ABSTRACT
The purpose of this study is to assess innovation system performance and identify the system-blocking mechanisms for AI healthcare technology innovations related to the life science industry. The socio-technical analytical framework Technological innovation systems (TIS) was used to assess the structural and functional dynamics of AI healthcare technology innovations related to the life science industry in West Sweden. The case study employs a mixed-method research approach, triangulating qualitative and quantitative data from secondary published sources and interviews with 21 experts and 25 life science business executives. The results reveal that innovation system performance is primarily restricted by the system weaknesses of limited resources and insufficient communication from leading healthcare professionals regarding their needs for improving healthcare using AI technology innovations. This study shows that to improve innovation system performance, policy interventions intended to increase available resources and to formulate vision and mission statements to improve healthcare with AI technology innovations may be encouraged. This study contributes to the understanding of the mechanisms and interdependencies between system functions using the socio-technical TIS framework in a healthcare context.
1. Introduction
Global societies are faced with a growing demand for high-quality healthcare as a consequence of an aging population with an increased prevalence of patients experiencing multiple and chronic conditions (Kingston et al. Citation2018). Increased demand in combination with cost pressures challenges societies to deliver healthcare in a sustainable way, which cannot be managed only by increasing resources. Quality improvement and innovation are fundamental drivers in the search for better medical outcomes without necessarily increasing costs (Bergman et al. Citation2015; Liddell and Ayling Citation2011). A potentially powerful technique that could act as a vehicle to accelerate innovation in healthcare is artificial intelligence (AI). Healthcare is full of data-rich processes, and the accessibility to large volumes of data, combined with the development of computer power and AI techniques, has created tremendous opportunities (Jiang et al. Citation2017).
Innovations based on AI aim to mimic human cognitive functions, and the ability of machines using AI algorithms to understand and manipulate large datasets has advanced rapidly. One advantage of applying AI in healthcare in the era of big data is that complex patterns and relationships within the data can be discovered algorithmically without having to hire more healthcare professionals for data analysis. AI innovations have various healthcare applications, such as predicting mortality after cardiac surgery (Nilsson et al. Citation2006), as intelligent artificial prostheses (Ortiz-Catalan, Brånemark, and Håkansson Citation2013), or diagnosing skin cancer as efficiently as (Esteva et al. Citation2017), or perhaps even better than, dermatologists (Haenssle et al. Citation2018). However, the digitalisation and pace of AI adoption in the healthcare sector are low compared to other fields (Laurenza et al. Citation2018), and various issues on the macro, meso and micro levels have been identified (Lennon et al. Citation2017).
Healthcare is a complex dynamic socio-technical system combining functional elements that evolve interdependently (Effken Citation2002). The socio-technical analytical framework Technological Innovation Systems (TIS) has emerged as a prominent concept to assess the structure and functions that influence innovation system performance (Bergek et al. Citation2008; Hekkert et al. Citation2007; Markard and Truffer Citation2008). Previous TIS studies have taken a functional dynamics approach in various fields, such as biorefinery development (Hellsmark et al. Citation2016) and off-shore wind technologies (Wieczorek et al. Citation2013). To the authors’ knowledge, there are few TIS studies illuminating the healthcare sector (Kukk, Moors, and Hekkert Citation2016; Larisch, Amer-Wåhlin, and Hidefjäll Citation2016); however, no specific research study or review have applied the TIS framework for assessment of innovation system performance related to AI healthcare technologies. Therefore, our intention is to make a contribution in this field and explore if and how the TIS framework can be expanded into new areas, identify possible shortcomings and development areas. Thus, to contribute to a more holistic view of innovation processes in the healthcare sector, the researchers used a TIS approach to examine the performance and system-blocking mechanisms of AI healthcare technology innovations related to the life science industry.
The purpose of this study is to assess innovation system performance and identify system-blocking mechanisms of AI healthcare technology innovations related to the life science industry. Accordingly, the researchers formulated the following research questions: (1) what system strengths and weaknesses can be identified and (2) are there implications for policy interventions for AI healthcare technology innovations related to the life science industry?
2. Theoretical background
AI refers to machines that are able to perform tasks that would require intelligence if done by humans. Machine learning encompasses various techniques one can use to achieve AI and refers to computer learning without being explicitly programmed. Artificial neural networks are an example of machine learning technology architecture, which simulates the human brain. The human brain consists of billions of nerve cells or neurons, organised in complex interconnected networks allowing us to generate complex thought patterns and actions. During a lifetime, neurons connect with other neurons, and complex patterns grow into functioning circuits. Similarly, artificial neural networks consist of neurons or nodes, organised in different layers that can capture complex non-linear relationships between data variables and an outcome. Deep learning is a specific subset of artificial neural network that use multiple hidden layers to solve complex problems. For example, convolutional deep neural networks are typically used in healthcare to analyse images, such as structured CT or MRI data. Recurrent deep neural networks are used to analyse sequential data, such as genetic sequencing. Natural language processing (NLP) is a popular AI technique used to analyse unstructured data, such as data from clinical examinations and electronic health record systems. In this study, the term AI technologies refer to all generations and techniques of artificial intelligence.
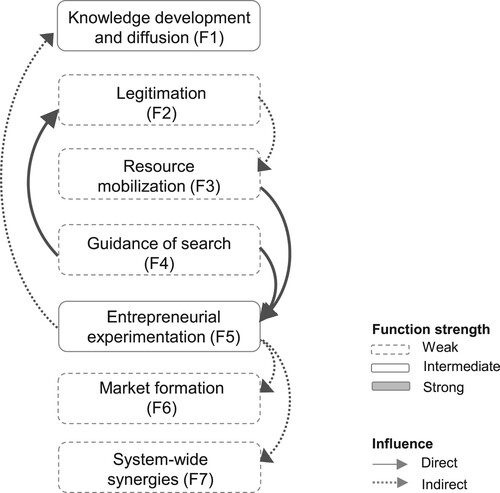
Innovation systems are a group of structural components contributing to the overall function of developing, diffusing and utilising innovations (Carlsson and Stankiewicz Citation1991). TIS analytical framework incorporates analysis of the structures and functions that influence innovation system performance (Bergek et al. Citation2008; Hekkert et al. Citation2007; Larisch, Amer-Wåhlin, and Hidefjäll Citation2016; Markard and Truffer Citation2008; Rickne Citation2000). As described in , the function knowledge development and diffusion (F1) relates to the breadth and depth of knowledge. The function strength can be assessed with various types of indicators, such as number of publications and qualitative assessments by experts. The function legitimation (F2) relates to the social acceptance of the technology with relevant institutions and can be assessed with qualitative data to ascertain how legitimacy influences other functions and the length of time it takes from developing applications to customer installations. The function resource mobilisation (F3) concerns the extent to which actors are able to mobilise capital to generate and diffuse technology innovation. Guidance of search (F4) relates to the expectations for growth potential as well as end-users’ needs relating to the technology innovation. The function entrepreneurial experimentation (F5) refers to the experimentation and testing of new technologies and applications and can be assessed with a variety of application types. The function market formation (F6) relates to when innovations are made widely available in markets. Indicators may include timing, size and type of market. When all functions are fulfilled, a reinforcing and synergistic system can function and spread positive effects within the innovation system and to other regions, countries or sectors. This process is sometimes referred to as positive externalities or system-wide synergies (F7) and can be assessed by the establishment of international standards and formal networks between actors.
Table 1. Functions of innovation systems and their indicators of strength (Bergek et al. Citation2008; Hekkert et al. Citation2007; Larisch, Amer-Wåhlin, and Hidefjäll Citation2016; Markard and Truffer Citation2008; Rickne Citation2000).
3. Material and methods
The nature of the problem addressed in this research requires an in-depth exploration of experts’ thoughts to understand the processes for AI innovations related to the life science industry. A qualitative case study is a suitable research technique in this context, as this approach is used to explore a phenomenon within a particular context (Eisenhardt Citation1989). For this case study, a mixed-methods approach was employed and deemed to be suitable since triangulation of qualitative and quantitative data were expected to provide an in-depth understanding of the research topic (Creswell et al. Citation2003).
3.1. Research setting
Sweden was ranked third on the Global Innovation Index 2018 (Dutta, Lanvin, and Wunsch-Vincent Citation2018). Although the Swedish healthcare system ranks high on healthcare outcome measures, population health and quality of care, it ranks low on technical efficiency (Anell, Glenngård, and Merkur Citation2012; Tchouaket et al. Citation2012). West Sweden is a key region for life science, constituting approximately 25% of the total national population (www.scb.se). West Sweden was chosen as the empirical setting due to the researchers’ access to data, and in this study, the structural components refer to actors, networks and institutions relating to AI healthcare technology innovations from a life science industry perspective. The life science industry consists of companies dedicated to developing, producing and distributing healthcare technologies such as pharmaceuticals, medical devices and equipment. In this study, the researchers categorised AI healthcare technology innovations based on end-user applications, as described in .
Table 2. Categorisation of AI healthcare technology innovations based on end-user applications.
3.2. Data collection and analysis
Data collection was based on qualitative interviews and secondary data from official and publicly available documents. The interviews were divided into three phases to (1) explore the field, (2) collect empirical evidence and (3) examine and explore experts’ experiences.
3.2.1. Phase I – in-depth interviews
In-depth interviews with eight stakeholders were conducted to inductively explore the field and gather more information on structural components and perceived weaknesses and strengths. An interview guide with open-ended questions was developed to allow participants to express their viewpoints and experiences (Flick Citation2018). Sample selection used the snowball sampling technique (Flick Citation2018). The interviews included one local policy-maker, three researchers, one medical doctor, two industry associates and one financial investor. Each interview lasted approximately 30−60 min. Interview notes were transcribed and analysed using the qualitative content analysis software program NVivo.
3.2.2. Phase II – phone surveys
Phone interviews were conducted to collect empirical evidence from life science companies that have developed, or are in the process of developing, AI innovations. To identify companies, secondary data and participants in phase I was consulted. Supplementary data, including revenues and number of employees, were collected through published data sources (www.allabolag.se). Thirty companies were identified and contacted by phone or email. Five companies did not yet consider applying AI technologies and were excluded from the research. Phone interviews were conducted during which each company representative was asked to define the company’s AI initiatives based on end-user application. On six occasions, more than one end-user application was applied, and the researchers decided to include all applications for further analysis. Three applications were specifically used for pharmaceutical development by the industry and were excluded from analysing commercially deployed innovations utilised in healthcare. The researchers categorised captured data into innovation types (OECD Citation2018). Each company representative was asked to rank the level of adoption, as described in . Interviews lasted 20−30 min.
Table 3. Metrics are used to rank the level of adoption AI healthcare technology innovations.
3.2.3. Phase III – semi-structured expert interviews
Semi-structured expert interviews were conducted with open-ended questions to allow for further exploration of the phenomena (Flick Citation2018). An interview guide was created based on the theoretical framework and knowledge about the phenomena gathered in phase I. In the event company representatives in phase II ranked the adoption as five or above, they were asked to appoint AI experts within their organisations. An expert was defined as an individual having technical, process-oriented and interpretive knowledge of AI technologies gained from research, development, computer science, IT, marketing or business development. All experts who were contacted agreed to participate in the study. A total of 13 expert interviews, each lasting approximately 60 min were conducted, as categorised in . Interview notes were transcribed and coded according to themes that corresponded to the research questions using the qualitative content analysis software program NVivo.
Table 4. Categorisation of participants from companies a, b, c, d, e and f.
4. Results and analysis
4.1. Structural components
The healthcare sectoral map illustrated in was developed based on information provided by study participants and secondary data from published sources and includes key actors classified into academic, market and governance spheres on the vertical axis and along the value chain on the horizontal axis. Chalmers University of Technology, Gothenburg University and regional universities and colleges were identified as central actors within the academic sphere. The market sphere included 25 qualified life science companies. The companies were classified according to the EU definition of small and medium enterprises (European Commission Citation2015). Companies that did not meet any SME definition were categorised as large-sized companies. The study included 5 large-sized companies, 2 medium-sized companies, 3 small-sized companies and 15 micro-sized companies.
Figure 1. Selection of key actors inspired by Larisch, Amer-Wåhlin, and Hidefjäll (Citation2016). Boxes indicate study participants included in phases I–III. Dotted boxes indicate actors who were contacted to identify participants for phases I–II.
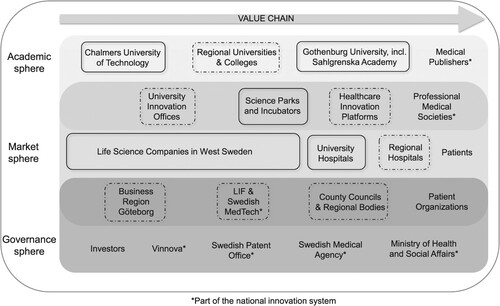
Sahlgrenska University Hospital is the largest university hospital in the region. University innovation offices and platforms were established to support innovations generated by healthcare professionals and researchers. Science parks and incubators were established to contribute to the commercialisation of innovations. The BioVenture Hub is an innovative ecosystem integrated at the AstraZeneca strategic research and development centre in Gothenburg, providing emerging life science companies and academic groups unique opportunities to interact with each other and with one of the largest incumbents on the market. The governance sphere included investors and national actors, such as the Swedish Agency for Innovation Systems (Vinnova), the Swedish Patent Office, the Swedish Medical Agency and the Ministry of Health and Social Affairs. Further, regional actors influencing the governance and market spheres included Business Region Göteborg (BRG), a non-profit company working to develop regional industry and regional county offices and regional bodies.
4.2. Functional assessment
This section describes the strengths (S) and weaknesses (W) of the system functions relating to AI healthcare technology innovations.
4.2.1. Knowledge development and diffusion (F1)
A knowledge gap between decision-makers and AI experts was identified in a few of the medium- and large-sized companies (W1). Not all decision-makers and managers had sufficient technological knowledge to understand the potential impact AI innovations could have on the market or organisations. In contrast, not all AI experts within the companies had the overall business perspective needed to provide arguments for larger AI investments. The general market knowledge was scarce among industrial actors, mainly due to a lack of experience with AI healthcare technology innovations.
I think our greatest barrier is within our internal organization. We have lots of data, but to be honest, we don’t know what we can do with it. (Business developer, company b, phase III)
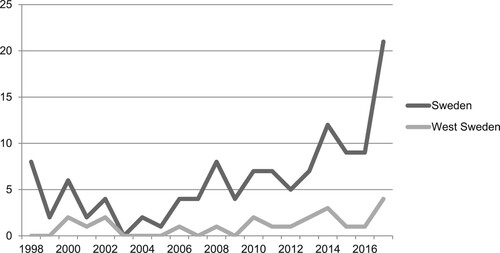
Interview data and bibliometric analysis were used to assess scientific and technological knowledge. There has been a slight increase in the number of scientific publications relating to AI healthcare technology innovations, as illustrated in ; however, the absolute number of scientific publications related to life science and healthcare was low (W2). In comparison, the number of publications related to AI technologies in all industrial sectors was analysed and researchers within West Sweden representing all industrial sectors published approximately six times more articles, compared to what would be expected when compared to global TIS. The large number of publications is partly explained by the intense collaboration between the local automotive industry and academia, which has resulted in a vast number of research projects related to electromobility. Hence, there was a broad technological knowledge base due to the large number of activities in other sectors (S1).
To be frank, we as researchers will focus our research on where the funding is. Volvo and Geely invest huge amounts of resources into advanced AI technologies for electromobility, whereas the local healthcare organizations support very few AI projects. (Senior AI researcher, phase I)
4.2.2. Legitimation (F2)
Qualitative and secondary data from published sources were analysed to assess the function strength of legitimation (F2). AI experts expressed frustration regarding difficulties with the use of health data, due to data liability and privacy concerns (W3). For example, re-using patient data collected from previous clinical trials can be a key asset during development; however, previously collected patient consent forms do not allow developers to use data originally intended for other research projects, thus creating uncertainties concerning data ownership.
I wish that I had access to more patient data. The more data I have, the better prediction models I can develop. (Senior developer, company a, phase III)
The length of time from application development to installation was analysed by assessing the level of technology adoption. As described in , it was discovered that there was a low level of adoption of healthcare technology innovations (W4). Only 9% of the service innovations were commercially deployed and 73% were in pre-development. According to study participants, service innovations were often delayed or postponed due to difficulties gaining internal and external acceptance for the business model. For example, many experts were frustrated to present return on investment projections and justify potential future revenue generation models. In contrast, approximately half of the product innovations were commercially deployed and launched to paying customers and niche markets. Apart from being either physical product innovation or digital-virtual service innovation, one of the main differences between product and service innovations was access to data. Thus, the researchers hypothesise that the less control of data, the longer it will take to commercialise AI healthcare technology innovations. As an example, decision support systems based on artificial neural networks depend on access to healthcare databases, while product innovations (e.g. artificial prosthesis) may only require data from individual patients. Hence, companies have more control over the innovation process for physical product innovations compared to service innovations relating to services or processes where access to external healthcare databases is needed.
Table 5. Level of commercially deployed AI healthcare technology innovations.
4.2.3. Resource mobilization (F3)
The function resource mobilisation (F3) was assessed with qualitative data and secondary data from published sources. Infrastructure and accessibility to data are key to success for AI projects, the core of which is the availability of relevant data to train and fine-tune AI functionality and accuracy. Sweden is well recognised for its highly developed healthcare quality registries with a tremendous amount of data for various medical procedures, such as the National Quality Register for hip fracture patients, RIKSHÖFT. However, not all actors are allowed to use the data from healthcare databases, and for non-academic actors in this study, the restrictions to use patient data from healthcare databases and quality registries (W5) challenged the further development of AI healthcare technology innovations. Two companies did not experience such difficulties; however, these companies were founded by medical researchers from the academic sphere and could therefore access healthcare databases.
The attractiveness of working with fast-moving sectors, such as electromobility, combined with the perceived slow pace of development in the healthcare sector, has caused difficulties in attracting AI-skilled talents in life science (W6).
Although I definitely value such projects, the pace of development in healthcare is way too slow for me. (Senior AI researcher, phase I)
AI experts in academia experienced low financial resources (W7), which also was confirmed by analysing the total number of funding calls by the Swedish Agency for Innovation Systems (Vinnova). Until the year 2017, there was one call related to the TIS; however, six calls were related to other sectors. Further, many AI experts in medium- and large-sized companies were challenged by management to present return on investment for new projects, and the lack of evidence or uncertainties often resulted in an unwillingness to invest. In contrast, micro- and small-sized companies often attracted seed and venture capital for early-stage development projects. This was also confirmed by one of the investors, who declared that local seed investors were willing to invest in new prospects relating to AI technology innovations. However, the absolute number of projects, and hence, the total amount of invested financial capital, was low.
We have decided to dedicate a full-time employee towards digitization technologies in order to identify new business opportunities and to support our portfolio companies. (Senior investor, phase I)
4.2.4. Guidance of search (F4)
The function guidance of search (F4) was assessed with qualitative interview data and secondary data from published sources from downstream market actors. Actors were chosen based on their proximity to healthcare and patients, and hence, their knowledge and understanding of end-user needs. For example, University Hospitals and Healthcare Innovation Platforms were included in the analysis. Although there were a few articles from leading healthcare professionals relating to the TIS, these were often not related to what is needed for AI technology innovations in healthcare. The finding was confirmed by upstream market actors who perceived insufficient articulation by leading healthcare professionals of the needs to improve healthcare with AI technology innovations (W8). Interestingly, two small-sized companies did not experience a lack of guidance from leading healthcare professionals; however, the researchers concluded that these companies were founded by renowned academic medical researchers with well-established networks in the healthcare system; therefore, they did not need guidance in the same way the non-academic actors did.
We don’t actually know what healthcare organizations need. If they can guide us how AI can be utilized in their daily operations, we would definitely be willing to invest. (Senior industry associate, phase I)
4.2.5. Entrepreneurial experimentation (F5)
The function entrepreneurial experimentation (F5) was assessed by the general promotion of innovation in the area, the number of actors and different types of applications. The function was strengthened by the strong culture of innovation in the region, such as well-established innovation offices, science parks and incubators that were actively involved in various activities to catalyse entrepreneurial activities and establish new companies based on university spin-offs. Further, the number of new company entrants relating to the TIS has increased (S2) by approximately 25% since 2014. A few established medium- and large-sized life science companies had not yet initiated AI innovation projects (W9), which had an overall negative effect on the function strength.
It has been difficult to move AI applications forward in the past. Recent high-profile events and the buzz around AI has increased our possibilities for developing new applications. (Business developer, company b, phase III)
This study showed a variety of end-user applications (S3); 31% of the identified end-user applications were related to decision support, 19% to patient support, 16% to education, 13% to medical device therapy, 12% to pharmaceutical development and 10% were related to diagnostic applications.
4.2.6. Market formation (F6)
The function market formation (F6) was assessed based on qualitative data on actors’ strategies to enhance market access and on the level of commercially deployed AI technology innovations. There was a general view that collaborations between the technology-driven small-sized companies and the medium- and large-sized companies would be help decrease the time to market.
We did not have the technology in house, so therefore we have recently acquired a start-up with very interesting AI innovations. (IT manager, company f, phase III)
As described in , there were no AI technology innovations for healthcare applications commercially deployed among medium- and large-sized companies (W10). In contrast, 31% of the AI healthcare technology innovations developed by micro- and small-sized companies were deployed commercially; however, these commercial AI innovations developed by micro- and small-sized companies were deployed to niche markets and did not yet generate significant revenues (W11). Hence, the researchers concluded that overall, the market size for AI healthcare technology innovations at the time of the study was small.
Table 6. Level of adoption of commercially deployed AI healthcare technology innovations according to study participants.
4.2.7. System-wide synergies (F7)
Many experts perceived difficulties initiating projects with actors represented by other spheres and formal networks were sought to further develop knowledge and increase interdisciplinary collaboration (W12). A few of the medium- and large-sized companies with domain knowledge lacked the technological knowledge, and were thereby keen to establish collaboration with technology-driven small-sized companies. In contrast, a majority (85%) of the small-sized companies in this study were founded based on specific technology innovations, and they typically lacked domain knowledge. Further, the so-called teacher exception rule regulating the intellectual property rights of academic staff (Regeringskansliet Citation1949, 345) and the restrictive collaboration rules between industry and healthcare (W13) (SKL Citation2013) negatively influenced interdisciplinary collaboration and initiation of innovation projects.
Just because I am knowledgeable in various fields of medicine, does not make me capable of developing innovations relating to my field. We need to collaborate with the companies. (Healthcare professional, phase I)
Innovations based on machine learning technologies become more intelligent the more they are being used, and thus can be improved once more data becomes available. Legal and regulatory requirements relating to the management of the dynamic nature of such innovations were unclear among many industrial actors. For example, it was unclear whether automatic upgrades of software were allowed from a legal standpoint. This was also confirmed in the literature, and according to the Swedish Institute for Standards (SIS), there is a lack of national and international standards and regulations for AI healthcare technology innovations (W14).
4.2.8. Summary of the functional assessment
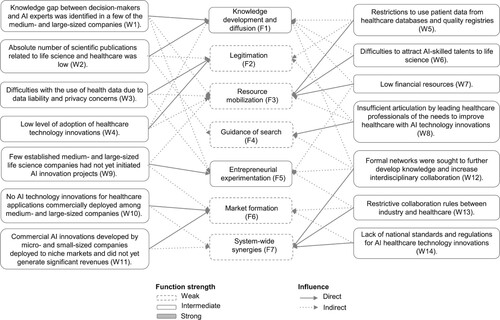
A summary of the functional assessment, including identified strengths and weaknesses of AI healthcare technology innovations from a life science industry perspective, is illustrated in . Two system functions, knowledge development and diffusion (F1) and entrepreneurial experimentation (F5), are assessed intermediate and can be regarded as cornerstones to further the TIS development and performance. However, this study showed that the remaining four functions were weak and may significantly restrict the development and innovation system performance.
Table 7. Identified system strengths and weaknesses.
4.3. Functional pattern
Many decision-makers expressed that the willingness to further investigate how AI technologies can be utilised would increase if the need for the technology could be justified. Decision-makers urged healthcare organisations to articulate their needs in relation to improving healthcare with AI technologies. Clearly communicated needs in combination with an increased legitimacy for AI innovations will provide incentives for more actors to enter the field. Consequently, more resources would be invested in new product development, resulting in a stronger knowledge base. Hence, the guidance of search (F4) influences legitimation (F2) and entrepreneurial experimentation (F5) and indirectly also impacts knowledge development and diffusion (F1) and resource mobilisation (F3).
Only a fraction of data within internal company databases are analysed. Increased access and structuring of such data would allow AI experts to experiment more. Furthermore, access to the large multilevel datasets available in healthcare would empower AI experts to experiment and potentially unlock novel insights. AI projects utilising internal and external data sources could increase the understanding of potential business opportunities. Furthermore, it could catalyse the alignment between regulatory requirements and technology developments, and moreover, the establishment of standards. Hence, resource mobilisation (F3) influences experimentation (F5), and indirectly influences knowledge development and diffusion (F1), market formation (F6) and system-wide synergies (S7).
Based on the functional pattern, the researchers conclude that strengthening resource mobilisation (F3) and guidance of search (F4) can start a cascade of activity with direct and indirect influence on all functions ().
4.4. System-blocking mechanisms
As illustrated in , addressing the function weakness insufficient articulation by healthcare professionals of the needs to improve healthcare with AI technology innovations (W8), will have a direct influence on the guidance of search (F4) and indirect influence on two other functions. Furthermore, restrictions to use patient data from healthcare databases and quality registries (W5), difficulties to attract AI-skilled talents to life science (W6) and low financial resources (W7) will influence resource mobilisation (F3) and indirectly impact three other functions.
Thus, this analysis showed that some system-blocking mechanisms are more important to address than others due to their direct and indirect influences on functions. Consequently, the researchers conclude that addressing the function weaknesses of resource mobilisation (F3) and guidance of search (F4) could have a significant impact on innovation system performance.
5. Discussion and conclusions
The purpose of this study was to assess the innovation system performance and identify the blocking mechanisms for AI healthcare technology innovations related to the life science industry. Based on the analysis of functional dynamics and system-blocking mechanisms, the researchers conclude that addressing the function weaknesses of resource mobilisation (F3) and guidance of search (F4) could have a significant impact on innovation system performance.
5.1. Implications for policy interventions
As functions interact and influence each other, policy interventions and activities can be targeted towards strengthening specific system-blocking mechanisms. This study shows that the innovation system performance is primarily restricted by the system weaknesses of limited resources and insufficient communication from leading healthcare professionals regarding their needs for improving healthcare using AI technology innovations.
One approach to address the system-blocking mechanisms identified in this study would be to establish a shared project portfolio platform, ideally funded by governmental bodies, to catalyse interdisciplinary collaboration, in which actors from the academic, market and governance spheres could work together on projects with clearly defined goals and objectives. An increased number of such projects would provide opportunities for more actors to experiment and gain experience with AI technologies. For example, increasing access to health data from databases and the Swedish quality registries would allow more actors to experiment with data. Such concrete projects would also catalyse discussion on data liability and privacy concerns encouraging institutional actors to align current regulations with recent technological developments.
Thus, to increase innovation system performance, policy interventions with the intention to increase available resources and to formulate a vision and mission statements to improve healthcare with AI healthcare technology innovations may be motivated.
5.2. Theoretical contributions
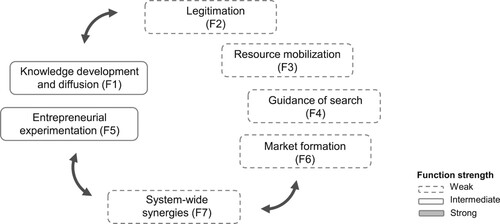
In other analyses of innovation systems (Hekkert and Negro Citation2009; Larisch, Amer-Wåhlin, and Hidefjäll Citation2016), knowledge development and diffusion (F1), legitimation (F2) and resource mobilisation (F3) have shown interdependencies by providing the initial conditions for entrepreneurial experimentation (F5) and guidance of search (F4) in early phases of technological development. However, as described in , our analysis shows that knowledge development and diffusion (F1) and entrepreneurial experimentation (F5) are interdependent and provide initial conditions for legitimation (F2), resource mobilisation (F3), guidance of search (F4) and market formation (F6). More specifically, the results reveal that innovation system performance is primarily restricted by the system weaknesses of limited resources and insufficient communication from leading healthcare professionals regarding their needs for improving healthcare using AI technology innovations. Hence, despite the limitations of using a single case study, the theoretical implications of our study show there may be a correlation between knowledge development and diffusion (F1) and entrepreneurial experimentation (F5) during the early phases of innovation system development. However, more research is needed to strengthen the empirical foundation, and therefore, we encourage more research studies applying the TIS in a healthcare setting to better understand the adoption of innovations.
5.3. Limitations
The researchers acknowledge the limitations of the present study, which should be considered when drawing conclusions and building on the findings. This case study was based on the triangulation of secondary data from published sources and interviews with 46 study participants, and may, therefore, not be indicative of the entire population. Thus, caution is needed when making generalisations based on this study’s findings. Another limitation of the study was the boundaries, which included innovations relating to the life science industry, while innovations developed within healthcare organisations were excluded. Hence, the authors call for further research of AI healthcare technology innovations developed within and by healthcare organisations. The complexity of healthcare systems and the rapid development of the innovation system from the time of data collection to the publication of this report constitute a call for further studies to analyse the temporal development of AI healthcare technology innovations.
Acknowledgments
The analysis includes data previously collected by the first author and supported by Sahlgrenska Science Park, Sweden, who is gratefully acknowledged. However, Sahlgrenska Science Park did not influence the analysis or conclusions of this research report.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
Petra Apell
Petra Apell is a PhD student at the Centre for Healthcare Improvement (CHI) at the Chalmers University of Technology, Gothenburg, Sweden. Her research interest is quality improvement in healthcare through digital innovations. Petra is a MedTech entrepreneur, the editor of ‘Fundamentals in Surgical Simulation: principles and practice’ (Springer, Ltd), and has worldwide patents relating to healthcare technology innovations.
Henrik Eriksson
Henrik Eriksson is a Professor at the Centre for Healthcare Improvement (CHI) at the Chalmers University of Technology, Gothenburg, Sweden. He has a number of research interests, like quality, quality management and quality improvement and excellent organisations. Examples of focussed research questions are: (1) How does one achieve high quality and good results? (2) What characterise excellent organisations? and (3) What are excellent organisations do differently from average organisations?
References
- Anell, A., A. H. Glenngård, and S. Merkur. 2012. “Health Systems in Transition – Sweden: Health System Review.” Health Systems Transition 14 (5): 1–159.
- Bergek, A., S. Jacobsson, B. Carlsson, S. Lindmark, and A. Rickne 2008. “Analyzing the Functional Dynamics of Technological Innovation Systems: A Scheme of Analysis.” Research Policy 37 (3): 407–429.
- Bergman, B., A. Hellström, S. Lifvergren, and S. M. Gustavsson. 2015. “An Emerging Science of Improvement in Health Care.” Quality Engineering 27 (1): 17–34.
- Carlsson, B., and R. Stankiewicz. 1991. “On the Nature, Function and Composition of Technological Systems.” Journal of Evolutionary Economics 1 (2): 93–118.
- Creswell, J. W., V. L. Clark, M. L. Gutmann, and W. E. Hanson. 2003. “Advanced Mixed Methods Research Designs.” In Handbook of Mixed Methods in Social and Behavioral Research, edited by A. Tashakkori and C. Teddlie, 209–240. Thousand Oaks, CA: Sage.
- Dutta, S., B. Lanvin, and S. Wunsch-Vincent. 2018. Global Innovation Index 2018: Energizing the World with Innovation. Ithaca, Fontainebleu, Geneva: Cornell University.
- Effken, J. A. 2002. “Different Lenses, Improved Outcomes: A New Approach to the Analysis and Design of Healthcare Information Systems.” International Journal of Medical Informatics 65 (1): 59–74.
- Eisenhardt, K. M. 1989. “Building Theories from Case Study Research.” Academy of Management Review 14 (4): 532–550.
- Esteva, A., B. Kuprel, R. A. Novoa, J. Ko, S. M. Swetter, H. M. Blau, and S. Thrun. 2017. “Dermatologist-level Classification of Skin Cancer with Deep Neural Networks.” Nature 542 (7639): 115–118.
- European Commission. 2015. “User Guide to the SME Definition.” Accessed April 10, 2018 https://ec.europa.eu/regional_policy/sources/conferences/state-aid/sme/smedefinitionguide_en.pdf.
- Flick, U. 2018. An Introduction to Qualitative Research, 6th ed. London: Sage Publications.
- Haenssle, H. A., C. Fink, R. Schneiderbauer, F. Toberer, T. Buhl, A. Blum, A. Kalloo, et al. 2018. “Man Against Machine: Diagnostic Performance of a Deep Learning Convolutional Neural Network for Dermoscopic Melanoma Recognition in Comparison to 58 Dermatologists.” Annals of Oncology 29 (8): 1836–1842.
- Hekkert, M. P., and S. O. Negro. 2009. “Functions of Innovation Systems as a Framework to Understand Sustainable Technological Change: Empirical Evidence for Earlier Claims.” Technological Forecasting and Social Change 76 (4): 584–594.
- Hekkert, M. P., R. A. A. Suurs, S. O. Negro, S. Kuhlmann, and R. E. H. M. Smits. 2007. “Functions of Innovation Systems: A New Approach for Analysing Technological Change.” Technological Forecasting and Social Change 74 (4): 413–432.
- Hellsmark, H., J. Mossberg, P. Söderholm, and J. Frishammar. 2016. “Innovation System Strengths and Weaknesses in Progressing Sustainable Technology: The Case of Swedish Biorefinery Development.” Journal of Cleaner Production 131: 702–715.
- Jiang, F., Y. Jiang, H. Zhi, Y. Dong, H. Li, S. Ma, Y. Wang, Q. Dong, H. Shen, and Y. Wang. 2017. “Artificial Intelligence in Healthcare: Past, Present and Future.” Stroke and Vascular Neurology 2 (4): 230–243.
- Kingston, A., L. Robinson, H. Booth, M. Knapp, and C. Jagger. 2018. “Projections of Multi-morbidity in the Older Population in England to 2035: Estimates from the Population Ageing and Care Simulation (PACSim) Model.” Age and Ageing 47 (3): 374–380.
- Kukk, P., E. H. M. Moors, and M. P. Hekkert. 2016. “Institutional Power Play in Innovation Systems: The Case of Herceptin®.” Research Policy 45 (8): 1558–1569.
- Larisch, L.-M., I. Amer-Wåhlin, P. Hidefjäll. 2016. “Understanding Healthcare Innovation Systems: The Stockholm Region Case.” Journal of Health Organization and Management 30 (8): 1221–1241.
- Laurenza, E., M. Quintano, F. Schiavone, and D. Vrontis. 2018. “The Effect of Digital Technologies Adoption in Healthcare Industry: A Case Based Analysis.” Business Process Management Journal 24 (5): 1124–1144.
- Lennon, M. R., M.-M. Bouamrane, A. M. Devlin, S. O'Connor, C. O'Donnell, U. Chetty, R. Agbakoba, et al. 2017. “Readiness for Delivering Digital Health at Scale: Lessons from a Longitudinal Qualitative Evaluation of a National Digital Health Innovation Program in the United Kingdom.” Journal of Medical Internet Research 19 (2): e42. https://doi.org/10.2196/jmir.6900
- Liddell, M., and G. Ayling. 2011. Reid, Innovation Health and Wealth, Accelerating Adoption and Diffusion in the NHS. Department of Health, NHS Improvement & Efficiency Directorate, Innovation and Service Improvement, UK.
- Markard, J., and B. Truffer. 2008. “Actor-oriented Analysis of Innovation Systems: Exploring Micro–Meso Level Linkages in the Case of Stationary Fuel Cells.” Technology Analysis and Strategic Management 20 (4): 443–464.
- Nilsson, J., M. Ohlsson, L. Thulin, P. Höglund, S. A. M. Nashef, and J. Brandt. 2006. “Risk Factor Identification and Mortality Prediction in Cardiac Surgery Using Artificial Neural Networks.” The Journal of Thoracic and Cardiovascular Surgery 132: 12–19.e1.
- OECD/Eurostat. 2018. Oslo Manual 2018: Guidelnes for Collecting, Reporting and Using Data on Innovation, 4th Edition. The Measurement of Scientific, Technological and Innovation Activities. Paris/Eurostat, Luxembourg: OECD Publishing, Paris/Eurostat.
- Ortiz-Catalan, M., R. Brånemark, and B. Håkansson. 2013. “BioPatRec: A Modular Research Platform for the Control of Artificial Limbs Based on Pattern Recognition Algorithms.” Source Code for Biology and Medicine 8 (1): 1–18.
- Regeringskansliet. 1949. Accessed May 20, 2018. http://rkrattsbaser.gov.se/sfsr?bet=1949:345.
- Rickne, A. 2000. “New Technology-based Firms and Industrial Dynamics: Evidence from the Technological System of Biomaterials in Sweden, Ohio and Massachusetts”, Doctoral Thesis. Chalmers University of Technology, no. 1659, pp. 1–340.
- SKL. 2013. Agreement Regarding Rules of Cooperation, Stockholm.
- Tchouaket, É. N., P. A. Lamarche, L. Goulet, and A. P. Contandriopoulos. 2012. “Health Care System Performance of 27 OECD Countries.” The International Journal of Health Planning and Management 27 (2): 104–129.
- Wieczorek, A. J., S. O. Negro, R. Harmsen, G. J. Heimeriks, L. Luo, and M. P. Hekkert. 2013. “A Review of the European Offshore Wind Innovation System.” Renewable and Sustainable Energy Reviews 26: 294–306.