Abstract
Horizontal platelet-rich fibrin (H-PRF) contains a variety of bioactive growth factors and cytokines that play a key role in the process of tissue healing and regeneration. The blood collection tubes used to produce Solid-PRF (plasmatrix (PM) tubes) have previously been shown to have a great impact on the morphology, strength and composition of the final H-PRF clot. Therefore, modification to PM tubes is an important step toward the future optimization of PRF. To this end, we innovatively modified the inner wall surface of the PM tubes with plasma and adjusted the gas environment inside the PM tubes to prepare super-hydrophilic anaerobic plasmatrix tubes (SHAP tubes). It was made anaerobic for the preparation of H-PRF with the aim of improving mechanical strength and bioactivity. The findings demonstrated that an anaerobic environment stimulated platelet activation within the PRF tubes. After compression, the prepared H-PRF membrane formed a fibrous cross-linked network with high fracture strength, ideal degradation characteristics, in addition to a significant increase in size. Thereafter, the H-PRF membranes prepared by the SHAP tubes significantly promoted collagen synthesis of gingival fibroblast and the mineralization of osteoblasts while maintaining excellent biocompatibility, and advantageous antibacterial properties. In conclusion, the newly modified PRF tubes had better platelet activation properties leading to better mechanical strength, a longer degradation period, and better regenerative properties in oral cell types including gingival fibroblast and alveolar osteoblasts. It also improves the success rate of H-PRF preparation in patients with coagulation dysfunction and expands the clinical application scenario.
Plain Language Summary
Why was the study done?
Direct anaerobic environment effects on fibrin formation have been insufficiently studied.
The effect of hydrophilic change caused by nitrogen plasma treatment on H-PRF coagulation has not been fully studied.
Optimal preparation of H-PRF in patients with poor coagulation function was needed in clinical application.
What is new?
The coagulation of H-PRF correlated with the level of dissolved oxygen concentrations. Anaerobic environment significantly accelerates fibrin formation and platelet activation.
Nitrogen plasma treatment can remarkably enhance the hydrophilicity of the inner surface of glass blood collecting tubes, thereby promoting the activation of platelets and the formation of fibrin network.
The H-PRF prepared in the tubes with anaerobic environment and hydrophilic surface showed high fracture strength, promoted collagen synthesis of gingival fibroblast and the mineralization of osteoblasts.
What is the impact?
The work is aimed at developing super-hydrophilic anaerobic plasmatrix tubes (SHAP tubes) for studying gas environment and hydrophilicity participation in fibrin formation in H-PRF preparation and investigating the influence of platelet activation in the anaerobic environment.
This study provides a successful trial to convert the physiological process into biotechnological application. The SHAP tubes proposed within this article was an effective versatile H-PRF preparation device, which provided a promising alternative for tissue engineering.
Introduction
Platelet concentrates have been used in regenerative medicine and dentistry for years by centrifugation of the patients’ own blood.Citation1 The first-generation was termed platelet-rich plasma (PRP) which was widely used in various fields of medicine. However, the preparation process involves the use of anticoagulants which prevents normal/optimal wound healing and presents potential risks, making clinical use inconvenient.Citation2,Citation3 As a result, the second-generation platelet concentrate, termed platelet-rich fibrin (PRF), was developed to avoid the use of anticoagulants. PRF has also been named leukocyte-rich platelet fibrin or L-PRF, due to its incorporation of leukocyte content.Citation4–6 To solve the problems of cell damage and uneven distribution caused by fixed-angular centrifugation during the preparation of PRF, it was demonstrated that PRF prepared via horizontal centrifugation (called horizontal PRF, or H-PRF) was shown to lead to up to four times more cell content when compared to fixed-angle. Furthermore, the problems of uneven distribution and cell damage in PRF produced by fixed-angle centrifugation could be entirely avoided using horizontal centrifugation.Citation7,Citation8
H-PRF is a three-dimensional fibrin matrix that has been shown to favor the regeneration of many tissues in both medicine and dentistry owing to its slow and gradual release of growth factors when compared to PRP.Citation9,Citation10 It is also considered an extremely safe and effective biomaterial for tissue regeneration.Citation11,Citation12 Due to its potential ability to modulate a vascular response, H-PRF can accelerate wound healing by promoting early angiogenesis and tissue growth.Citation13 The prepared H-PRF membrane is also considered to contain certain toughness and tensile strength when compared to a standard fibrin clot, which has often been used as a surgical adjuvant in oral and maxillofacial surgery, acting as a tensile barrier in many clinical situations.Citation13–16 Furthermore, H-PRF can significantly improve the mechanical properties of bone substitutes, facilitate clinical operation, reduce micromovement of bone grafts during the healing process of the surgical area, and enhance soft/hard tissue regeneration.Citation17–20
Nevertheless, there are still many open questions regarding research using PRF in terms of studies such as the final PRF membrane mechanical strength and biological activity as a result. Several studies have suggested that the age of the patient, the time between the start of the blood draw to the start of centrifugation, and various centrifugation techniques have an impact on the final outcomes of the PRF preparation. Correspondingly, PRF derived from female and elderly patients tends to be of higher quality. In addition, shorter centrifugation time and lower centrifugation speed can also improve the quality of PRF.Citation21–23 As a tool that directly affects the clotting ability of PRF, several studies have now clearly demonstrated that the blood collection tubes have a distinct effect on the final production of PRF and the strength and size of the membranes.Citation24–26 To date, there are no studies investigating the effect of the gas environment inside the blood collection tubes on the PRF outcomes. It has been reported that anaerobic environment promotes platelet activation and adhesion by the up-regulation of calpain activity.Citation27–29 Plasma treatment can enhance the hydrophilicity and change the nanoscale morphology of the inner glass surface, which promotes the adhesion and activation of platelets.Citation30–34 Therefore, it is reasonable to speculate that the anaerobic environment combined with plasma treatment in the blood collection tubes can also accelerate H-PRF formation and enhance its mechanical and biological properties.
In the process of clinical H-PRF preparation, especially for patients with poor clotting ability (medication, advanced age, etc.), H-PRF may not always coagulate immediately after centrifugation. By studying the coagulation process of PRF clots, it was routinely observed that the flocculent fibers formed at the lower layers of the PRF clots where the oxygen content is obviously reduced. As a result, the present study aimed to modify the inner wall surface of the PRF tubes with plasma to increase hydrophilicity and create an anaerobic environment inside the blood collection tubes. These modified tubes were termed “super-hydrophilic anaerobic plasmatrix tubes” (SHAP tubes). Afterward, we evaluated the size, mechanical properties, biological properties, and platelet activation of the H-PRF clots prepared by SHAP tubes and provided data to support the fabrication of a stronger H-PRF membrane with better mechanical properties and tensile strength.
Materials and methods
Preparation of PRF
Informed consent was collected from 21 healthy volunteers (11 males and 10 females, average age 26 years old) for the collection of their blood samples. In this study, all procedures were performed according to the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. There was no evidence of any illness among the participants.Citation1,Citation8 In a single blood collection step, disposable needles and tubes (Plasmatrident, Weiyin Technology Co., Ltd., Wuhan, China) were used to collect whole blood from their median cubital vein. Volunteers were asked to donate six tubes of blood, each containing 10 mL. The blood collection tubes were horizontally centrifuged at 700 RCF for 8 min, and the H-PRF was collected after centrifugation.
Oxygen content detection
Sodium citrate does not affect platelet activation but prevents clotting by chelating calcium ions and interrupting the coagulation cascade, thus forming a liquid state of H-PRF, which helps the detection of dissolved oxygen and flow cytometry.Citation35,Citation36 H-PRF in the liquid state was prepared by adding 1 mL of 109 mmol/L sodium citrate to the blood collection tube and subsequently centrifuging the blood. The liquid PRF was divided into upper, middle and lower layers according to its depth, and the dissolved oxygen meter AR8210 (Smart Sensor, China) probe was inserted into the PRF layers of different layers to read the corresponding dissolved oxygen concentrations.
Preparation of the SHAP tubes
The standard glass tubes were ultrasonically cleaned with ultrapure water, 100% ethanol and ultrapure water for 20 minutes successively and then dried at high temperature for 30 min in a local 10 000-level layer protected tunnel oven while being sterilized. The cleaned tubes were then treated with plasma. The power of the plasma treatment was 210 W, the flow was 1.0 L/min, and the time was 5 min, respectively. The treated tubes were immediately vacuumed and injected with anaerobic gas simultaneously. The proportion of anaerobic gas mixture was nitrogen: hydrogen: carbon dioxide at 8:1:1. The tubes were sealed with a rubber stopper in a closed chamber at 101.325 kPa standard atmospheric pressure, 25°C and 50% vacuum at −0.8 kPa, and the vacuum in the tubes was set at −5.33 Pa by means of a vacuum pump and solenoid valve adjustment.
Evaluation of the mechanical properties of H-PRF membranes
A universal testing machine was used to evaluate the mechanical properties of H-PRF membranes at room temperature and 50% relative humidity. In order to ensure the rupture occurred near the middle region of the membrane at maximum stress, the H-PRF membranes were sheared into dumbbells with a middle part width of 6 mm. An unstressed H-PRF membrane was fixed to the universal testing machine’s upper and lower clamps by adjusting the distance between them. The membranes were measured before stretching to determine their initial length. Afterward, the H-PRF membranes were stretched at a constant speed of 2 mm/s, and strain and tensile stress measurements were taken for each group. Next, the maximum stress and strain were determined by plotting the stress-strain curve.
Examination of the stability of H-PRF
H-PRF membranes were prepared and incubated in 5 mL of phosphate-buffered saline (PBS) containing 1% penicillin-streptomycin (Hyclone, Logan, UT, USA). At each experimental time point (1, 2, 3, 4, 5, 7, 9, and 13 days), the characteristics of the H-PRF membranes were observed during this 13-day degradation period.
Scanning electron microscopy (SEM)
The fixation of H-PRF was performed with 2.5% glutaraldehyde at 4°C overnight. The samples were then washed three times with PBS and double-distilled water (ddH2O), sequentially dehydrated with 30%, 50%, 70%, 80%, and 95% ethanol, and then dehydrated twice with 100% ethanol for five minutes each. Spray coating with gold particles was then performed followed by critical point drying. SEM (Zeiss SIGMA, Carl Zeiss AG, UK) was used to examine the surface and cross-section of each sample.
Flow cytometry
H-PRF in the liquid state was prepared by pre-filling blood collection tubes with 1 mL of 109 mmol/L sodium citrate, followed by blood sampling and centrifugation. 50 μL of H-PRF was drawn from each of the upper, middle and lower layers and incubated with phycoerythrin (PE) anti-human CD41 (1:200, #303705, BioLegend, California, USA) and platelet activation marker FITC-labeled anti-human CD62P antibodies (1:200, #304903, BioLegend, California, USA) for 45 min at room temperature, and platelets were detected by flow cytometry. The activation status of platelets was also detected using flow cytometry.
Inhibition ring test of the H-PRF membranes
We obtained H-PRF by centrifugation at 700 RCF for 8 minutes in 10 mL glass tubes, then compressed and converted them into a standardized PRF membrane 1 mm in thickness to evaluate their antibacterial properties. 500 μL of S. aureus (1 × 106 CFU· mL−1) cultivated on tryptic soy agar (TSA) plates were then added. S. aureus was directly in contact with the H-PRF membranes on the TSA plate. A 24-hour incubation period was used for the samples. Using horizontal (length) and vertical (width) lines drawn at 90° angles from the midpoints, each PRF membrane was measured after 24 hours.
Primary human gingival fibroblasts culture
Experiments were conducted according to the principles outlined in the Declaration of Helsinki. Our study involved the collection of gingival tissues from healthy volunteers (n = 3, aged 18–30 years old) who had undergone the extraction of the third molar but indicated no clinical signs of any oral or systemic diseases. The tissues were placed on ice in Dulbecco’s modified eagle’s medium (DMEM; Gibco, NY, USA) containing 10% fetal bovine serum (FBS; Gibco, NY, USA) and 1% penicillin-streptomycin. Hank’s Balanced Salt Solution (HBSS, Hyclone, UT, USA) containing 1% penicillin-streptomycin was used to rinse pooled tissues, which were then removed from epithelium, cut into smaller pieces, and incubated with 2 mM type I collagenase (Sigma-Aldrich, Steinheim, Germany) at 37°C for 60 min. Cells were resuspended in DMEM after centrifugation (200 g, 3 min) and plated on T25 culture flasks (Corning, NY, USA) in a humidified atmosphere with 5% CO2. Further experiments were carried out with cells P3-P8.
Human osteoblasts culture and osteogenic induction
Human osteoblasts (hFOB 1.19) were obtained from Shanghai Yu Bo Biotech Co., Ltd. and incubated under standard cell culture conditions of 5% CO2 at 37°C in DMEM supplemented with 10% fetal bovine serum and 1% antibiotics (100 U/ml penicillin G, 100 µg/mL streptomycin, HyClone). At 80% confluence, the culture media was substituted for an osteoblast-inducing medium containing 550 μg·mL−1 ascorbic acid (Sigma-Aldrich, USA), 10 nM dexamethasone, and 10 mmol·L−1 β-glycerophosphate. A medium replacement was performed every three days at 37°C under 5% CO2.
Cell culture model
Transwell chambers (Corning, USA) with 3 μm pore membranes were selected as cell culture models, according to previous studies.Citation37 The upper chambers were used to hold H-PRF and the lower chambers were used to culture cells.
Cell proliferation determination using the cell counting kit-8 (CCK-8) assay
After 1, 3, and 5 days of culture with the corresponding H-PRF membranes, CCK-8 (Beyotime, China) was used to assess the proliferation of HGFs and human osteoblasts. Briefly, in 96-well plates, human osteoblasts and HGFs were seeded at a density of 5 × 103 cells per well at 37°C in DMEM supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. During the third and fifth days of the experiment, the culture medium was replaced by 10 μL of the original CCK-8 solution. Incubation at 37°C for 1 h followed by 450 nm absorbance measurement was performed immediately with a microplate reader (PowerWave XS2, BioTek, USA).
Collagen type I staining
HGFs were plated in 24-well plates at a density of 50 000 cells per structure, with the medium being changed every three days. A seven-day fixation procedure was performed after washing with PBS and fixing them with 4% formaldehyde at room temperature (RT) for 10 minutes. Subsequently, cells were incubated with polyclonal rabbit to collagen type I (1:100, Boster Biological Technology Ltd., Wuhan, China) diluted in PBS containing 2% bovine serum albumin (BSA, Roche, Shanghai, China) for 1 h, followed by incubation with FITC-conjugated-goat-anti-rabbit (1:200, Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) diluted in PBS for 1 h. DAPI staining was followed by three washes in PBS following each step. A fluorescence microscope (Olympus Co., Tokyo, Japan) was used to capture images of each surface.
ALP staining and Alizarin red S (ARS) staining
Mineralization levels of the extracellular matrix were assessed in vitro by ALP and ARS staining. Medium containing osteogenic ingredients were used to culture human osteoblasts in 24-well plates at a density of 1 × 105 cells/well. Human osteoblasts were washed three times with PBS and fixed in 4% formaldehyde for 20 minutes during osteogenic differentiation. Staining was performed using an ALP kit (Beyotime, China) according to the manufacturer’s instructions. Cell fixation was performed with 4% formaldehyde for 20 minutes after 14 days of culture. In the next step, we washed the cells three times with ddH2O, then stained the cells for 10 minutes at 37°C with 0.1% ARS staining solution (pH 4.5). As a final step, the cells were gently rinsed with ddH2O to terminate the reaction. A microscope was used to photograph the mineralized nodules that formed after the culture. After being dissolved in 10% cetylpyridinium chloride for two hours, the absorbance at 562 nm was detected to determine the amount of ARS staining.
Assay for ALP activity
Washed with PBS three times after supersonic decomposition, the cells were then lysed for 30 minutes with 0.1% Triton X-100 at 4°C. It took 15 minutes for the centrifugation to be completed at 14 000 g. With the Alkaline Phosphate Assay Kit (Beyotime, China), alkaline phosphatase activity in the supernatant was determined according to the manufacturer’s instructions. BCA Protein Assay Kit was used to measure the protein concentration of each group, and ALP activity was normalized to the total protein concentration.
Statistical analysis
A total of 24 volunteers were enrolled in this study, and each quantitative experiment consisted of six repetitions from different volunteers. GraphPad Prism software 7.0 was used to analyze the data by one-way analysis of variance. Mean results for each procedure were compared in a pairwise test with a post hoc Bonferroni adjustment for multiple comparisons and a confidence level of 95%. A Shapiro–Wilk test was used to test the data for normal distribution. Data was graphed as mean ± SD. *p < .05, **p < .01, and ***p < .001 were considered statistically significant.
Results
Coagulation pattern of H-PRF after centrifugation
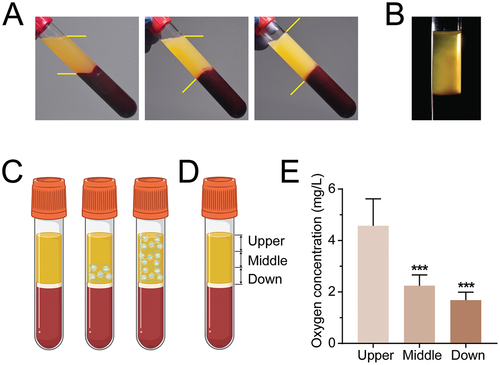
By observing the coagulation process, it was observed that the coagulation process of liquid H-PRF started at the junction of the plasma and erythrocyte layer, and eventually, the overall coagulation of the entire H-PRF clots occurred (). Noteworthy, the internal crosslinked fibrin network was first formed on the lower layer of H-PRF (). By stratifying the H-PRF and examining the dissolved oxygen content in the upper, middle and lower layers, it was found that the dissolved oxygen content in the middle and lower layers of the H-PRF was significantly and markedly lower than that in the upper layer ().
Figure 1. H-PRF shows the coagulation sequence from the yellow-red junction to the coronal part. (A) Coagulation state of H-PRF with time. (B) Fiber formation during coagulation of H-PRF. (C) Schematic diagram of coagulation of H-PRF. (D) Layer demarcation of H-PRF. (E) The oxygen content of H-PRF in different layers. (n = 6, ***p < 0.001).
The preparation and characterization of the SHAP tubes
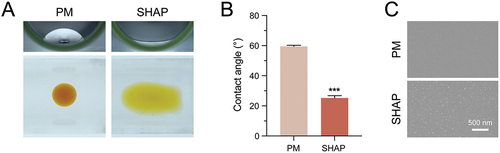
To further investigate whether the oxygen environment would have an effect on the preparation of H-PRF. We pre-treated PRF tubes with nitrogen gas plasma, followed by vacuuming to maintain a negative pressure environment (). Dropping 30 ml of double distilled water droplets on the inner wall of the blood collection tube to conduct the contact angle test. The results have shown that the treated blood collection tube (SHAP tube) exhibited superior hydrophilicity relative to the conventional blood collection tube (PM tube) (). The droplets remained intact as droplets in the PM group, but spread out with a larger surface area in the SHAP group after treatment. The contact angle measurements also demonstrated the enhanced hydrophilicity (). Meanwhile, observations using SEM revealed that the surface morphology of the inner wall of the tube in the SHAP group changed, forming a large number of uniformly distributed raised particles with a slightly increased surface roughness ().
Figure 3. SHAP tubes show good hydrophilicity and different surface topography compared to PM tubes. (A) Comparison of hydrophilicity between PM and SHAP tubes. (B) Contact angle measurement of the inner surface of the blood collection tube. (C) Comparison of morphology between PM and SHAP tubes by SEM. (n = 6, ***p < 0.001).
H-PRF membranes prepared by the SHAP tubes show significant improvements in size and mechanical properties
Comparing the PRF membranes prepared by both tube groups, the results revealed that the size of the H-PRF clot was significantly higher in the SHAP group than that in the PM group. The surface area of the SHAP membrane was 1.6 times larger than that in the PM group ().
Figure 4. H-PRF prepared by SHAP tubes has a larger size, higher mechanical strength and longer degradation time. (A) Comparison of H-PRF clots prepared by PM and SHAP tubes. (B) Comparison of H-PRF membranes prepared by PM and SHAP tubes. (C) Length analysis of H-PRF clots prepared by PM and SHAP tubes. (D) Area analysis of H-PRF membranes prepared by PM and SHAP tubes. (E) Fracture strain analysis of H-PRF membranes prepared by PM and SHAP tubes. (F) Fracture stress analysis of H-PRF membranes prepared by PM and SHAP tubes. (G) Stress-strain curves of H-PRF membranes prepared by PM and SHAP tubes. (H) SEM observation of the microstructure on the surface and cross-section of H-PRF membranes prepared by PM and SHAP tubes. (I) Degradation of H-PRF membranes prepared by PM and SHAP tubes with time. (n = 6, ***p < 0.001).
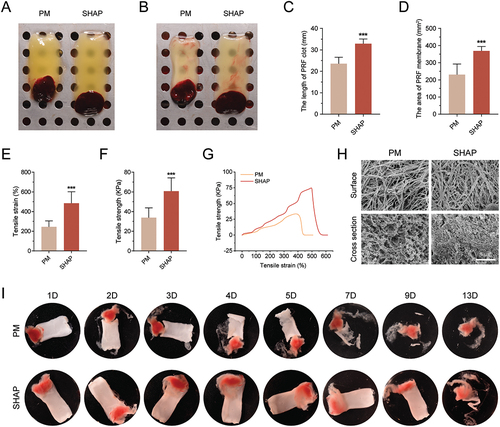
To gain insight into the differences in device performance between the H-PRF prepared from both tube types, the tensile properties of the H-PRF membranes were tested. The results have shown that the fracture strain and fracture stress of the H-PRF membranes in the SHAP tube group were 1.98 and 1.80 times higher than those produced in the PM group, respectively (). The H-PRF in the SHAP group clearly had a more significant improvement in mechanical strength (). By observing the surface and cross-sections of the H-PRF membranes, it was found that the fiber density of the H-PRF in the SHAP group was also denser as revealed by SEM. The PM group had a relatively looser fiber structure and the cross-sectional view was more porous (). To further assess the stability of the fibrous cross-linking within H-PRF, we performed an in vitro degradation assay. During a 13-day degradation period, it was observed that the H-PRF prepared in the PM group disintegrated by day 7, whereas the H-PRF clots from the SHAP group maintained good morphology even by day 9 without significant differences in morphology between days 0 and 9 ().
SHAP tubes are more conducive to platelet activation in the preparation of the H-PRF
As the main component within the H-PRF, platelets play a crucial role in the activation of coagulation and the clotting of H-PRF.Citation38 We performed SEM on the internal platelets of both groups of H-PRF and found that some of the internal platelets in the SHAP group showed cell rupture/activation, while the internal platelet structure of H-PRF in the PM group was relatively intact ().
Figure 5. SHAP tubes promote platelet activation within H-PRF. (A) The internal platelet morphology of H-PRF prepared by PM tubes was observed by scanning electron microscope. (B) The internal platelet morphology of H-PRF prepared by SHAP tubes was observed by scanning electron microscope. (C) Flow cytometry compared the platelet activation in different layers of H-PRF prepared by PM and SHAP tubes (n = 6).
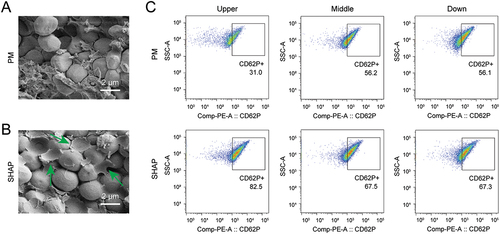
The analysis of platelet activation within both groups of H-PRF revealed that platelet activation was found to be generally higher in the SHAP tube group when compared to the PM group. Particularly, for the uppermost layer closest to the anaerobic environment in the blood collection tubes, the percentage of platelet activation within the H-PRF in the SHAP group was approximately 2.66 times higher than that in the PM group (). Even for the middle and lower layers, the percentage of platelet activation within the H-PRF was approximately 20% higher in the SHAP group when compared to the PM group. By flow cytometry, we found that with the decrease of dissolved oxygen in plasma, the proportion of platelet activation increased, proving that anaerobic environment can promote platelet activation and subsequently promote the formation of H-PRF.
H-PRF prepared from SHAP tubes have better biological effects
Considering the clinical applications of H-PRF, two main focuses are its antibacterial properties and the ability to promote either soft or hard tissue formation/regeneration. Therefore, we selected Staphylococcus aureus, the most common species of graft infection, as a representative, to explore the antibacterial properties of H-PRF prepared from either blood collection tube. Thereafter, the biological roles of gingival fibroblasts and human osteoblasts were also explored.
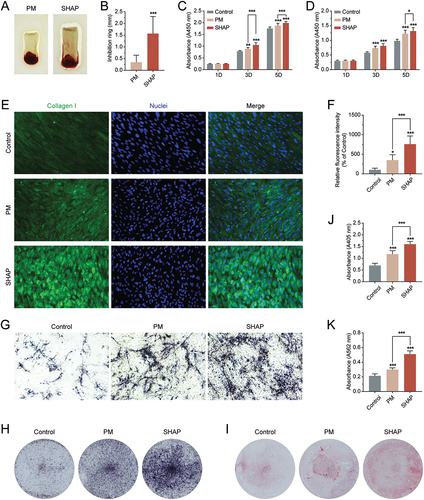
The results have shown that the inhibition effect of H-PRF on S. aureus in the SHAP group was significantly higher than that in the PM group, and the size of its inhibition ring was 4.55 times larger than that in the PM group (). For the proliferation of human gingival fibroblasts and human osteoblasts, both the PM and SHAP groups showed improved cellular effects (). Although it was observed from the data that the SHAP group had a slightly better proliferation-promoting effect than the PM group, there was no statistical difference. Subsequently, we investigated the secretion of collagen fibers by H-PRF-stimulated human gingival fibroblasts. Fluorescence staining of type I collagen revealed that the promotion of collagen formation in gingival fibroblasts by H-PRF was more pronounced in the SHAP group (). The mineralization results on human osteoblasts have also shown that H-PRF prepared by the SHAP tubes had the most significant promotion effect on osteogenesis (). The quantitative data demonstrated that the SHAP group had 2.32 times more expression of alkaline phosphatase than the control group and 1.36 times more expression than the PM group (). Meanwhile, mineralization nodule staining of human osteoblasts mineralized for 2 weeks using alizarin red staining showed a remarkable superior mineralization effect in the SHAP group ().
Figure 6. H-PRF prepared by SHAP tubes has better antibacterial, collagen secretion and mineralization promotion effects. (A) Comparison of the inhibition rings of H-PRF membranes prepared by PM and SHAP tubes. (B) Quantification of the inhibition rings in (A). (C) CCK-8 experiment to analyze the effect of H-PRF prepared by PM and SHAP tubes on the proliferation of human gingival fibroblasts. (D) CCK-8 experiment to analyze the effect of H-PRF prepared by PM and SHAP tubes on the proliferation of human osteoblasts; (E) fluorescence staining of the effect of H-PRF prepared by PM and SHAP tubes on collagen secretion of human gingival fibroblasts. (F) Quantification of fluorescence staining in (E). (G, H). ALP staining of human osteoblasts after 7 days of mineralization. (I) Alizarin red staining of human osteoblasts after 14 days of mineralization. (J) ALP activity analysis of human osteoblasts after 7 days of mineralization. (K) Quantification of staining in (I). (n = 6, ***p < 0.01 and ***p < 0.001).
H-PRF prepared by SHAP tubes improved significantly in patients with coagulation dysfunction
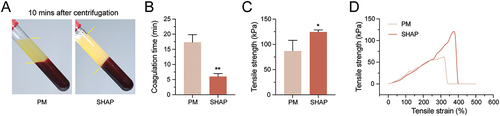
In clinical practice, it was found that blood from patients with coagulation disorders cannot produce a solid H-PRF after centrifugation. To determine whether SHAP tubes could solve this problem. We screened and enrolled three patients with poor coagulation (2 males and 1 female, aged 18–30 years old) in the clinic before dental implant placement. Subsequently, H-PRF was prepared using PM tubes and SHAP tubes. Ten mins after centrifugation, the results showed that H-PRF in PM tubes failed to coagulate, while H-PRF in SHAP tubes was clotted (). Twenty mins later, H-PRF membranes were prepared and tested for tensile properties. It was found that the H-PRF membranes prepared in the SHAP group showed significantly improved mechanical properties ().
Figure 7. H-PRF prepared by SHAP tubes improved significantly in patients with poor coagulation function. (A) Coagulation effect of H-PRF prepared by PM and SHAP tubes after centrifugation. (B) Coagulation time of H-PRF prepared by PM and SHAP tubes in patients with poor coagulation function. (C) Fracture stress analysis of H-PRF membranes prepared by PM and SHAP tubes. (D) Stress-strain curves of H-PRF membranes prepared by PM and SHAP tubes. (n = 3, ***p < 0.05 and ***p < 0.01).
Discussion
As a new biomaterial in the field of oral regeneration, H-PRF has been increasingly applied in the fields of antibacterial, promoting soft tissue healing, improving the performance of bone graft materials, and accelerating hard tissue regeneration.Citation6,Citation8,Citation39,Citation40 As a membrane with added mechanical strength, H-PRF membranes can play a more robust role in the thickening of soft tissue, as an aid in the repair of maxillary sinus mucosal perforations, and as a membrane for the closure of extraction sockets.Citation40–45 Furthermore, PRF bone blocks prepared using H-PRF and bone grafting materials can stabilize the graft complex, preventing micromotion of the graft material during healing, and favoring regeneration.Citation17–19,Citation46 However, all of the above present a greater challenge to the effectiveness of H-PRF preparation. The ability to fabricate large-scale, high-mechanical-strength H-PRF membranes is of great clinical significance for tissue regeneration applications.
In the clinical preparation of H-PRF, for patients with poor coagulation function, H-PRF sometimes failed to coagulate immediately and presented a fluid state at the end of the centrifugation procedure. As the resting time was extended, the liquid H-PRF would gradually solidify and form the common solid H-PRF clot in the clinic. This procedure highly increases the operating time, and such slow-forming H-PRF clots tend to have poorer mechanical properties. Therefore, we need to explore the process of H-PRF coagulation and find a better solution.
Observing the physiological coagulation process of H-PRF, we first found that the coagulation of liquid H-PRF started at the junction of the plasmatrix layer and the erythrocyte layer, and eventually, the overall coagulation of the H-PRF occurred. As oxygen remained above the liquid level, the dissolved oxygen content in the middle and lower layers of the H-PRF was remarkably lower than that in the upper layer. In the current literature, the role of oxygen in the preparation of coagulation and plasma mechanisms has not been studied in great detail, nor has the direct involvement of oxygen in prothrombin activation been reported. However, it has been shown that platelets exposed to low oxygen concentration (5% O2) had significantly higher expression of platelet activation-related proteins compared to platelets exposed to normal oxygen concentration (21% O2) in mice and human platelets.Citation28 Therefore, during the preparation of H-PRF, creating an anaerobic environment for the coagulation of PRF inside the blood collection tubes was hypothesized to potentially promote platelet activation. In the present study, we innovatively modify the inner wall of the blood collection tubes with plasma and adjust the gas environment inside the tubes to prepare the super-hydrophilic anaerobic plasmatrix tubes (SHAP tubes).
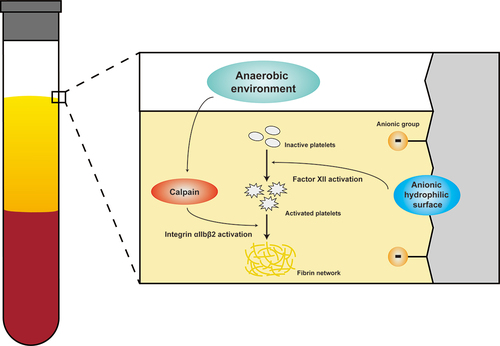
According to the diagram, at the end of centrifugation, erythrocytes are pushed to the bottom of PRF tubes, whereas the H-PRF layer contains a large number of platelets, fibrinogen, and thrombin.Citation47,Citation48 Under the action of thrombin, a fibrinogen molecule releases four small peptides (two fibrinopeptides A and two fibrinopeptides B) to form fibrin monomers.Citation49–53 Fibrin monomers can spontaneously form fibrin multimers by hydrogen bonding and form a reticulated fibrin bundle. This reticulum is capable of capturing a large number of platelets. The anaerobic environment in the SHAP tubes can significantly upregulate calpain activity and thus increase platelet reactivity. The anaerobic environment can also promote the aggregation and activation of platelets, increase the transmembrane proteins on the surface of platelets, and promote the release of alpha particles and dense particles from the activated platelets. They can release a variety of procoagulant and growth factors, accelerate the occurrence of the coagulation process, and promote cell proliferation and osteogenic differentiation. In addition, plasma treatment improves the hydrophilicity of the inner tube wall, results in the emergence of a surface-energy-dependent catalytic potential, significantly reduces the adsorption competition between coagulation factor FXII and other plasma proteins, and enhances the surface contact efficiency of FXII, thus promoting platelet activation.Citation54 Subsequently, the activated platelets in the SHAP group released more FXIII which could catalyze a covalent cross-linking reaction between fibrin monomers when activated by thrombin and promoted the formation of fibrin in H-PRF ().Citation55–57
Figure 8. The schematic diagram illustrate the mechanism that the SHAP tubes with the anaerobic gas environment and the super-hydrophilic surface can activate the platelet clotting cascade and thus promote the fibrin network and H-PRF formation.
After platelet activation, in addition to stimulating the coagulation process, there is a continuous release of growth factors that promote tissue regeneration in the defect area, mainly because α-granules contained within platelets stimulate the release of platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), epidermal growth factor (EGF), vascular endothelial growth factor (VEGF), among others.Citation58–60 PDGF mainly promotes the migration and proliferation of precursor cells, TGF-β promotes cell growth and secretion of extracellular matrix, and VEGF promotes neo-angiogenesis.Citation61 The synergistic effect of multiple cytokines simultaneously was shown to promote collagen secretion from human gingival fibroblasts and mineralization of human osteoblasts. In addition, platelets are multifunctional host defense cells that express a variety of proteins and receptors, including Fc, complement, as well as antimicrobial peptides that help coordinate immune defense against bacteria, and platelets interact directly with microorganisms to help remove pathogens from the blood.Citation62–64 Furthermore, activated platelets can bond to leukocytes and stimulate leukocyte function.Citation65 In the present study, the anaerobic environment stimulated platelet activation and significantly enhanced the antibacterial effects on S. aureus.
In general, a new modification scheme for H-PRF preparation of blood collection tubes was proposed in this study. Unlike conventional reagent chemical additives, the SHAP tubes ensure good biosafety of H-PRF, improve the size, tensile properties and density of H-PRF, and provide better barrier protection against incoming pathogens. The activated platelets release more bioactive substances to exert anti-bacterial properties locally and accelerate soft and hard tissue healing and regeneration, thus providing a new and improved strategy for the repair and treatment of their defects. Through the application of clinical scenarios, we proved that the SHAP tubes are effective solutions to the problem of difficult preparation of H-PRF in patients with poor coagulation.
Conclusion
This study focuses on proposing a new blood collection tube for H-PRF preparation, which has more optimized hydrophilic and anaerobic interior properties. The activation of platelets induced by hypoxia was able to promote the cross-linking of fibrin, improve the density of the internal fibrous network, enhance the fracture strength and maximum strain of H-PRF, and prolong their degradation time. Further biological evaluation has shown that H-PRF prepared by the SHAP tubes had a better proliferation-promoting effect on both human gingival fibroblasts and human osteoblasts. Moreover, its antibacterial properties, pro-collagen formation ability and promotion of mineralization were significantly improved suggesting better potential of the SHAP tubes for clinical applications.
Author contribution
Yan Wei and Yihong Cheng contributed equally. Yan Wei and Shanglan Qing conceived and designed the research. Yan Wei, Yihong Cheng and Hongjiang Wei performed the experiments and data acquisition. Yan Wei and Xiaoxin Zhang performed the analysis, interpretation and wrote the manuscript. Yan Wei, Richard J. Miron, Yulan Wang, and Shanglan Qingcritically revised the manuscript. All authors approved and agreed to the final revision.
Ethical approval
The study was approved by the Ethics Committee of the School and Hospital of Stomatology, Wuhan University (B52/2020).
Informed consent
For this study, informed written consent was provided to conduct the outlined experiments prior to the blood draw.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Wei Y, Cheng Y, Wang Y, Zhang X, Miron RJ, Zhang Y. The effect of resting and compression time post-centrifugation on the characteristics of platelet rich fibrin (PRF) membranes. Clin Oral Investig. 2022;26(8):5281–12. doi:10.1007/s00784-022-04496-9.
- Honda Y, Morishima Y. Thrombin generation induced by tissue factor plus ADP in human platelet rich plasma: a potential new measurement to assess the effect of the concomitant use of an oral factor Xa inhibitor edoxaban and P2Y12 receptor antagonists. Thromb Res. 2015;135(5):958–62. doi:10.1016/j.thromres.2015.03.001.
- Lee JS, Guo P, Klett K, Hall M, Sinha K, Ravuri S, Huard J, Murphy WL. VEGF-attenuated platelet-rich plasma improves therapeutic effect on cartilage repair. Biomater Sci. 2022;10:2172–81. doi:10.1039/d1bm01873f.
- Lei L, Yu Y, Han J, Shi D, Sun W, Zhang D, Chen L. Quantification of growth factors in advanced platelet-rich fibrin and concentrated growth factors and their clinical efficacy as adjunctive to the GTR procedure in periodontal intrabony defects. J Periodontol. 2020;91:462–72. doi:10.1002/JPER.19-0290.
- Li Z, Liu L, Wang L, Song D. The effects and potential applications of concentrated growth factor in dentin-pulp complex regeneration. Stem Cell Res Ther. 2021;12:357. doi:10.1186/s13287-021-02446-y.
- Anitua E, Sanchez M, Nurden AT, Nurden P, Orive G, Andia I. New insights into and novel applications for platelet-rich fibrin therapies. Trends Biotechnol. 2006;24:227–34. doi:10.1016/j.tibtech.2006.02.010.
- Miron RJ, Chai J, Zheng S, Feng M, Sculean A, Zhang Y. A novel method for evaluating and quantifying cell types in platelet rich fibrin and an introduction to horizontal centrifugation. J Biomed Mater Res A. 2019;107:2257–71. doi:10.1002/jbm.a.36734.
- Feng M, Wang Y, Zhang P, Zhao Q, Yu S, Shen K, Miron RJ, Zhang Y. Antibacterial effects of platelet-rich fibrin produced by horizontal centrifugation. Int J Oral Sci. 2020;12:32. doi:10.1038/s41368-020-00099-w.
- Miron RJ. Understanding platelet rich fibrin: quintessence. 2021.
- Kobayashi E, Fluckiger L, Fujioka-Kobayashi M, Sawada K, Sculean A, Schaller B, Miron RJ. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig. 2016;20:2353–60. doi:10.1007/s00784-016-1719-1.
- Castro AB, Meschi N, Temmerman A, Pinto N, Lambrechts P, Teughels W, Quirynen M. Regenerative potential of leucocyte- and platelet-rich fibrin. Part A: intra-bony defects, furcation defects and periodontal plastic surgery. A systematic review and meta-analysis. J Clin Periodontol. 2017;44:67–82. doi:10.1111/jcpe.12643.
- Egle K, Salma I, Dubnika A. From blood to regenerative tissue: how autologous platelet-rich fibrin can Be combined with other materials to ensure controlled drug and growth factor release. IJMS. 2021;22:11553. doi:10.3390/ijms222111553.
- Bi J, Intriago MFB, Koivisto L, Jiang G, Hakkinen L, Larjava H. Leucocyte- and platelet-rich fibrin regulates expression of genes related to early wound healing in human gingival fibroblasts. J Clin Periodontol. 2020;47:851–62. doi:10.1111/jcpe.13293.
- Mirhaj M, Tavakoli M, Varshosaz J, Labbaf S, Jafarpour F, Ahmaditabar P, Salehi S, Kazemi N. Platelet rich fibrin containing nanofibrous dressing for wound healing application: fabrication, characterization and biological evaluations. Biomater Adv. 2022;134:112541. doi:10.1016/j.msec.2021.112541.
- Wang J, Sun Y, Liu Y, Yu J, Sun X, Wang L, Zhou Y. Effects of platelet-rich fibrin on osteogenic differentiation of schneiderian membrane derived mesenchymal stem cells and bone formation in maxillary sinus. Cell Commun Signal. 2022;20:88. doi:10.1186/s12964-022-00844-0.
- Kardos D, Hornyak I, Simon M, Hinsenkamp A, Marschall B, Vardai R, Kallay-Menyhard A, Pinke B, Meszaros L, Kuten O. et al. Biological and mechanical properties of platelet-rich fibrin membranes after thermal manipulation and preparation in a single-syringe closed system. IJMS. 2018;19:3433. doi:10.3390/ijms19113433.
- Kargarpour Z, Panahipour L, Miron RJ, Gruber R. Fibrinogen concentrations in liquid PRF using various centrifugation protocols. Molecules. 2022;27. doi:10.3390/molecules27072043.
- Barbu HM, Iancu SA, Rapani A, Stacchi C. Guided bone regeneration with concentrated growth factor enriched bone graft matrix (sticky bone) vs. bone-shell technique in horizontal ridge augmentation: a retrospective study. JCM. 2021;10:3953. doi:10.3390/jcm10173953.
- Tony JB, Parthasarathy H, Tadepalli A, Ponnaiyan D, Alamoudi A, Kamil MA, Alzahrani KJ, Alsharif KF, Halawani IF, Alnfiai MM. et al. CBCT evaluation of sticky bone in horizontal Ridge augmentation with and without collagen membrane—a randomized parallel arm clinical trial. JFB. 2022;13:194. doi:10.3390/jfb13040194.
- Miron RJ, Zucchelli G, Pikos MA, Salama M, Lee S, Guillemette V, Fujioka-Kobayashi M, Bishara M, Zhang Y, Wang HL. et al. Use of platelet-rich fibrin in regenerative dentistry: a systematic review. Clin Oral Investig. 2017;21(6):1913–27. doi:10.1007/s00784-017-2133-z.
- Miron RJ, Dham A, Dham U, Zhang Y, Pikos MA, Sculean A. The effect of age, gender, and time between blood draw and start of centrifugation on the size outcomes of platelet-rich fibrin (PRF) membranes. Clin Oral Investig. 2019;23:2179–85. doi:10.1007/s00784-018-2673-x.
- Al-Maawi S, Dohle E, Lim J, Weigl P, Teoh SH, Sader R, Ghanaati S. Biologization of pcl-mesh using platelet rich fibrin (Prf) enhances its regenerative potential in vitro. IJMS. 2021;22:2159. doi:10.3390/ijms22042159.
- Reksodiputro MH, Harahap AR, Setiawan L, Yosia M. A modified preparation method of ideal platelet-rich fibrin matrix from whole blood. Front Med. 2021;8:724488. doi:10.3389/fmed.2021.724488.
- Aizawa H, Tsujino T, Watanabe T, Isobe K, Kitamura Y, Sato A, Yamaguchi S, Okudera H, Okuda K, Kawase T. Quantitative near-infrared imaging of platelets in platelet-rich fibrin (PRF) matrices: comparative analysis of bio-PRF, leukocyte-rich PRF, advanced-PRF and concentrated growth factors. IJMS. 2020;21:4426. doi:10.3390/ijms21124426.
- Tsujino T, Masuki H, Nakamura M, Isobe K, Kawabata H, Aizawa H, Watanabe T, Kitamura Y, Okudera H, Okuda K. et al. Striking differences in platelet distribution between advanced-platelet-rich fibrin and concentrated growth factors: effects of silica-containing plastic tubes. JFB. 2019;10:43. doi:10.3390/jfb10030043.
- Dohan Ehrenfest DM, Del Corso M, Diss A, Mouhyi J, Charrier JB. Three-dimensional architecture and cell composition of a Choukroun’s platelet-rich fibrin clot and membrane. J Periodontol. 2010;81:546–55. doi:10.1902/jop.2009.090531.
- Cameron SJ, Mix DS, Ture SK, Schmidt RA, Mohan A, Pariser D, Stoner MC, Shah P, Chen L, Zhang H. et al. Hypoxia and ischemia promote a maladaptive platelet phenotype. Arterioscler Thromb Vasc Biol. 2018;38:1594–606. doi:10.1161/ATVBAHA.118.311186.
- Kusanto B, Gordon A, Naylor-Adamson L, Atkinson L, Coupland C, Booth Z, Ahmed Y, Pires IM, Stasiuk GJ, Sturmey R. et al. Practical considerations of dissolved oxygen levels for platelet function under hypoxia. IJMS. 2021;22:13223. doi:10.3390/ijms222413223.
- Tyagi T, Ahmad S, Gupta N, Sahu A, Ahmad Y, Nair V, Chatterjee T, Bajaj N, Sengupta S, Ganju L. et al. Altered expression of platelet proteins and calpain activity mediate hypoxia-induced prothrombotic phenotype. Blood. 2014;123(8):1250–60. doi:10.1182/blood-2013-05-501924.
- Vogler EA, Graper JC, Harper GR, Sugg HW, Lander LM, Brittain WJ. Contact activation of the plasma coagulation cascade. I. Procoagulant surface chemistry and energy. J Biomed Mater Res. 1995;29:1005–16. doi:10.1002/jbm.820290813.
- Zhuo R, Miller R, Bussard KM, Siedlecki CA, Vogler EA. Procoagulant stimulus processing by the intrinsic pathway of blood plasma coagulation. Biomaterials. 2005;26:2965–73. doi:10.1016/j.biomaterials.2004.08.008.
- Wittenburg G, Lauer G, Oswald S, Labudde D, Franz CM. Nanoscale topographic changes on sterilized glass surfaces affect cell adhesion and spreading. J Biomed Mater Res A. 2014;102:2755–66. doi:10.1002/jbm.a.34943.
- Crespin M, Moreau N, Masereel B, Feron O, Gallez B, Vander Borght T, Michiels C, Lucas S. Surface properties and cell adhesion onto allylamine-plasma and amine-plasma coated glass coverslips. J Mater Sci Mater Med. 2011;22:671–82. doi:10.1007/s10856-011-4245-3.
- Moradi S, Hadjesfandiari N, Toosi SF, Kizhakkedathu JN, Hatzikiriakos SG. Effect of extreme wettability on platelet adhesion on metallic implants: from superhydrophilicity to Superhydrophobicity. ACS Appl Mater Interfaces. 2016;8:17631–41. doi:10.1021/acsami.6b03644.
- van Rensburg WJ J, van der Merwe P. Comparison of commercially available blood collection tubes containing sodium citrate and hirudin in platelet aggregation testing. Med Sci Monit Basic Res. 2017;23:264–9. doi:10.12659/msmbr.905246.
- Schneider DJ, Tracy PB, Mann KG, Sobel BE. Differential effects of anticoagulants on the activation of platelets ex vivo. Circulation. 1997;96:2877–83. doi:10.1161/01.cir.96.9.2877.
- Wei Y, Zhu G, Zhao Z, Yin C, Zhao Q, Xu H, Wang J, Zhang J, Zhang X, Zhang Y. et al. Individualized plasticity autograft mimic with efficient bioactivity inducing osteogenesis. Int J Oral Sci. 2021;13(1):14. doi:10.1038/s41368-021-00120-w.
- Di Liddo R, Bertalot T, Borean A, Pirola I, Argentoni A, Schrenk S, Cenzi C, Capelli S, Conconi MT, Parnigotto PP. Leucocyte and platelet-rich fibrin: a carrier of autologous multipotent cells for regenerative medicine. J Cell Mol Med. 2018;22:1840–54. doi:10.1111/jcmm.13468.
- Polak D, Clemer-Shamai N, Shapira L. Incorporating antibiotics into platelet-rich fibrin: a novel antibiotics slow-release biological device. J Clin Periodontol. 2019;46:241–7. doi:10.1111/jcpe.13063.
- Wang X, Fok MR, Pelekos G, Jin L, Tonetti MS. Increased local concentrations of growth factors from leucocyte- and platelet-rich fibrin do not translate into improved alveolar ridge preservation: an intra-individual mechanistic randomized controlled trial. J Clin Periodontol. 2022;49:889–98. doi:10.1111/jcpe.13688.
- Koca-Unsal RB, Unsal G, Kasnak G, Firatli Y, Ozcan I, Orhan K, Firatli E. Ultrasonographic evaluation of the titanium-prepared platelet-rich fibrin effect in free gingival graft procedures. J Periodontol. 2022;93:187–94. doi:10.1002/JPER.21-0076.
- Keceli HG, Kamak G, Erdemir EO, Evginer MS, Dolgun A. The adjunctive effect of platelet-rich fibrin to connective tissue graft in the treatment of buccal recession defects: results of a randomized, parallel-group controlled trial. J Periodontol. 2015;86:1221–30. doi:10.1902/jop.2015.150015.
- Xin L, Yuan S, Mu Z, Li D, Song J, Chen T. Histological and histomorphometric evaluation of applying a bioactive advanced platelet-rich fibrin to a perforated schneiderian membrane in a maxillary sinus elevation model. Front Bioeng Biotechnol. 2020;8:600032. doi:10.3389/fbioe.2020.600032.
- Oncu E, Kaymaz E. Assessment of the effectiveness of platelet rich fibrin in the treatment of schneiderian membrane perforation. Clin Implant Dent Relat Res. 2017;19:1009–14. doi:10.1111/cid.12528.
- Sultan T, Cheah CW, Ibrahim NB, Asif MK, Vaithilingam RD. Three-dimensional assessment of the extraction sockets, augmented with platelet-rich fibrin and calcium sulfate: a clinical pilot study. J Dent. 2020;101:103455. doi:10.1016/j.jdent.2020.103455.
- Gheno E, Alves GG, Ghiretti R, Mello-Machado RC, Signore A, Lourenco ES, Leite PEC, Mourao C, Sohn DS, Calasans-Maia MD. “Sticky Bone” preparation device: a pilot study on the release of cytokines and growth factors. Mater (Basel). 2022;15:1474. doi:10.3390/ma15041474.
- Miron RJ, Zhang Y. Autologous liquid platelet rich fibrin: a novel drug delivery system. Acta Biomater. 2018;75:35–51. doi:10.1016/j.actbio.2018.05.021.
- Serafini G, Lopreiato M, Lollobrigida M, Lamazza L, Mazzucchi G, Fortunato L, Mariano A, Scotto d’Abusco A, Fontana M, De Biase A. Platelet rich fibrin (PRF) and its related products: biomolecular characterization of the liquid fibrinogen. J Clin Med. 2020;9:1099. doi:10.3390/jcm9041099.
- Soe G, Kohno I, Inuzuka K, Itoh Y, Matsuda M. A monoclonal antibody that recognizes a neo-antigen exposed in the E domain of fibrin monomer complexed with fibrinogen or its derivatives: its application to the measurement of soluble fibrin in plasma. Blood. 1996;88(6):2109–2117. doi:10.1182/blood.V88.6.2109.bloodjournal8862109.
- Mosesson MW. Fibrinogen and fibrin structure and functions. J Thromb Haemost. 2005;3:1894–904. doi:10.1111/j.1538-7836.2005.01365.x.
- Mosesson MW, Siebenlist KR, Meh DA. The structure and biological features of fibrinogen and fibrin. Ann N Y Acad Sci. 2001;936:11–30. doi:10.1111/j.1749-6632.2001.tb03491.x.
- Yang Z, Mochalkin I, Doolittle RF. A model of fibrin formation based on crystal structures of fibrinogen and fibrin fragments complexed with synthetic peptides. Proc Natl Acad Sci U S A. 2000;97:14156–61. doi:10.1073/pnas.97.26.14156.
- Bilezikian SB, Nossel HL, Butler VP Jr., Canfield RE. Radioimmunoassay of human fibrinopeptide B and kinetics of fibrinopeptide cleavage by different enzymes. J Clin Invest. 1975;56:438–45. doi:10.1172/JCI108110.
- Zhuo R, Siedlecki CA, Vogler EA. Autoactivation of blood factor XII at hydrophilic and hydrophobic surfaces. Biomaterials. 2006;27:4325–32. doi:10.1016/j.biomaterials.2006.04.001.
- Mitchell JL, Lionikiene AS, Fraser SR, Whyte CS, Booth NA, Mutch NJ. Functional factor XIII-A is exposed on the stimulated platelet surface. Blood. 2014;124:3982–90. doi:10.1182/blood-2014-06-583070.
- Somodi L, Beke Debreceni I, Kis G, Cozzolino M, Kappelmayer J, Antal M, Panyi G, Bardos H, Mutch NJ, Muszbek L. Activation mechanism dependent surface exposure of cellular factor XIII on activated platelets and platelet microparticles. J Thromb Haemost. 2022;20:1223–35. doi:10.1111/jth.15668.
- Ariens RA, Philippou H, Nagaswami C, Weisel JW, Lane DA, Grant PJ. The factor XIII V34L polymorphism accelerates thrombin activation of factor XIII and affects cross-linked fibrin structure. Blood. 2000;96:988–95. doi:10.1182/blood.V96.3.988.
- van der Meijden PEJ, Heemskerk JWM. Platelet biology and functions: new concepts and clinical perspectives. Nat Rev Cardiol. 2019;16:166–79. doi:10.1038/s41569-018-0110-0.
- Rodrigues BL, Montalvao SAL, Cancela RBB, Silva FAR, Urban A, Huber SC, Junior J, Lana J, Annichinno-Bizzacchi JM. Treatment of male pattern alopecia with platelet-rich plasma: a double-blind controlled study with analysis of platelet number and growth factor levels. J Am Acad Dermatol. 2019;80:694–700. doi:10.1016/j.jaad.2018.09.033.
- Hamilton B, Tol JL, Knez W, Chalabi H. Exercise and the platelet activator calcium chloride both influence the growth factor content of platelet-rich plasma (PRP): overlooked biochemical factors that could influence PRP treatment. Br J Sports Med. 2015;49:957–60. doi:10.1136/bjsports-2012-091916.
- Ruvalcaba-Ontiveros RI, Gonzalez-Chavez SA, Carrasco-Hernandez AR, Lopez-Loeza SM, Castellanos-Ponce I, Vazquez-Olvera G, Neri-Flores MA, Espino-Solis GP, Duarte-Moller JA, Pacheco-Tena C. et al. Treatment with silica–gold nanostructures decreases inflammation-related gene expression in collagen-induced arthritis. Biomater Sci. 2022;10:5216–29. doi:10.1039/d2bm00498d.
- Ungerer M, Rosport K, Bultmann A, Piechatzek R, Uhland K, Schlieper P, Gawaz M, Munch G. Novel antiplatelet drug revacept (Dimeric Glycoprotein VI-Fc) specifically and efficiently inhibited collagen-induced platelet aggregation without affecting general hemostasis in humans. Circulation. 2011;123:1891–9. doi:10.1161/CIRCULATIONAHA.110.980623.
- Arbesu I, Bucsaiova M, Fischer MB, Mannhalter C. Platelet-borne complement proteins and their role in platelet-bacteria interactions. J Thromb Haemost. 2016;14:2241–52. doi:10.1111/jth.13495.
- Cognasse F, Laradi S, Berthelot P, Bourlet T, Marotte H, Mismetti P, Garraud O, Hamzeh-Cognasse H. Platelet inflammatory response to stress. Front Immunol. 2019;10:1478. doi:10.3389/fimmu.2019.01478.
- Kossmann S, Lagrange J, Jackel S, Jurk K, Ehlken M, Schonfelder T, Weihert Y, Knorr M, Brandt M, Xia N. et al. Platelet-localized FXI promotes a vascular coagulation-inflammatory circuit in arterial hypertension. Sci Transl Med. 2017;9. doi:10.1126/scitranslmed.aah4923.