdi K, Hakimi P, Niknafs B. Intraovarian injection of autologous platelet-rich plasma improves therapeutic approaches in the patients with poor ovarian response: a before-after study. Int J Fertil Steril. 2022;16(2):90–94. doi:10.22074/IJFS.2021.533576.1154.
- Keikha F, Shahsavari S, Salari Y, Roozbeh N, Haghollahi F, Tarazjani MD, Razavi M, Shariat M, Bagheri M. One side ovarian rejuvenation: a quasi-experimental study of the effect of the autologous platelet rich plasma in poor ovarian responders in IVF. Ethiop J Health Sci. 2022;32(6):1133–1140. doi:10.4314/ejhs.v32i6.10.
- Pacu I, Zygouropoulos N, Dimitriu M, Rosu G, Ionescu CA. Use of platelet-rich plasma in the treatment of infertility in poor responders in assisted human reproduction procedures. Exp Ther Med. 2021;22(6):1412. doi:10.3892/etm.2021.10848.
- Farimani M, Nazari A, Mohammadi S, Anvari Aliabad R. Evaluation of intra-ovarian platelet-rich plasma administration on oocytes-dependent variables in patients with poor ovarian response: a retrospective study according to the POSEIDON criteria. Reprod Biol Endocrinol. 2021;19(1):137. doi:10.1186/s12958-021-00826-w.
- Cakiroglu Y, Yuceturk A, Karaosmanoglu O, Kopuk SY, Korun ZEU, Herlihy N, Scott RT, Tiras B, Seli E. Ovarian reserve parameters and IVF outcomes in 510 women with poor ovarian response (POR) treated with intraovarian injection of autologous platelet rich plasma (PRP). Aging. 2022;14(6):2513–2523. doi:10.18632/aging.203972.
- Tülek F, Kahraman A. The effects of intra-ovarian autologous platelet rich plasma injection on IVF outcomes of poor responder women and women with premature ovarian insufficiency. J Turkish German Gynecol Assoc. 2022;23(1):14–21. doi:10.4274/jtgga.galenos.2021.2021.0134.
- Aflatoonian A, Lotfi M, Saeed L, Tabibnejad N. Effects of intraovarian injection of autologous platelet-rich plasma on ovarian rejuvenation in poor responders and women with primary ovarian insufficiency. Reprod Sci (Thousand Oaks, Calif). 2021;28(7):2050–2059. doi:10.1007/s43032-021-00483-9.
- Sfakianoudis K, Simopoulou M, Grigoriadis S, Pantou A, Tsioulou P, Maziotis E, Rapani A, Giannelou P, Nitsos N, Kokkali G, et al. Reactivating ovarian function through autologous platelet-rich plasma intraovarian infusion: Pilot data on premature ovarian insufficiency, perimenopausal, menopausal, and poor responder women. J Clin Med. 2020;9(6):1809. doi:10.3390/jcm9061809.
- Parvanov D, Ganeva R, Vidolova N, Nikolova K, Vasileva M, Totev T, Stamenov G. Autologous ovarian platelet rich plasma treatment improves oocyte and embryo quality: a before-after prospective study. Biotechnol Biotechnol Equip. 2022;36(1):425–32. doi:10.1080/13102818.2022.2090280.
- Stojkovska S, Dimitrov G, Stamenkovska N, Hadzi-Lega M, Petanovski Z. Live birth rates in poor responders’ group after previous treatment with autologous platelet-rich plasma and low dose ovarian stimulation compared with poor responders used only low dose ovarian stimulation before in vitro fertilization. Open Access Maced J Med Sci. 2019;7(19):3184–8. doi:10.3889/oamjms.2019.825.
- Farimani M, Heshmati S, Poorolajal J, Bahmanzadeh M. A report on three live births in women with poor ovarian response following intra-ovarian injection of platelet-rich plasma (PRP). Mol Biol Rep. 2019;46(2):1611–16. doi:10.1007/s11033-019-04609-w.
- Garner AL, Frelinger AL 3rd, Gerrits AJ, Gremmel T, Forde EE, Carmichael SL, Michelson AD, Neculaes VB. Using extracellular calcium concentration and electric pulse conditions to tune platelet-rich plasma growth factor release and clotting. Med Hypotheses. 2019 Apr;125:100–105. doi:10.1016/j.mehy.2019.02.036.
- Irmak G, Demirtaş TT, Gümüşderelioğlu M. Sustained release of growth factors from photoactivated platelet rich plasma (PRP). Eur J Pharm Biopharm. 2020;148:67–76. doi:10.1016/j.ejpb.2019.11.011.
- Martineau I, Lacoste E, Fau - Gagnon G, Gagnon G. Effects of calcium and thrombin on growth factor release from platelet concentrates: kinetics and regulation of endothelial cell proliferation. Biomaterials. 2004 Aug;25(18):4489–502. doi:10.1016/j.biomaterials.2003.11.013.
- Sills ES, Wood SH, Walsh APH. Intraovarian condensed platelet cytokines for infertility and menopause-mirage or miracle? Biochimie. 2023 Jan;204:41–47. doi:10.1016/j.biochi.2022.08.020.
- Fadadu P-O, Mazzola AJ, Hunter CW, Davis TT. Review of concentration yields in commercially available platelet-rich plasma (PRP) systems: a call for PRP standardization. Reg Anesth Pain Med. 2019 Apr;16(1532–8651). doi:10.1136/rapm-2018-100356.
- Sills ES, Rickers N, Wood S. Regenerative effect of intraovarian injection of activated autologous platelet rich plasma: serum anti-mullerian hormone levels measured among poor prognosis IVF patients. Int J Regenr Med. 2020;2613–5914:1–5. doi:10.31487/j.RGM.2020.01.02.
- Atkinson L, Martin F, Sturmey RG. Intraovarian injection of platelet-rich plasma in assisted reproduction: too much too soon? Hum Reprod (Oxford, England). 2021;36(7):1737–50. doi:10.1093/humrep/deab106.
- Hsu CC, Hsu I, Hsu L, Chiu YJ, Dorjee S. Resumed ovarian function and pregnancy in early menopausal women by whole dimension subcortical ovarian administration of platelet-rich plasma and gonadotropins. Menopause (New York, NY). 2021;28(6):660–6. doi:10.1097/GME.0000000000001746.
- Sharara FI, Lelea LL, Rahman S, Klebanoff JS, Moawad GN. A narrative review of platelet-rich plasma (PRP) in reproductive medicine. J Assist Reprod Genet. 2021;38(5):1003–12. doi:10.1007/s10815-021-02146-9.
- Callejo J, Salvador C, González-Nuñez S, Almeida L, Rodriguez L, Marqués L, Valls A, Lailla JM. Live birth in a woman without ovaries after autograft of frozen-thawed ovarian tissue combined with growth factors. J Ovarian Res. 2013;6(1):33. doi:10.1186/1757-2215-6-33.
- Ahmadian S, Sheshpari S, Pazhang M, Bedate AM, Beheshti R, Abbasi MM, Nouri M, Rahbarghazi R, Mahdipour M. Intra-ovarian injection of platelet-rich plasma into ovarian tissue promoted rejuvenation in the rat model of premature ovarian insufficiency and restored ovulation rate via angiogenesis modulation. Reprod Biol Endocrinol. 2020;18(1):78. doi:10.1186/s12958-020-00638-4.
- Abu-Musa A, Haahr T, Humaidan P. Novel physiology and definition of poor ovarian response; clinical recommendations. Int J Mol Sci. 2020;21(6):2110. doi:10.3390/ijms21062110.