Abstract
Currently, the standard treatment for patients who have undergone percutaneous coronary intervention (PCI) following acute myocardial infarction (MI) involves dual antiplatelet therapy (DAPT) with a combination of aspirin and a potent P2Y12 receptor inhibitor. However, the potential benefits of aspirin were partially constrained by the intolerance of some patients. The safety and efficacy of indobufen, an alternative antiplatelet agents to aspirin, in patients with AMI after PCI are yet to be thoroughly investigated.
This retrospective study was conducted at a single center and utilized propensity score matching. The enrollment spanned from January 2019 to June 2022, incorporating patients with AMI after PCI. The participants were categorized into two groups based on discharged prescriptions: the aspirin DAPT group and the indobufen DAPT group. The primary endpoint focused on net adverse clinical event (NACE), defined as a composite outcome, including cardiac death, recurrence of MI, definite or probable stent thrombosis (ST), target lesion revascularization (TLR), ischemic stroke and Bleeding Academic Research Consortium (BARC) criteria type 2, 3, or 5. All the patients underwent a one-year follow-up period.
A total of 1451 patients were enrolled in this study, with 258 assigned to the indobufen DAPT group and 1193 to the aspirin DAPT group. Following 1:1 propensity score matching, 224 patients were retained in each group. In the indobufen DAPT group, 58 individuals (25.9%) experienced the primary endpoint within one year, compared to 52 individuals (23.2%) in the aspirin DAPT group (HR 1.128, 95% CI 0.776–1.639, p = .527). Specifically, no significant differences were observed in either the efficacy endpoint (MACCE, 20.1% vs. 14.7%, HR 1.392, 95% CI 0.893–2.170, p = .146) or the safety endpoint (BARC 2,3 or 5, 8.04% vs. 10.30%, HR 0.779, p = .427). These findings remained consistent at 1, 3, or 6 months. Additionally, the incidence of gastrointestinal symptoms were significantly lower in indobufen DAPT group compared to the aspirin DAPT group (7.1% vs. 14.3%, p = .022).
Our research reveals that the efficacy and safety of indobufen are comparable to aspirin in Chinese patients with AMI following PCI. Given the potential advantages of indobufen in alleviating gastrointestinal symptoms, we propose it as a viable alternative for individuals intolerant to aspirin.
Plain Language Summary
What is the context?
Currently, the standard treatment for patients who have undergone percutaneous coronary intervention following acute myocardial infarction involves dual antiplatelet therapy with a combination of aspirin and a potent P2Y12 receptor inhibitor.
However, the potential benefits of aspirin were partially constrained by the intolerance of some patients.
The safety and efficacy of indobufen, an alternative antiplatelet agents to aspirin, in patients with AMI after PCI are yet to be thoroughly investigated.
What is new?
While both American and European clinical guidelines recommend the use of indobufen as an alternative treatment for patients who cannot tolerate aspirin, there exists a limited body of research on this subject.
Our research is the first to address this gap by comparing the efficacy and safety of indobufen and aspirin in patients with AMI.
Our research reveals that the efficacy and safety of indobufen are comparable to aspirin in Chinese patients with AMI following PCI. Given the potential advantages of indobufen in alleviating gastrointestinal symptoms, we propose it as a viable alternative for individuals intolerant to aspirin.
What is the impact?
These findings might pave the way for further exploration of alternatives to aspirin in patients with AMI.
Introduction
In the context of acute myocardial infarction (AMI), effective antiplatelet therapy plays a crucial role in minimizing the recurrence of thrombotic events. Current guidelines recommend the use of dual antiplatelet therapy (DAPT) involving aspirin in patients with AMI after percutaneous coronary intervention (PCI).Citation1 Despite its robust antiplatelet efficacy, the associated side effects cannot be ignored.Citation2 Previous studies have reported that aspirin resistance, affecting 5%-60% of patients, contributes to an increased recurrence rate of thrombotic events.Citation3 Findings from the SYMPHONY large sample study indicate that among aspirin users, 5.4% experienced nausea, 1.7% had vomiting, 2.6% reported dyspepsia or discomfort, and 2.2% suffered from abdominal pain. Even in acute coronary syndrome (ACS) patients with high ischemic risk, approximately 9% of patients discontinued aspirin over an extended follow-up period due to issues such as bleeding, intolerance, and various other factors.Citation4 Meta-analysis showed that 2–3% of aspirin users experienced gastrointestinal bleeding, with a 50–60% increased risk compared to a placebo. Even with a reduced dose of aspirin, there has been no significant improvement in the associated bleeding tendency.Citation5
Indobufen, considered one of the alternative antiplatelet agents to aspirin, exerts its influence by reversibly inhibiting platelet aggregation. Its effect stems from the reversible inhibition of cyclooxygenase 1 (COX-1), thereby blocking the formation of thromboxane (TX) A2, while having little effect on prostacyclin (PGI2) production.Citation6,Citation7 Previous studies have shown that indobufen exhibited a comparable antiplatelet effect to aspirin, particularly in patients with aspirin allergy or intolerance.Citation8,Citation9 In individuals with chronic coronary syndromes (CCS), indobufen has demonstrated the ability to decrease bleeding events without elevating ischemic risks.Citation10 There have been studies suggesting that indobufen can serve as a viable alternative to aspirin.Citation10,Citation11 However, its safety and efficacy in patients with AMI after PCI are yet to be thoroughly investigated.
This study seeks to address the gap by retrospectively analyzing the 1-year follow-up data to evaluate the safety and efficacy of combining indobufen with clopidogrel in individuals with AMI after PCI.
Methods
Study design and participants
This retrospective study was conducted as a single-center investigation at the Department of Cardiology, Second Hospital of Tianjin Medical University in China. The study adhered to the principles outlined in the Declaration of Helsinki and the protocol received approval from the Clinical Research Ethics Committee of the Second Hospital of Tianjin Medical University on 26 October 2023 (approved number: KY2023K205). Informed consent was waived due to the retrospective nature of the study.
This study enrolled patients with AMI, comprising both ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation myocardial infarction (NSTEMI), admitted to the Second Hospital of Tianjin Medical University between 10 January 2019, and 30 June 2022. The diagnosis was based on the fourth universal definition of myocardial infarction.Citation12 All the participants fulfilled the following criteria: (1) age over 18 years old, (2) positive biomarkers, (3) definitive clinical evidence of ischemia, (4) successful PCI with contemporary drug-eluting stents (DES). Exclusion criteria included: (1) use of antiplatelet drugs other than aspirin, indobufen, and clopidogrel; (2) treatment with single or triple antiplatelet therapy; (3) non-standardized dosage of dual antiplatelet therapy; (4) withdrawal or alteration of the treatment regimen; (5) patients who did not fulfill the 1-year follow-up requirements.
Data collection
Comprehensive data on patient demographics, medication, medical history, and laboratory results including cardiac enzymes and blood lipids were extracted from the medical records. Additionally, clinical observations during hospitalization and interventional details, such as the quantity and location of vascular lesions, as well as the number and placement of stents, were thoroughly analyzed. The patients were categorized into two groups based on discharged DAPT prescriptions: the aspirin DAPT group and the indobufen DAPT group. Prior to the procedure, all patients received a loading dose of antiplatelet drugs orally, typically 300 mg of aspirin or 200 mg of indobufen, and 300 mg of clopidogrel. The techniques and strategies of PCI were determined by the operator’s discretion. The indobufen DAPT group received a regimen of 100 mg of indobufen twice a day along with 75 mg of clopidogrel daily for 12 months. In contrast, the aspirin DAPT group was treated with 100 mg of aspirin daily combined with 75 mg of clopidogrel daily for the same duration. Follow-up assessments were conducted at 1, 3, 6, and 12 months after PCI, mainly through telephone interactions. Some patients who returned to our hospital took the time of further consultation as the time of follow-up. During these follow-up sessions, clinical data, such as health status, medication (adherence or medication prescription history), occurrence of thrombotic events, and any adverse reactions were meticulously recorded. Patients who did not consistently adhere to the prescribed medication regimen after discharge were excluded from the follow-up analysis.
Endpoints
The primary endpoint event in this study was the occurrence of net adverse clinical event (NACE), which is a composite measure including cardiac death, nonfatal MI, definite or probable stent thrombosis (ST), target lesion revascularization (TLR), ischemic stroke and bleeding classified according to, Bleeding Academic Research Consortium (BARC) criteria as type 2, 3, or 5 within a 1-year time-frame. The secondary efficacy endpoint, Major Adverse Cardiac and Cerebral Events (MACCE) was characterized by a composite of cardiac death, recurrence of MI, definite or probable ST, TLR, and ischemic stroke. The secondary safety endpoint included assessing bleeding events according to BARC criteria, specifically type 2, 3, or 5. Additionally, gastrointestinal symptoms, a composite of gastric burning, nausea and vomiting, dyspepsia, and gastric ulcer, were also considered as one of the endpoint events.
Statistical analysis
Data were processed using R version 4.0.4 software. Categorical variables were presented as percentage, and group comparisons were made using the chi-square test. Continuous variable data were expressed as mean ± standard deviation, and comparisons between the two groups were conducted using the independent sample t-test. To minimize bias in observational studies, propensity score matching was employed at a 1:1 ratio between the two groups, utilizing the nearest neighbor approach based on Mahalanobis distance with a caliper of 0.02. The propensity score for each patient was calculated as a probability from a logistic regression model, incorporating 15 clinically important covariates impacting patient’s prognosis: sex; age; clinical Indication of PCI (NSTEMI/STEMI); previous PCI; previous MI; hypertension; diabetes; chronic kidney disease (CKD); previous ischemic stroke; previous cerebral hemorrhage; anemia (Hb < 90 g/L); previous gastrointestinal bleeding; the Academic Research Consortium-High Bleeding Risk (ARC-HBR) criteriaCitation13; smoking history; proton pump inhibitor (PPI) use. Kaplan-Meier curves were utilized to illustrate survival curves for NACE, MACCE, and BARC 2, 3, or 5 bleeding events, with log-rank tests employed for comparisons. Subgroup analyses of endpoints were conducted based on important characteristics, including age (≥75 years or <75 years), sex (male or female), clinical indication of PCI (NSTEMI/STEMI), previous MI, hypertension, diabetes, eGFR (<60 ml/min/1.73 m2 or ≥60 ml/min/1.73 m2), stroke history, PPI use, ARC-HBR. All tests were two-tailed, and a significance level of p < .05 was applied.
Results
Patient enrollment
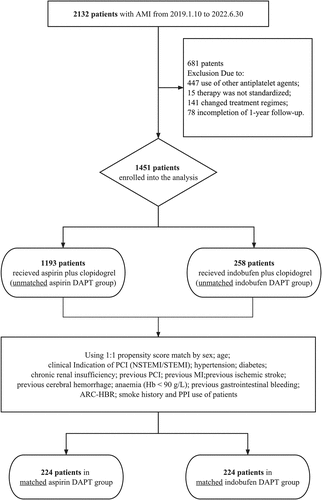
From 10 January 2019 to 30 June 2022, a total of 2132 patients with AMI underwent PCI at our center. Among them, 447 patients received alternative antiplatelet drugs, 15 patients underwent non-standardized treatment, 141 patients modified their treatment regimens, and 78 patients were excluded due to incomplete 1-year follow-up. Ultimately, this cohort study included 1451 patients, with 258 patients in the indobufen DAPT group and 1193 patients in the aspirin DAPT group. Following 1:1 propensity score matching, 224 patients were included in both groups ().
Figure 1. Patient enrollment.
Baseline characteristics pre and post propensity score matching
illustrates the baseline characteristics of the patients, categorized by medication, for both the unmatched and matched samples. Before matching, the indobufen DAPT group displayed noteworthy discrepancies compared to the aspirin DAPT group. These differences included a higher percentage of elderly patients, males, individuals at higher risk of bleeding, lower levels of low-density lipoprotein cholesterol (LDLc), and fewer patients with a history of smoking in the Indobufen DAPT group as opposed to the Aspirin DAPT group. Regarding medical history, the Indobufen DAPT group exhibited notably higher rates of prior PCI, CKD, previous cerebral hemorrhage, and a history of gastrointestinal bleeding. Significant differences were also observed in medication usage (including β-blockers, angiotensin converting enzyme inhibitors (ACEI) or angiotensin receptor blockers (ARB), PPIs, and statins) between the two groups (p < 0.05). Additionally, the Indobufen DAPT group demonstrated a higher prevalence of diseased vessels in terms of interventional characteristics.
Table I. Clinical characteristics of patients.
Following a 1:1 propensity score matching, post-matching absolute standardized differences were consistently below 10% for all covariates (Figure S1). A total of 448 patients were successfully matched, resulting in 224 patients in each of the two groups.
The median follow-up duration for the matched patients was 2.1 years. These patients, with an average age of 72 ± 11 years, included 291 (65.0%) males. Among them, 293 (65.4%) were diagnosed with NSTEMI, and 155 (34.6%) were STEMI. Smoking history was reported in 162 (36.2%) patients, while 182 (40.6%) had a history of diabetes, and 333 (74.3%) had hypertension (refer to ). Additionally, 42 (9.38%) patients presented with left main coronary artery disease, 429 (95.8%) had lesions in the left anterior descending artery, and 420 (93.8%) had multi-vessel coronary artery disease. The average number of diseased vessels for all patients was 2.90 ± 0.67, with a mean of 1.36 ± 0.89 stents implanted(see ).
Table II. One-year clinical endpoints in patients with AMI.
Primary endpoints
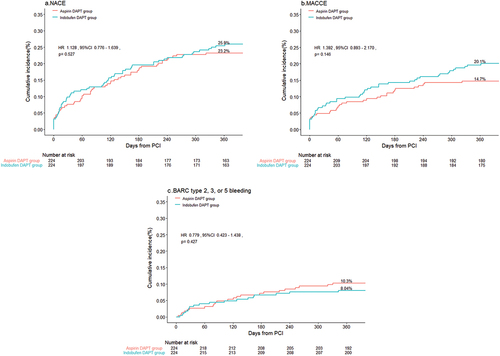
The primary endpoint was observed in 58 (25.9%) patients in the indobufen DAPT group and 52 (23.2%) patients in the aspirin DAPT group. Log-rank test showed no significant difference in the risk of the primary endpoint between the two groups (HR 1.128, 95% CI, 0.776–1.639, p = .527) (, ).
Figure 2. Cumulative Kaplan-Meier curve estimates of NACE (A), MACCE (B), and BARC 2, 3, or 5 (C) at 1 year in patients with AMI.
Efficacy endpoints
In terms of the secondary efficacy endpoints, the occurrence of MACCE was noted in 45 (20.1%) patients in the indobufen DAPT, compared to 33 (14.7%) patients in the aspirin DAPT group (HR 1.392, 95%CI 0.893–2.170, p = .146) (, ). The remaining efficacy endpoints, including recurrence of cardiac death, MI, definite or probable ST, TLR, and Ischemic stroke, displayed similar rates between the two groups (p > .05) ().
Safety endpoints
Regarding secondary safety endpoints, bleeding events of BARC type 2, 3, or 5 developed in 18 (8.04%) patients in the indobufen DAPT group, and 23 (10.3%) patients in the aspirin DAPT group(HR 0.779, 95% CI 0.423–1.438, p = .427). Specifically, BARC type 2 bleeding events were observed in 13 (5.80%) patients in the indobufen DAPT group, compared to 19 (8.48%) patients in the aspirin DAPT group (HR 0.681, 95%CI 0.341–1.363, p = .284). The incidence of BARC type 3 or 5 bleeding was similar between the two groups (2.23% vs. 1.79%, p = .744) ().
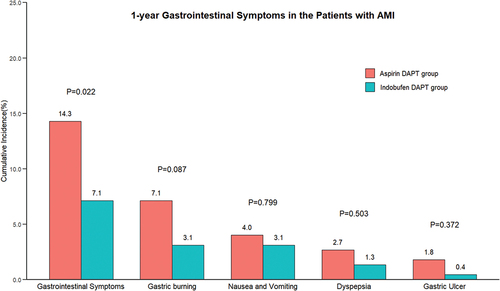
Gastrointestinal symptoms
Aspirin DAPT patients were more likely to suffer from gastrointestinal symptoms (a composite of gastric burning, nausea and vomiting, dyspepsia, gastric ulcer) than indobufen DAPT patients (14.3% vs. 7.1%, p = .022). The incidence of the individual components of gastrointestinal symptoms (i.e., gastric burning, nausea and vomitting, dyspepsia, gastric ulcer) was similar between two groups ().
Sensitivity analysis
Upon follow-up at 1, 3, or 6 months, reanalysis of the data revealed no substantial changes. Endpoint rates of NACE, MACCE, or BARC 2, 3, or 5 were comparable between the two groups at each time point (Figure S2-S4).
Subgroup analysis
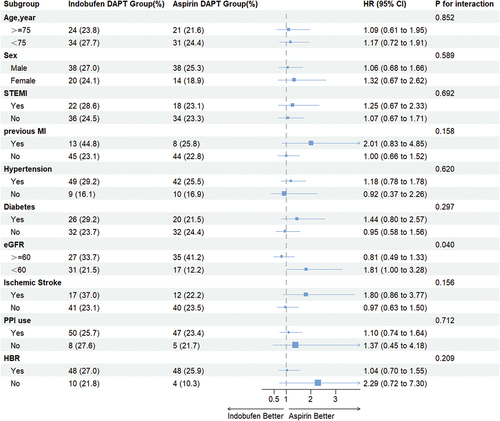
Subgroup analysis was conducted for clinically relevant factors, including age (≥75 years or <75 years), sex (male or female), clinical indication of PCI (NSTEMI/STEMI), previous MI, hypertension, diabetes, eGFR (<60 ml/min/1.73 m2 or ≥60 ml/min/1.73 m2), stroke history, PPI use, ARC-HBR. For the primary endpoint, the indobufen DAPT group exhibited comparable risks of NACE in most subgroups when compared to aspirin DAPT. It’s worth noting that CKD stages 3–5 modified the association between indobufen and NACE. In patients with CKD stages 3–5, the indobufen DAPT group showed a 1.8-fold higher risk of NACE (HR, 1.81; 95% CI, 1.00–3.28), whereas in patients with eGFR ≥60 ml/min/1.73 m2, the risk was 0.8-fold (HR, 0.81; 95% CI, 0.49–1.33 [p value for interaction = 0.040]). No other significant interactions were detected ().
Figure 4. Subgroup analyses for primary endpoints (NACE) of the patients with AMI.
Concerning secondary efficacy endpoints, although no significant interactions were observed in all subgroups (all p for interaction >0.05), the use of indobufen was associated with an increased incidence of MACCE in female patients, those with CKD stages 3–5, and individuals with a history of ischemic stroke. The indobufen DAPT group showed a 2.3-fold higher risk of MACCE in female patients (HR, 2.27; 95% CI, 1.00–5.20), a 2.2-fold higher risk in patients with CKD stages 3–5 (HR, 2.16; 95% CI, 1.06–4.42), and a 2.7-fold higher risk in patients with previous ischemic stroke (HR, 2.7; 95% CI, 1.03–7.16) (Figure S5).
Regarding secondary safety endpoints, the two groups were generally consistent across all subgroups with no significant interaction (Figure S6).
Discussions
Currently, while both American and European clinical guidelines recommend the use of indobufen as an alternative treatment for patients who cannot tolerate aspirin,Citation1,Citation14 there exists a limited body of research on this subject. Our research is the first to address this gap by comparing the efficacy and safety of indobufen and aspirin in patients with AMI. In the context of Chinese patients with AMI after PCI, our findings indicate a non-significant difference in 1-year NACE rates between the indobufen DAPT and aspirin DAPT groups (25.9% vs. 23.2%, p = .527). Specifically, no significant differences were noted in either the efficacy endpoint (MACCE, 20.1% vs. 14.7%, HR 1.392, 95% CI 0.893–2.170, p = .146) or the safety endpoint (BARC 2,3, or 5, 8.04% vs. 10.30%, HR 0.779, p = .427). These results suggest that the efficacy and safety of indobufen may be comparable to aspirin in Chinese patients with AMI after PCI.
The standard treatment for patients with AMI after PCI involves a dual antiplatelet therapy comprising aspirin and a potent P2Y12 receptor inhibitor. However, aspirin use is frequently linked to complications such as resistance, gastrointestinal reactions, allergic responses and bleeding events.Citation3,Citation4 In a study conducted by Latib involving 127 patients intolerant to aspirin, indobufen emerged as the predominant alternative. Remarkably, 64.6% of the participants chose indobufen over aspirin in conjunction with clopidogrel.Citation9 Indobufen functions as a platelet COX inhibitor, presenting a distinctive advantage over aspirin by reversibly inhibiting COX − 1 activity. This mechanism effectively hinders the activation of the TxA2 pathway in platelets. Recognizing its efficacy, the American College of Chest Physicians identified indobufen as a potent platelet COX-1 inhibitor in 2008, with both biochemical and clinical effectiveness comparable to standard-dose aspirin. A single 200 mg dose of indobufen can rapidly inhibit TxA2 generation by more than 95% within a mere 2 hours.Citation15 In healthy volunteers, the initial inhibition of platelet aggregation induced by indobufen (200 mg twice a day) closely mirrors that of aspirin (200 mg once a day). However, the anti-aggregation effect of indobufen diminished more rapidly than that observed with aspirin. This suggests that indobufen achieves a maximum antiplatelet comparable to aspirin while displaying characteristics of swift recovery.Citation16
The adverse effects associated with aspirin stem from the inhibition of COX, affecting pathways beyond platelets and resulting in a reduction in PGI2 generation. In healthy volunteers, indobufen (200 mg twice daily) and aspirin (300 mg once daily) were administrated over one week. While there was no significant difference in the degree of platelet inhibition between the two groups, the indobufen DAPT group exhibited a significantly lower impact on PG production, compared to the aspirin DAPT group (p < .001). This suggests that indobufen demonstrates higher selectivity for platelets and enhanced gastrointestinal tolerance.Citation17 Endoscopic studies corroborated these findings, demonstrating that indobufen, in comparison with aspirin, significantly mitigated damage to the gastrointestinal tract.Citation18 Therefore, gastrointestinal symptoms were considered as one of the clinical prognostic indicators. We observed a significantly lower incidence of gastrointestinal symptoms in the indobufen DAPT group.
The OPTION study provided evidence supporting indobufen as an alternative to aspirin, showing its capacity to reduce bleeding events (2.97% vs. 4.71%) without an associated increase in ischemic events (1.51% vs. 1.40%) in Chinese cardiac troponin negative patients undergoing DES implantation.Citation10 A real-world study found that indobufen-based DAPT had the same MACCE risk (6.5% vs. 6.5%) but a lower bleeding risk (3.0% vs. 11.9%) compared with aspirin-based DAPT.Citation19 Conversely, insights from the INSURE study hint that indobufen appears to be less effective than aspirin in reducing the risk of recurrent strokes within 90 days.Citation20 The conflicting conclusions underscore the need for a more comprehensive exploration into the efficacy and safety of indobufen in comparison to aspirin.
Our study revealed no substantial variance in primary and secondary endpoints between the two groups. However, the incidence of gastrointestinal symptoms were significantly lower in indobufen DAPT group. This observation supports the notion of considering indobufen as a viable alternative to aspirin for individuals with AMI after PCI.
Of note, in our subgroup analysis, the presence of CKD stages 3–5 significantly influenced the relationship between indobufen and the composite endpoint of NACE.
CKD is associated with increased platelet reactivity, systemic inflammation, oxidative stress, and a pro-coagulant state, contributing to the occurrence of ischemic events. Paradoxically, these patients also face an elevated risk of bleeding, significantly complicating their treatment.Citation21 As kidney function deteriorates, the risks of both ischemic and bleeding events markedly rise. In a study involving 8593 patients with ACS, rates of ischemic and bleeding events within 24 months increased significantly across various stages of CKD. In patients with moderately decreased kidney function, a 5 ml/min decrease in creatinine clearance was associated with a 20–40% increased risk of ischemic and bleeding events.Citation22 Therefore, achieving a delicate balance between ischemic and bleeding risks is crucial in devising antiplatelet strategies. Unfortunately, patients with CKD are often excluded from major clinical trials due to inadequate representation. Despite our subgroup analysis suggesting a preference for aspirin use in patients with CKD stages 3–5, given the observational nature of the study and the limitations of the subgroup analysis, this association should be interpreted with caution. It’s worth noting that a large-scale intentional clinical trial (ATTACK, NCT03796156) is currently underway to investigate the primary preventive effects of daily 75 mg aspirin on cardiovascular complications, bleeding, and CKD progression in patients with CKD stages 3–5.Citation23
Actually, indobufen not only exerts antiplatelet effects but also demonstrates anticoagulant properties. Studies indicate that in animal experiments, indobufen significantly lowers the levels of APTT, PT, TT, PF3, FI, II, V, VIII, and X in plasma.Citation24 We had intended to analyze the benefit of indobufen in the subgroup of patients with atrial fibrillation in the AMI population. However, we should recognize the small sample size of atrial fibrillation patients in our study, compromising the depth of meaningful subgroup analysis. Further investigation involving a larger, AMI with atrial fibrillation-specific cohort is warranted to establish conclusive findings.
There were several limitations in this study, with its retrospective nature being the primary constraint. This study has several limitations, with its retrospective nature being the primary constraint. Due to the tendency for gastrointestinal reactions to be overlooked, precise onset times were challenging to ascertain. However, we recorded whether patients experienced gastrointestinal reactions within the 1-year follow-up. Moreover, there have been studies consistently emphasize a relatively lower incidence of gastrointestinal adverse reactions associated with indobufen compared to aspirin.Citation7,Citation18 While retrospective studies inherently carry bias in outcome reporting and ascertainment, we employed rigorous methods, using propensity score matching to address discrepancies in disease severity among patients. Another constraint stems from the practices specific to a single center, with very few patients receiving ticagrelor or prasugrel on top of indobufen. Hence, this study exclusively investigates the combined use of indobufen and clopidogrel, while the safety and efficacy of indobufen with other potent P2Y12 receptor inhibitors require additional assessment in separate cohorts to enhance the generalizability of the findings. Additionally, the study sample size was relatively small. Therefore, large randomized clinical trial are necessary to more definitely assess the efficacy and safety of indobufen versus aspirin in patients with AMI.
In conclusion, our research reveals that the efficacy and safety of indobufen are comparable to aspirin in Chinese patients with AMI following PCI. Given the potential advantages of indobufen in alleviating gastrointestinal symptoms, we propose it as a viable alternative for individuals intolerant to aspirin.
List of abbreviations
| ACS | = | Acute coronary syndrome; |
| ADP | = | Adenosine -diphosphate; |
| AMI | = | Acute myocardial infarction; |
| ARC-HBR | = | The Academic Research Consortium-High Bleeding Risk; |
| BARC | = | Bleeding Academic Research Consortium; |
| CI | = | Confidence interval; |
| COX-1 | = | Cyclooxygenase-1; |
| CKD | = | Chronic kidney disease; |
| DAPT | = | Dual antiplatelet therapy; |
| DES | = | Drug-eluting stents; |
| eGFR | = | Estimated Glomerular Filtration Rate; |
| HR | = | Hazard ratio; |
| MACCE | = | Major adverse cardiac and cerebral events; |
| NACE | = | Net adverse clinical event; |
| NSTEMI | = | Non-ST segment elevation myocardial infarction; |
| PCI | = | Percutaneous coronary intervention; |
| PGI2 | = | Prostacyclin; |
| PPI | = | Proton pump inhibitor; |
| ST | = | Stent thrombosis; |
| STEMI | = | ST segment elevation myocardial infarction; |
| TLR | = | Target lesion revascularization; |
| TX | = | Thromboxane; |
Authors’ contributions
The conception and study design were put forward by Tong Liu and Kang-Yin Chen. Wen-Bo Dai, Tian-Shu Gu, Jia-Yi Ren, Su-Tao Hu and Yu-Kun Zhang contributed to the analysis and interpretation of data, while Wen-Bo Dai, Xue Wu, Jing-Kun Zhang and Guang-Ping Li were responsible for drafting of the manuscript or revising it. Jing-Jin Che and Xiang-Hong Ma contributed to final approval of the manuscript submitted. All authors have read and approved the final version.
Ethics approval and consent to participate
The study was performed in accordance with the Declaration of Helsinki and protocol was approved by the Clinical Research Ethics Committee of the Second Hospital of Tianjin Medical University (approval date: 26 October 2023, approved number: KY2023K205). Informed consent was exempted due to the retrospective nature.
Supplementary Material
Download ()Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets generated during and/or analyzed during the current study are not publicly available as the data also forms part of another ongoing study but are available from the corresponding author on reasonable request.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/09537104.2024.2364748
Additional information
Funding
References
- Byrne RA, Rossello X, Coughlan JJ, Barbato E, Berry C, Chieffo A, Claeys MJ, Dan G-A, Dweck MR, Galbraith M. et al. 2023 ESC guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44(38):3720–10. doi:10.1093/eurheartj/ehad191.
- Thakker RA, Salazar L, Jazar DA, Bhakta P, Baker B, Patel C, Elbadawi A, Agarwal M, Albaeni A, Saleh M. et al. Coronary artery disease and aspirin intolerance: background and insights on current management. Cardiol Ther. 2022;11(2):175–83. doi:10.1007/s40119-022-00255-9.
- Michos ED, Ardehali R, Blumenthal RS, Lange RA, Ardehali H. Aspirin and clopidogrel resistance. Mayo Clin Proc. 2006;81(4):518–26. doi:10.4065/81.4.518.
- Newby LK, Bhapkar MV, White HD, Moliterno DJ, LaPointe NMA, Kandzari DE, Verheugt FWA, Kramer JM, Armstrong PW, Califf RM. Symphony and 2nd symphony investigators. Aspirin use post-acute coronary syndromes: intolerance, bleeding and discontinuation. J Thromb Thrombolysis. 2003;16(3):119–28. doi:10.1023/B:THRO.0000024050.78728.35.
- Derry S, Loke YK. Risk of gastrointestinal haemorrhage with long term use of Aspirin: meta-analysis. BMJ. 2000;321(7270):1183–7. doi:10.1136/bmj.321.7270.1183.
- Yang M, Ye Z, Mei L, Ullah I, Tan C, Wang G, Gu Q, Lu Y, Abdus S, Shi L. et al. Pharmacodynamic effects of indobufen compared with aspirin in patients with coronary atherosclerosis. Eur J Clin Pharmacol. 2021;77(12):1815–23. doi:10.1007/s00228-021-03177-y.
- Liu J, Sun P, Qi X. Reversible and non-competitive inhibition of cyclooxygenase by indobufen for efficient antiplatelet action and relief of gastrointestinal irritation. Pharmaceutics. 2023;15(8):2135. doi:10.3390/pharmaceutics15082135.
- Barillà F, Pulcinelli FM, Mangieri E, Torromeo C, Tanzilli G, Dominici T, Pellicano M, Paravati V, Acconcia MC, Gaudio C. Clopidogrel plus Indobufen in acute coronary syndrome patients with hypersensitivity to aspirin undergoing percutaneous coronary intervention. Platelets. 2013;24(3):183–8. doi:10.3109/09537104.2012.686072.
- Latib A, Ielasi A, Ferri L, Chieffo A, Godino C, Carlino M, Montorfano M, Colombo A. Aspirin intolerance and the need for dual antiplatelet therapy after stent implantation: a proposed alternative regimen. Int J Cardiol. 2013;165(3):444–7. doi:10.1016/j.ijcard.2011.08.080.
- Wu H, Xu L, Zhao X, Zhang H, Cheng K, Wang X, Chen M, Li G, Huang J, Lan J. et al. Option investigators. Indobufen or aspirin on top of clopidogrel after coronary drug-eluting stent implantation (OPTION): a randomized, open-label, end point-blinded, noninferiority trial. Circulation. 2023;147(3):212–22. doi:10.1161/CIRCULATIONAHA.122.062762.
- Shi Q-P, Luo X-Y, Zhang B, Wang X-G, Zhao J, Xie Q-F, Liu J-H, Liu Y-K, Jiang J, Zheng B. Effect of indobufen vs. aspirin on platelet accumulation in patients with stable coronary heart disease after percutaneous coronary intervention: an open-label crossover study. Front Pharmacol. 2022;13:950719. doi:10.3389/fphar.2022.950719.
- Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD, Thygesen K, Alpert JS, Jaffe AS. et al. ESC scientific document group. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2019;40(3):237–69. doi:10.1093/eurheartj/ehy462.
- Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, Cuisset T, Cutlip D, Eerdmans P, Eikelboom J. et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention. Circulation. 2019;140(3):240–61. doi:10.1161/CIRCULATIONAHA.119.040167.
- Review Committee Members E, Bittl JA, Baber U, Bradley SM. Duration of dual antiplatelet therapy: a systematic review for the 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2016;134(10):e156–78. doi:10.1161/CIR.0000000000000405.
- Patrono C, Baigent C, Hirsh J, Roth G. Antiplatelet drugs: American college of chest physicians evidence-based clinical practice guidelines (8th Edition). Chest. 2008;133(6 Suppl):199S–233S. doi:10.1378/chest.08-0672.
- Lee J-Y, Sung K-C, Choi H-I. Comparison of aspirin and Indobufen in healthy volunteers. Platelets. 2016;27(2):105–9. doi:10.3109/09537104.2015.1042853.
- De Caterina R, Giannessi D, Bernini W, Lazzerini G, Lavezzari M, Stragliotto E, Biagi G, Coccheri S. A prostacyclin-sparing effect of Indobufen vs. Aspirin. Thromb Haemost. 1996;75(3):510–4. doi:10.1055/s-0038-1650306.
- Marzo A, Crestani S, Fumagalli I, Giusti A, Lowenthal DT. Endoscopic evaluation of the effects of Indobufen and aspirin in healthy volunteers. Am J Ther. 2004;11(2):98–102. doi:10.1097/00045391-200403000-00004.
- Dai C, Liu M, Yang Z, Li Y, Zhou Y, Lu D, Xia Y, Chen A, Li C, Lu H. et al. Real-world performance of indobufen versus aspirin after percutaneous coronary intervention: insights from the aspiration registry. BMC Med. 2024;22(1):148. doi:10.1186/s12916-024-03374-3.
- Pan Y, Meng X, Yuan B, Johnston SC, Li H, Bath PM, Dong Q, Xu A, Jing J, Lin J. et al. INSURE investigators. Indobufen versus aspirin in patients with acute ischaemic stroke in China (INSURE): a randomised, double-blind, double-dummy, active control, non-inferiority trial. Lancet Neurol. 2023;22(6):485–93. doi:10.1016/S1474-4422(23)00113-8.
- Baaten CCFMJ, Schröer JR, Floege J, Marx N, Jankowski J, Berger M, Noels H. Platelet abnormalities in CKD and their implications for antiplatelet therapy. Clin J Am Soc Nephrol CJASN. 2022;17(1):155–70. doi:10.2215/CJN.04100321.
- Melloni C, Cornel JH, Hafley G, Neely ML, Clemmensen P, Zamoryakhin D, Prabhakaran D, White HD, Fox KA, Ohman EM. et al. Impact of chronic kidney disease on long-term ischemic and bleeding outcomes in medically managed patients with acute coronary syndromes: insights from the trilogy ACS trial. Eur Heart J Acute Cardiovasc Care. 2016;5(6):443–54. doi:10.1177/2048872615598631.
- Gallagher H, Dumbleton J, Maishman T, Whitehead A, Moore MV, Fuat A, Fitzmaurice D, Henderson RA, Lord J, Griffith KE. et al. Aspirin to target arterial events in chronic kidney disease (ATTACK): study protocol for a multicentre, prospective, randomised, open-label, blinded endpoint, parallel group trial of low-dose aspirin vs. standard care for the primary prevention of cardiovascular disease in people with chronic kidney disease. Trials. 2022;23(1):331. doi:10.1186/s13063-022-06132-z.
- Liu J, Xu D, Xia N, Hou K, Chen S, Wang Y, Li Y. Anticoagulant activities of Indobufen, an antiplatelet drug. Mol Basel Switz. 2018;23(6):1452. doi:10.3390/molecules23061452.