Abstract
Platelets are terminally differentiated anucleated cells, but they still have cell-like functions and can even produce progeny platelets. However, the mechanism of platelet sprouting has not been elucidated so far. Here, we show that when platelet-rich plasma(PRP) was cultured at 37°C, platelets showed a spore phenomenon. The number of platelets increased when given a specific shear force. It is found that AMP-related signaling pathways, such as PKA and AMPK are activated in platelets in the spore state. Meanwhile, the mRNA expression levels of genes, such as CNN3, CAPZB, DBNL, KRT19, and ESPN related to PLS1 skeleton proteins also changed. Moreover, when we use the AMPK activator AICAR(AI) to treat washed platelets, cultured platelets can still appear spore phenomenon. We further demonstrate that washed platelets treated with Forskolin, an activator of PKA, not only platelet sprouting after culture but also the AMPK is activated. Taken together, these data demonstrate that AMPK plays a key role in the process of platelet budding and proliferation, suggesting a novel strategy to solve the problem of clinical platelet shortage.
Plain Language Summary
What is new?
In this study, we showed that when platelet-rich plasma(PRP) was cultured at 37°C, platelets showed spore phenomenon and increased.
It was found that AMP-related signaling pathways, such as PKA and AMPK were activated in platelets in the spore state.
In addition, we found that PKA acts as an upstream kinase of AMPK.
In the process of platelet sprouting and proliferation, the mRNA expression levels of skeleton protein PLS1 and its related genes, such as CNN3, CAPZB, DBNL, KRT19, andESPN also changed.
What is the impact?
Our study proposes a new strategy to solve the problem of clinical platelet shortage.
Keywords:
Introduction
Platelets are anuclear cell debris produced by the shedding of the cytoplasm of bone marrow precursor megakaryocytes.Citation1,Citation2 Their diameter varies from 2 μm to 5 μm in healthy people. The survival time of platelets in blood circulation is 7–10 days.Citation3–5 Current studies have found that platelets play an important role in thrombosis and hemostasis,Citation6–8 tumor growth, and metastasis. In the tumor microenvironment, platelet surface receptors such as P2Y2 conduct signal transduction with tumor cells to promote their migration or invasion; some proteins in platelets affect the proliferation of tumor cells.Citation9–11 In the past two decades, although there is no direct evidence that platelets have the function of normal cell division in the understanding of whether platelets have the function of cells, the results of some scholars have found that platelets still have relatively unexpected cell-like functions, such as the ability to edit and cut mRNA and convert mRNA into proteins,Citation12–15 and even synthesize some related essential proteins when stored in vitro, such as GPIIIa.Citation16 In addition, some studies have found that after in vitro use of M199 medium to specifically culture platelets, new progeny platelets can be produced after the formation of spore shape.Citation17 For other conditions, such as whether the “budding” phenomenon can be produced in PRP, further research and discussions are still needed. Besides, in this study, it was found that the expression of various proteins in the generated offspring platelets changed, but the molecular mechanism of platelet generation has not been elucidated.
It is well known that AMPK is a complex composed of multiple subunits, including three subunits: α, β, and γ.Citation18–20 Studies have shown that AMPK affects platelets by participating in some signaling pathways in platelets. For example, AMPK increases the content of platelet phospholipids required to produce arachidonic acid by affecting the ACC signaling pathway, thereby increasing the level of arachidonic acid and promoting platelet activation.Citation21 Furthermore, AMPK also directly affects platelet function and even skeleton remodeling through phosphorylation of α-subunits.Citation22,Citation23 The role of AMPK in the process of platelet sprouting and proliferation has not been reported so far.
In our study, we show that platelet germination and proliferation are found in PRP-cultured platelets. Furthermore, When AMPK is activated during the culture of washed platelets, platelets also show a spore state, indicating that AMPK is essential for platelet germination and proliferation.
Materials and methods
Healthy volunteers
Approval for obtaining whole-blood samples from healthy volunteers was obtained from the Ethics Committee of The First Affiliated Hospital of Soochow University, and informed consent was obtained from all subjects according to the Declaration of Helsinki.
Antibodies and reagents
Phospho-VASP antibody (Ser157,3111s), phospho-AMPKɑ antibody(Thr172,2535),CaMKII antibody(4436s) and β-actin (4970s) were purchased from Cell signaling technology. Calcein acetoxymethyl-ester (Calcein-AM) was purchased from Dojindo. JC-1 (C2005) was purchased from Beyotime(Shanghai). FITC-conjugated Bovine Lactadherin(BLAC-FITC) was purchased from Biosciences(San Jose, CA, USA).
Platelet-rich plasma (PRP)
The platelets from healthy volunteers were prepared as previously described. Briefly, whole blood was drawn from the inferior vena cava and anti-coagulated with a 1:7 volume of acid–citrate–dextrose (ACD: 2.5% trisodium citrate, 2.0% D-glucose, 1.5% citric acid). Platelet-rich plasma (PRP) was collected from the whole blood by centrifugation at 200 × g for 11 minutes (min). Platelet counting was performed with the Sysmex XP-100 Hematologic Analyzer (Sysmex Corporation).
Platelet culture and germination rate analysis
Platelets in PRP or treated with AMPK or PKA activators after washing are placed in a 37°C incubator for cultivation. Cultivated platelets fixed with 4% paraformaldehyde. Platelets were immunofluorescence stained with phalloidin dye. Three random fields were captured from each independent experiment and at least 500 total cells, which encompassed individual platelets and platelets with 2 or more distinct cell bodies were counted. Platelet germination rate=the number of platelets that encompassed 2 or more distinct cell bodies/total number of platelets or the increase in platelet count after cultivation under shear force.
Flow cytometry
For detecting human platelet activation and apoptosis, mitochondrial inner transmembrane potential (ΔΨm) depolarization in human platelets was measured by JC-1 (2 μg/mL). PS exposure of human platelets was detected by FITC-labeled lactadherin (10 μg/mL). Platelets were measured by a flow cytometer (FC 500; Beckman-Coulter).
Western blot
The samples were centrifuged at 4000 rpm for 10 min to obtain supernatants and platelet pellets, and platelet pellets were lysed with an equal volume of 2 × lysis buffer on ice for 30 min. Proteins were separated and visualized by the ECL Chemiluminescence System on Kodak film.
RT-PCR
PRP at different time points(0 h,24 h, or 48 h) was cultured at 37°C. Washed human platelets (3 × 108/mL) were detected for the expression of different genes by RT-PCR. PLS1(FORWARD:TTGAATGTGTTATCGGATCTTGGAGAGG,REVERSE:CACTTCTACGAGGTCATCAGGTAATGC),KRT19(FORWARD:TACAGCCACTACTACACGACCATCC,REVERSE:CTTCGCATGTCACTCAGGATCTTGG),DNBL(FORWARD:AAGCAGAGAAGGAGGAGGAGAACC,REVERSE:GAGGACTGGAGATGGAGGTGGTG),CAPZB(FORWARD:TGGCGTCTCATCTGTCTACCTCTG,REVERSE:GGGAGCAGTCACTCACAGTTTCATC),CNN3(FORWARD:AAGAGGTGACAGGCATGAGCATTG,REVERSE:CTGGCTGGCACATTTGTTGGTTC),ESPN(FORWARD:GACCACAGTGTTCTCAGGCATCG,REVERSE:TCCGCTTCTGCTCCCTCTCTTC),RASL10A(FORWARD:CATCCGCCAGTTCCTGTTCG,REVERSE:CTGTCCCGCTTGTTGCCTAC).
Platelet aggregation
Washed human platelets (3 × 108/mL) were stimulated with 0.01 U thrombin. Platelet aggregation was recorded in a ChronoLog aggregometer at a stirring speed of 1200 rpm at 37°C. Platelet aggregation was monitored continuously over 10 min.
Platelet clot retraction
Washed human platelets (3 × 108/mL) were stimulated with 150 μg/mL fibrinogen and 0.01 U thrombin at 37°C. Photographic observation was carried out every 10 minutes until half an hour later.
Statistical analysis
All data were presented as mean ± SD. Numeric data were analyzed using one-way (for a single variant) ANOVA. The two groups were compared by the two-tailed Student’s t test. The significance of data was assessed using GraphPad Prism 9 software. Differences were considered significant at p < .05.
Results
The number of platelets in PRP increases after being cultured at 37°C
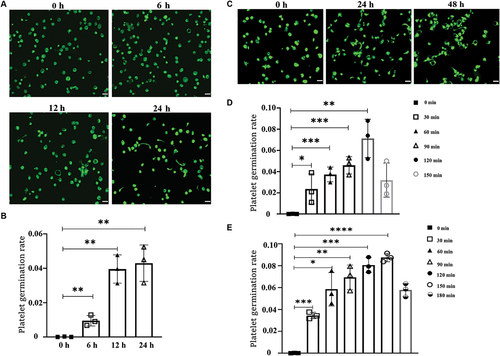
It has been reported that platelets can form the offspring of new spores after being cultured in a specific medium.Citation17 Nevertheless, we found that spore-like platelets also appeared after culture in human platelet-rich plasma(PRP) at 37°C under sterile conditions (). The results also showed that the longer the culture time was, the more the spore platelets were (). Therefore, the time of culture is an important factor affecting the state of platelet sprouting in PRP. At the same time, we were also thinking about whether the temperature would have an impact on it. To verify this idea, we set up 6°C, 22°C and 37°C culture conditions, and found that only 37°C culture conditions can appear a platelet spore state (Supplemental Figure S1A). Therefore, we believe that temperature is also one of the key factors for platelet sprouting in PRP. Moreover, we detected the changes in apoptosis and activation of platelets in PRP and found that platelets did not change significantly even after 48 hours of culture (Supplemental Figure S1B-D). Finally, after giving PRP a fixed shear force after 24 hours and 48 hours of culture, we found that the number of platelets increased with the prolongation of treatment time (). The above data indicate that platelets in PRP will produce offspring after culture, but this will be affected by culture time and temperature.
Figure 1. Platelets in PRP cultured at 37°C show “spores” and increase in number.
Special ingredients in M199 medium promote the germination of human-washed platelets
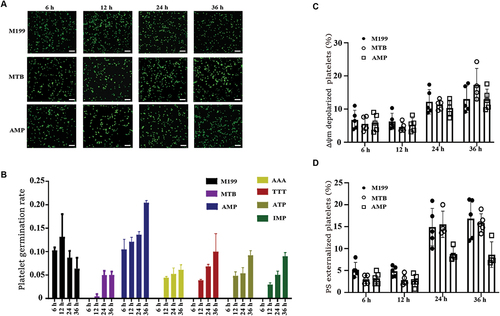
To explore what specific components in PRP can promote platelet “budding” proliferation, the components in PRP are too complex. In the existing research reports, only the M199 medium was found to be able to produce offspring by culturing platelets.Citation17 Therefore, we found that adenine(AAA), thymine(TTT), hypoxanthine(IMP), adenosine diphosphate(AMP), and adenosine triphosphate(ATP) are unique components by analyzing and comparing the components of M199 medium with several other commonly used medium(including DMEM, 1640, MEM and F12, etc). The washed sterile platelets were cultured in vitro at 37°C according to the concentration of each unique component in the M199 medium (MTB buffer as negative control, M199 medium as positive control), and it was found that platelets could sprout in each group with the prolongation of time ( and Supplemental Figure S2). We found that especially in the AMP group, the platelet germination rate was the highest compared to other components, and even reached 20% at 36 hours of culture (). In addition, we also detected the apoptosis of cultured platelets in each group and found that the apoptosis level of the AMP group was also slightly lower than that in the M199 group. (, Supplemental Figure S3, and Supplemental Figure S4).It is possible that the decrease in platelet germination rate in the M199 group is due to an increase in platelet activation. In summary, AMP plays an important role in the process of platelet budding.
Figure 2. Different components in M199 medium promote platelet sprouting.
Activation of AMPK promotes platelet sprouting and proliferation
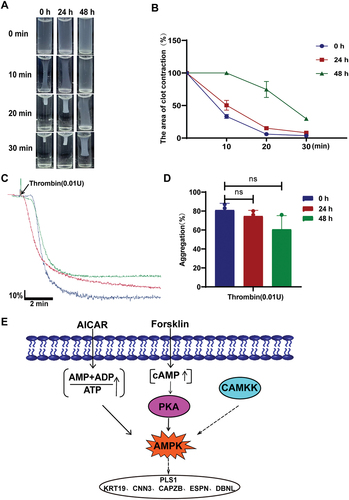
In our results, we have found that AMP can promote the proliferation of platelets. Therefore, we consider whether this phenomenon is caused by changes in AMP-related signaling pathways within platelets. To verify this idea, we detected and analyzed the proteins of AMP-related signaling pathways. The results showed that the phosphorylation levels of VASP and AMPK proteins were significantly increased (). This means that PKACitation24,Citation25 and AMPK are activated during platelet budding and proliferation. Furthermore, the expression level of CAMKK was also significantly increased after 48 hours of cultivation ().
Figure 3. Activation of AMPK promotes sporulation of platelets.
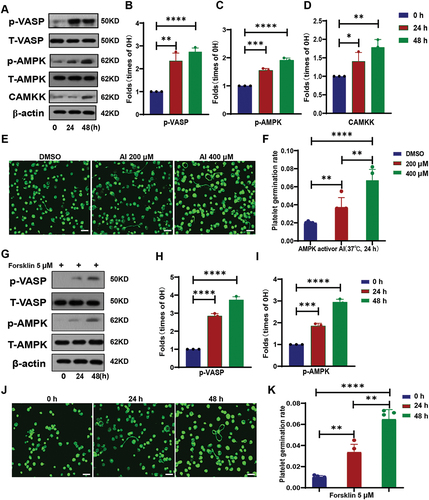
To further verify the role of AMPK and PKA in platelet budding and proliferation, we used their activators AICAR(AI) and Forsklin to treat washed human platelets for culture. The results further demonstrated that the activation of AMPK and PKA in platelets promoted the proliferation of platelets (). We were also surprised to find that after PKA activation, AMPK in platelets was also activated (). This indicates that PKA acts as an upstream kinase of AMPK. The above data demonstrates that the activation of AMPK in platelets can promote the budding and proliferation of platelets.
The expression levels of cytoskeleton protein PLS1 and its related genes are changed during platelet sprouting and proliferation
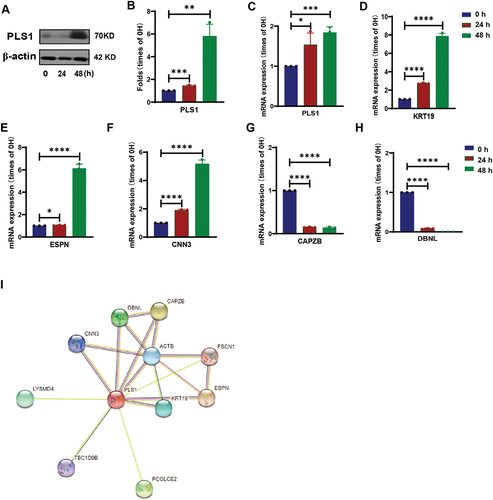
Although platelets are highly differentiated anucleated cells, there are still processes of gene transcription and protein translation inside them,Citation12–15 and even there are differences in the transcripts of platelet gene expression between human and mice.Citation26 Both the existing researchCitation17 and our research results have observed the morphological changes of platelets in the process of sprouting and proliferation. Therefore, we consider whether the skeleton-related genes in platelets will change. We detected some genes that were not expressed or low expressed in human platelets by PCR, including PLS1 related to cytoskeleton protein (Supplemental Figure S5A). The results showed that the expression levels of RASL10 A and PLS1 increased with the proliferation of platelets. Moreover, we also detected the protein expression level of PLS1 and found that it was consistent with the results of the mRNA level (). Through the database STRING to analyze and predict the genes related to PLS1 (). We detected the mRNA levels of these related genes by PCR, and the results showed that the expression levels of PLS1, KRT19, ESPN, and CNN3 were increased, while the expression levels of CAPZB and DBNL were decreased (, Supplemental Figure S5B). In summary, the expression of skeleton protein PLS1 and related genes changed during the process of platelet sprouting and proliferation.
Figure 4. The cytoskeleton protein of spore-like platelets cultured in PRP has significantly a different expression.
Proliferated platelets still have blood clot contraction and aggregation ability
At present, there is still a problem of platelet shortage in clinical practice.Citation27–29 Therefore, our findings can provide a new way to solve this situation. However, whether the sprouted and proliferated platelets still have normal platelet function we need some functional experimental tests to further determine. The results showed that whether cultured for 24 hours or 48 hours, the sprouted and proliferated platelets still had a certain blood clot contraction and aggregation ability under the action of the stimulant thrombin (). According to the results of the functional test, the platelets that may be cultured for 24 hours are slightly better than the platelets that may be cultured for 48 hours, which provides a time reference for clinical transformation and treatment ().
Figure 5. Spore platelets cultured in PRP still have platelet function.
Discussion
The data of this study show that 1) platelet sprouting and proliferation can be cultured in platelet-rich plasma at 37°C; 2) PKA, CAMKK, and AMPK were activated in platelets with sprouting proliferation, and PKA could act as an upstream kinase of AMPK to promote platelet sprouting proliferation (); 3) platelet proliferation can cause changes in the expression of skeleton protein PLS1 and its related genes; 4) The platelets after budding and proliferation still have a certain function.
Platelets are anuclear cell debris produced by megakaryocytes.Citation1,Citation2 Although it has been found in recent decades that platelets are in a terminal differentiation state, they also have functions similar to cells.Citation12–16 Some scholars have found that platelets can produce offspring in M199 medium,Citation17 however, the mechanism of platelet sprouting and proliferation and the thrombus and hemostatic function of platelets after proliferation are still unknown. In our study, platelets in platelet-rich plasma(PRP) were cultured at 37°C in vitro. Furthermore, Our research shows that in vitro platelet aggregation and clot contraction experiments proved that the proliferated platelets still have the function of platelet thrombosis and hemostasis. But, in the study of Schwertz H et al. only the platelet metabolic activity, platelet expression of P-selectin, and αIIbβIII protein were carried out on the platelet progeny.
Most importantly, the activation of PKA, AMPK, and CAMKK was found in our study. Moreover, PKA acts as an upstream of AMPK. PKA plays a positive role in prolonging the life of platelets.Citation24,Citation30 In cells, studies have shown that CAMKK is an upstream kinase of AMPK,Citation31,Citation32 suggesting that CAMKK may play a role in platelet proliferation by activating AMPK. It is well known that AMPK is a switch for energy metabolism regulation in cells.Citation33–35 Similarly, AMPK is involved in the metabolic process of platelets, and even in the process of autophagy, which in turn affects the function of platelets.Citation21,Citation36 At the same time, in the process of studying cell morphology changes, it was found that AMPK promoted the activation of downstream tubulin Tau and CLIP170, which in turn affected the cytoskeleton of cells.Citation37–41 In this study, the mRNA levels of Tau and CLIP170 were detected by PCR, but not detected. The reason may be that the mRNA levels contained in platelets themselves are extremely low. Fortunately, in the screening of related cytoskeleton proteins, it was found that the gene expression level of PLS1 increased with the increase of platelet proliferation. Besides, we also found that the expression of CNN3, CAPZB, DBNL, KRT19, and ESPN related to PLS1 also changed. Nevertheless, it is not clear whether there is a certain interaction between AMPK and cytoskeleton protein PLS1 and related genes. Onselaer MB et al. found that AMPKα1 can be activated by thrombin in human platelets, and phosphorylation of key cytoskeleton targets and actin cytoskeleton remodeling in the process of controlling platelet aggregation.Citation23 This suggests that AMPK may be associated with these skeleton-related genes, but how AMPK affects the changes of cytoskeleton proteins after activation needs further research and exploration.
In summary, this study is the first time to discover that platelet proliferation occurs after platelet-rich plasma (PRP) culture, and it is also the first to discover that AMPK activation can promote platelet proliferation. More importantly, it is suggested that our study can provide a new idea for solving the problem of insufficient platelet supply in clinical practice.
Author contributions
T. Z designed and performed the experiments, analyzed data, and wrote the manuscript. M.Y and S.L helped revise the paper. R. Y and K.D. initiated and supervised the project, designed research, analyzed and interpreted results, and wrote the paper. All authors reviewed the manuscript.
Supplemental Material
Download PDF (1.7 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/09537104.2024.2334701
Additional information
Funding
References
- Geddis AE, Kaushansky KI. The root of platelet production. Science. 2007;317(5845):1689–9. doi:10.1126/science.1148946.
- Montenont E, Bhatlekar S, Jacob S, Kosaka Y, Manne BK, Lee O, Parra-Izquierdo I, Tugolukova E, Tolley ND, Rondina MT, et al. CRISPR-edited megakaryocytes for rapid screening of platelet gene functions. Blood Adv. 2021;5(9):2362–74. doi:10.1182/bloodadvances.2020004112.
- Machlus KR, Italiano JE, Jr. The incredible journey: from megakaryocyte development to platelet formation. J Cell Biol. 2013;201(6):785–96. doi:10.1083/jcb.201304054.
- Koupenova M, Clancy L, Corkrey HA, Freedman JE. Circulating platelets as mediators of immunity, inflammation, and thrombosis. Circ Res. 2018;122(2):337–51. doi:10.1161/CIRCRESAHA.117.310795.
- Koupenova M, Livada AC, Morrell CN. Platelet and megakaryocyte roles in innate and adaptive immunity. Circ Res. 2022;130(2):288–308. doi:10.1161/CIRCRESAHA.121.319821.
- Colman RW, Stewart G, Budzynski A, Ittyerah TR, Olexa S, Kirby E, Fukami M, Piperno J. Thrombosis and haemostasis. Nature. 1979;282(5740):676. doi:10.1038/282676a0.
- Mosnier LO, Buijtenhuijs P, Marx PF, Meijers JC, Bouma BN. Identification of Thrombin Activatable Fibrinolysis Inhibitor (TAFI) in human platelets. Blood. 2003;101(12):4844–6. doi:10.1182/blood-2002-09-2944.
- Remijn JA, Wu YP, Jeninga EH, IJsseldijk MJW, van Willigen G, de Groot PG, Sixma JJ, Nurden AT, Nurden P. Role of ADP receptor P2Y(12) in platelet adhesion and thrombus formation in flowing blood. Arterioscler Thromb Vasc Biol. 2002;22(4):686–91. doi:10.1161/01.ATV.0000012805.49079.23.
- Haemmerle M, Bottsford-Miller J, Pradeep S, Taylor ML, Choi H-J, Hansen JM, Dalton HJ, Stone RL, Cho MS, Nick AM, et al. FAK regulates platelet extravasation and tumor growth after antiangiogenic therapy withdrawal. J Clin Invest. 2016;126(5):1885–96. doi:10.1172/JCI85086.
- Schumacher D, Strilic B, Sivaraj KK, Wettschureck N, Offermanns S. Platelet-derived nucleotides promote tumor-cell transendothelial migration and metastasis via P2Y2 receptor. Cancer Cell. 2013;24(1):130–7. doi:10.1016/j.ccr.2013.05.008.
- Suzuki-Inoue K, Tsukiji N. Platelet CLEC-2 and lung development. Res Pract Thromb Haemost. 2020;4(4):481–90. doi:10.1002/rth2.12338.
- Shashkin PN, Brown GT, Ghosh A, Marathe GK, McIntyre TM. Lipopolysaccharide is a direct agonist for platelet RNA splicing. J Immunol. 2008;181(5):3495–502. doi:10.4049/jimmunol.181.5.3495.
- Denis MM, Tolley ND, Bunting M, Schwertz H, Jiang H, Lindemann S, Yost CC, Rubner FJ, Albertine KH, Swoboda KJ, et al. Escaping the nuclear confines: signal-dependent pre-mRNA splicing in anucleate platelets. Cell. 2005;122(3):379–91. doi:10.1016/j.cell.2005.06.015.
- Weyrich AS, Dixon DA, Pabla R, Elstad MR, McIntyre TM, Prescott SM, Zimmerman GA. Signal-dependent translation of a regulatory protein, bcl-3, in activated human platelets. Proc Natl Acad Sci U S A. 1998;95(10):5556–61. doi:10.1073/pnas.95.10.5556.
- Panes O, Matus V, Saez CG, Quiroga T, Pereira J, Mezzano D. Human platelets synthesize and express functional tissue factor. Blood. 2007;109(12):5242–50. doi:10.1182/blood-2006-06-030619.
- Thon JN, Devine DV. Translation of glycoprotein IIIa in stored blood platelets. Transfusion. 2007;47(12):2260–70. doi:10.1111/j.1537-2995.2007.01455.x.
- Schwertz H, Köster S, Kahr WH, Michetti N, Kraemer BF, Weitz DA, Blaylock RC, Kraiss LW, Greinacher A, Zimmerman GA, et al. Anucleate platelets generate progeny. Blood. 2010;115(18):3801–9. doi:10.1182/blood-2009-08-239558.
- Carling D. The AMP-activated protein kinase cascade–a unifying system for energy control. Trends Biochem Sci. 2004;29(1):18–24. doi:10.1016/j.tibs.2003.11.005.
- Steinberg GR, Kemp BE. AMPK in health and disease. Physiol Rev. 2009;89(3):1025–78. doi:10.1152/physrev.00011.2008.
- Carling D, Mayer FV, Sanders MJ, Gamblin SJ. AMP-activated protein kinase: Nature’s energy sensor. Nat Chem Biol. 2011;7(8):512–8. doi:10.1038/nchembio.610.
- Lepropre S, Kautbally S, Octave M, Ginion A, Onselaer M-B, Steinberg GR, Kemp BE, Hego A, Wéra O, Brouns S, et al. AMPK-ACC signaling modulates platelet phospholipids and potentiates thrombus formation. Blood. 2018;132(11):1180–92. doi:10.1182/blood-2018-02-831503.
- Randriamboavonjy V, Isaak J, Frömel T, Viollet B, Fisslthaler B, Preissner KT, Fleming I. AMPK α2 subunit is involved in platelet signaling, clot retraction, and thrombus stability. Blood. 2010;116(12):2134–40. doi:10.1182/blood-2010-04-279612.
- Onselaer MB, Oury C, Hunter RW, Eeckhoudt S, Barile N, Lecut C, Morel N, Viollet B, Jacquet LM, Bertrand L, et al. The Ca2+/calmodulin-dependent kinase kinase β-AMP-activated protein kinase-α1 pathway regulates phosphorylation of cytoskeletal targets in thrombin-stimulated human platelets. J Thromb Haemost. 2014;12(6):973–86. doi:10.1111/jth.12568.
- Zhao L, Liu J, He C, Yan R, Zhou K, Cui Q, Meng X, Li X, Zhang Y, Nie Y, et al. Protein kinase a determines platelet life span and survival by regulating apoptosis. J Clin Invest. 2017;127(12):4338–51. doi:10.1172/JCI95109.
- Rukoyatkina N, Butt E, Subramanian H, Nikolaev VO, Mindukshev I, Walter U, Gambaryan S, Benz PM. Protein kinase a activation by the anti-cancer drugs ABT-737 and thymoquinone is caspase-3-dependent and correlates with platelet inhibition and apoptosis. Cell Death Dis. 2017 Jun 29;8(6):e2898. doi:10.1038/cddis.2017.290.
- Rowley JW, Oler AJ, Tolley ND, Hunter BN, Low EN, Nix DA, Yost CC, Zimmerman GA, Weyrich AS. Genome-wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood. 2014 Jun 12;123(24):3843.
- Rock G, Sherring VA, Tittley P. Five-day storage of platelet concentrates. Transfusion. 1984;24(2):147–52. doi:10.1046/j.1537-2995.1984.24284173347.x.
- Bode AP. Platelet activation may explain the storage lesion in platelet concentrates. Blood Cells. 1990;16:125–6.
- Rinder HM, Murphy M, Mitchell JG, Stocks J, Ault KA, Hillman RS. Progressive platelet activation with storage: evidence for shortened survival of activated platelets after transfusion. Transfusion. 1991;31(5):409–14. doi:10.1046/j.1537-2995.1991.31591263195.x.
- Chen M, Yan R, Zhou K, Li X, Zhang Y, Liu C, Jiang M, Ye H, Meng X, Pang N, et al. Akt-mediated platelet apoptosis and its therapeutic implications in immune thrombocytopenia. Proc Natl Acad Sci U S A. 2018;115(45):10682–91. doi:10.1073/pnas.1808217115.
- Sun B, Ou H, Ren F, Huan Y, Zhong T, Gao M, Cai H. Propofol inhibited autophagy through Ca2+/CaMKKβ/AMPK/mTOR pathway in OGD/R-induced neuron injury. Mol Med. 2018;24(1):58. doi:10.1186/s10020-018-0054-1.
- Jin L, Chun J, Pan C, Kumar A, Zhang G, Ha Y, Li D, Alesi GN, Kang Y, Zhou L, et al. The PLAG1-GDH1 axis promotes anoikis resistance and tumor metastasis through CamKK2-AMPK signaling in LKB1-deficient lung cancer. Mol Cell. 2018;69(1):87–99.e7. doi:10.1016/j.molcel.2017.11.025.
- Wu H, Liu B, Chen Z, Li G, Zhang Z. MSC-induced lncRNA HCP5 drove fatty acid oxidation through miR-3619-5p/AMPK/PGC1α/CEBPB axis to promote stemness and chemo-resistance of gastric cancer. Cell Death Dis. 2020;11(4):233. doi:10.1038/s41419-020-2426-z.
- Yan LS, Zhang SF, Luo G, Cheng BCY, Zhang C, Wang Y-W, Qiu X-Y, Zhou X-H, Wang Q-G, Song X-L, et al. Schisandrin B mitigates hepatic steatosis and promotes fatty acid oxidation by inducing autophagy through AMPK/mTOR signaling pathway. Metabolism. 2022;131:155200. doi:10.1016/j.metabol.2022.155200.
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8(11):1288–95. doi:10.1038/nm788.
- Lee TY, Lu WJ, Changou CA, Hsiung Y-C, Trang NTT, Lee C-Y, Chang T-H, Jayakumar T, Hsieh C-Y, Yang C-H, et al. Platelet autophagic machinery involved in thrombosis through a novel linkage of AMPK-MTOR to sphingolipid metabolism. Autophagy. 2021;17(12):4141–58. doi:10.1080/15548627.2021.1904495.
- Yoshida H, Goedert M. Phosphorylation of microtubule-associated protein tau by AMPK-related kinases. J Neurochem. 2012;120(1):165–76. doi:10.1111/j.1471-4159.2011.07523.x.
- Vingtdeux V, Davies P, Dickson DW, Marambaud P. AMPK is abnormally activated in tangle- and pre-tangle-bearing neurons in Alzheimer’s disease and other tauopathies. Acta Neuropathol. 2011;121(3):337–49. doi:10.1007/s00401-010-0759-x.
- Kickstein E, Krauss S, Thornhill P, Rutschow D, Zeller R, Sharkey J, Williamson R, Fuchs M, Köhler A, Glossmann H, et al. Biguanide metformin acts on tau phosphorylation via mTOR/protein phosphatase 2A (PP2A) signaling. Proc Natl Acad Sci U S A. 2010;107(50):21830–5. doi:10.1073/pnas.0912793107.
- Nakano A, Kato H, Watanabe T, Min K-D, Yamazaki S, Asano Y, Seguchi O, Higo S, Shintani Y, Asanuma H, et al. AMPK controls the speed of microtubule polymerization and directional cell migration through CLIP-170 phosphorylation. Nat Cell Biol. 2010;12(6):583–90. doi:10.1038/ncb2060.
- Yashirogi S, Nagao T, Nishida Y, Takahashi Y, Qaqorh T, Yazawa I, Katayama T, Kioka H, Matsui TS, Saito S, et al. AMPK regulates cell shape of cardiomyocytes by modulating turnover of microtubules through CLIP-170. EMBO Rep. 2021;22(1):e50949. doi:10.15252/embr.202050949.

