Abstract
Natural selection shaped the foraging-related processes of honeybees in such a way that a colony can react to changing environmental conditions optimally. To investigate this complex dynamic social system, we developed a multi-agent model of the nectar flow inside and outside of a honeybee colony. In a honeybee colony, a temporal caste collects nectar in the environment. These foragers bring their harvest into the colony, where they unload their nectar loads to one or more storer bees. Our model predicts that a cohort of foragers, collecting nectar from a single nectar source, is able to detect changes in quality in other food sources they have never visited, via the nectar processing system of the colony. We identified two novel pathways of forager-to-forager communication. Foragers can gain information about changes in the nectar flow in the environment via changes in their mean waiting time for unloadings and the number of experienced multiple unloadings. This way two distinct groups of foragers that forage on different nectar sources and that never communicate directly can share information via a third cohort of worker bees. We show that this noisy and loosely knotted social network allows a colony to perform collective information processing, so that a single forager has all necessary information available to be able to ‘tune’ its social behaviour, like dancing or dance-following. This way the net nectar gain of the colony is increased.
1. Introduction
Honeybees collect nectar in the environment to accommodate their energy demands. Nectar collecting is performed by a special cohort of bees, the forager bees. These bees fly out into the environment and collect nectar from flowering plants. A forager that returns successfully from a nectar source communicates the position of the found source to other bees via waggle dances (Frisch Citation1965; Seeley Citation1995). Other forager bees usually follow these dances and then start to fly to these sources by themselves. This mechanism of recruitment is the main positive feedback loop of the foraging system found in honeybees (Biesmeijer and DeVries Citation2001). After returning to the colony, the forager bees transfer the collected nectar to another special cohort of bees (storer bees, also called ‘receiver bees’ in the literature), which then place the nectar into honeycombs. In some cases, a returning forager bee does not transfer its whole nectar load to a single storer bee but utilises several storers. This process is called ‘multiple unloading’ (Seeley, Camazine and Sneyd Citation1991; Hart and Ratnieks Citation2001; Huang and Seeley Citation2003). The probability of the occurrence of multiple unloadings correlates with the mean crop load of the returning foragers, because an instance of multiple unloading occurs when the crop load of the foragers is greater than the available empty space in the crop of the storer bee it encounters (Huang and Seeley Citation2003).
The crop loads of returning forager bees are influenced by environmental conditions during the foraging trip. Under conditions of low nectar flow in the environment, forager bees tend to return with small amounts of nectar. Under conditions of high nectar flow, forager bees return to the colony with almost full nectar crops (Huang and Seeley Citation2003). It was shown in Seeley et al. Citation(1991) that a honeybee colony as a whole can differentiate between several nectar sources in the environment and that it is able to exploit massively the most profitable source through the dance-based recruitment mechanisms described above. This ability is a remarkable example of social learning, because no single individual forager bee directly compares all available foraging sources by visiting them, yet the decision is made within the colony, a good example of ‘swarm intelligence’ (Anderson and Ratnieks Citation1999; Bonabeau, Dorigo, and Theraulaz Citation1999; Kennedy and Eberhart Citation2001). The decision-making is driven by the two important feedback loops mentioned above: the waggle-dance recruitment of new foragers (positive feedback loop) and competition for ‘empty’ storage bees by the various forager groups visiting different nectar sources (negative feedback loop). As detailed by Seeley Citation(1995), an individual forager does not directly compare the competing sources or the dances that advertise those sources. Usually, an experienced forager visits the same source repeatedly and a naive recruit follows just one single dance before leaving the colony. Also the storer bees do not seem to compare the nectar offered by the returning forager bees. A colony's decision-making is especially impressive, as it requires ‘learning’ about the environment on the level of the colony, and not on the level of a single forager. For the colony, it suffices that adaptations to new (changed) environmental conditions are performed on the colony level and not on the individual level. Using the model described in this article, we will show that, without any individual adaptations, a colony can spread information about environmental changes through all its members. Members that never had direct contact to the environmental changes can retrieve information from the collective ‘brain’ of the colony and may alter their behaviour accordingly. We show that, by implementing the possibility to adapt the individual foraging and social behaviour to this information, the net nectar gain of the colony is increased.
Studies of DeGrandi-Hoffman and Hagler Citation(2000) showed that the collected nectar brought by the foragers is quickly distributed among the entire colony population. The first bees that receive this nectar in the hive are the storer bees. It has been established (Seeley et al. Citation1991; Seeley Citation1995) that the storer bees are needed for unloading of all forager bees and that there is competition among foragers for this shared limited resource. Because the crop loads of returning forager bees depend on the quality of the nectar sources they visit, the storer bees may allow an indirect form of communication between a group of foragers concentrating on one source and that concentrating on another. To investigate this possibility, we developed an individual-based multi-agent model and explored potential communication channels that could enable forager bees to detect changes in nectar flow on a source they have never visited.
2. The model
The main aspects of honeybee foraging our model focuses on are the dynamics of the crop load volume during a foraging trip (Schmidt-Hempel, Kacelnik, and Houston Citation1985), the distribution of crop load volumes under different environmental conditions (Huang and Seeley Citation2003), and changes in the frequency of multiple unloadings caused by changes in environmental conditions. We simplified the environment to consist of only two nectar sources. Allocation of foragers between the two sources was based on probability measures based on factors that are known to influence foraging behaviour (Anderson and Ratnieks Citation1999). For the sake of simplicity, the nectar transfer area in the hive was reduced to two one-dimensional lines, and the nectar transfer to the honeycomb was reduced to a simple delay, representing the time when storer bees are absent from the nectar transfer area. By simulating the metabolism rate of the agents (bees), we are able to study the economics of the emerging foraging strategies. The programming was done in ‘NetLogo 3.0’ (Wilensky Citation1999), which enabled us to create a temporally discrete and spatially discrete multi-agent model.
2.1 Some basic assumptions and constraints
The model treats only forager and storer bees. All other working cohorts in the colony (builders, nurses, etc.) are excluded. In our model, no shifts of workers from the cohort of foragers to the cohort of storers or vice versa are allowed. In nature, a limitation of the foraging capacity due to a low number of available storage bees leads to frequent tremble dances of forager bees. These dances recruit additional storer bees. The simulation runs presented in this article do not incorporate tremble dances, because we wanted to investigate the situation when storer bees are a shared limited resource. These simulation runs represent a honeybee colony that has already recruited all available bees for the storing task.
The design of the simulated experiment was inspired by the experiments published in Seeley Citation(1995), in which the experimenter offered two ad-libitum feeders with sugar solution of differing sugar concentration. Sudden changes in the sugar concentration and analysis of the number of the recruited bees, as well as of the dancing behaviour of forager bees, revealed some highly interesting details about the recruiting mechanisms of honeybees and the colonies mode to react to changes in the environment. Analogously to the empirical experiment, the conditions simulated in our model are rather abstract, but they enable us to investigate the possible mechanisms of decision-making and social learning in honeybee colonies without disturbance by any natural fluctuations. As this paper shows, this is especially important because these fluctuations might influence the reactions of honeybees, even those which are not even in direct touch with these fluctuations.
2.2 The environment
In our model, we use discrete time steps of Δ t=1 s. The model area outside the hive, as well as the area inside the hive, is implemented as a two-dimensional grid of ‘patches’.
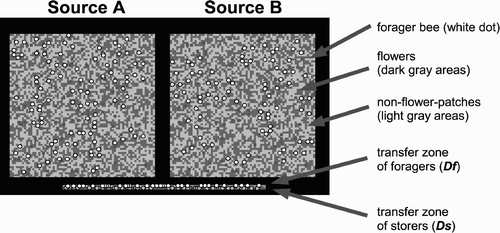
The ‘outside’ area of the model consists of two patch areas. These represent two nectar sources (areas of meadow of equal size) in the environment (). Each meadow m∈{A, B} consists of flower patches (fp) and non-flower patches (nfp). Flower patches are distributed with a variable density Md m and with variable amounts of nectar per flower Ma m . If a bee lands on a flower b in meadow m, it is able to upload nectar from the flowers bn b with an uploading rate F fl until the flower b is empty. After the bee has left, bn b is set to Ma m again. In a non-flower patch, bn b is always set to 0.
Figure 1. Interface of the model. The two upper square areas represent two meadows, nectar sources A and B. The dark grey areas within the nectar source areas represent flowers, while the light grey areas are nfps. The white dots (for the sake of visibility drawn bigger) are foragers searching for flowers or collecting nectar. The two lines in the lower part of the picture represent areas within the hive: the transfer zone for the foragers and the transfer zone for the storers (for the sake of visibility drawn bigger, as grey dots), where interactions between foragers and storers take place.
We used finite-state automatons to implement our simulated honeybee. Each simulated honeybee has its own metabolism. This metabolism uses nectar of a defined sugar concentration NC as the energy source. The biometric data of honeybees, such as honeybee weight, metabolic rate, crop volume, and flight speed, were taken from the literature (e.g. Hrassnigg and Crailsheim Citation1999; Huang and Seeley Citation2003; Perez and Farina Citation2004) and implemented into our model. Foragers fly out of the colony, visit flowers in the meadow areas, return to the colony, and transfer the collected nectar to one or more storer bees. Each single forager bee assesses the quality of the source it has just visited, dances for it, and then starts to forage again. The forager bee has a probability of abandoning its source, and being recruited by the dance of another forager bee to another source (, ). Storer bees wait near the hive entrance, receive nectar from returning foragers, and start to store the nectar in the honeycombs as soon as their honey crops are full (, ). Each of these behaviours is associated with a defined metabolic rate (EquationEquation (1)).
Figure 2. State diagram of a forager bee. Every behavioural state is associated with a defined metabolic rate and a defined behaviour. States associated with a high metabolic rate are shown in grey shading, those associated with a low metabolic rate in white. Abbreviations are explained in . Please keep in mind that there exists an own set of values for each source (A, B) for each transmission probability, taking place outside the colony (p lf, p af, p ff, p rc, p as, p sr).
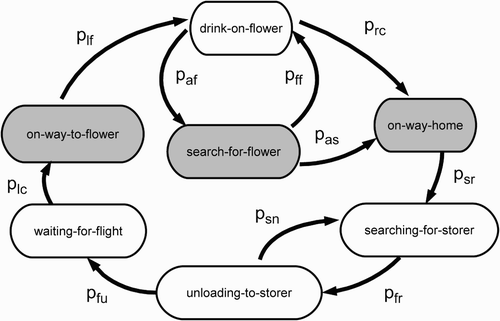
Figure 3. State diagram of a storer bee. All of the states of a storer bee are associated with the low metabolic rate, because storer bees do not leave the colony to fly out in the environment. Every state is associated with a defined behaviour. Abbreviations are explained in .
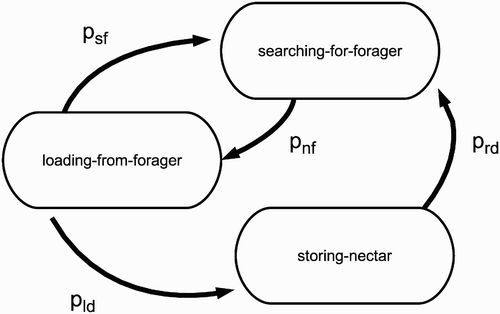
Table 1. Probabilities that a forager bee changes from one behavioural state to another.
Table 2. Probabilities that a storer changes from one behavioural state to another.
The ‘inside’ area of the model () represents the comb area near the hive's entrance, where the interactions between the honeybees usually take place (‘transfer zone’). We implemented this ‘transfer zone’ as two one-dimensional patch-arrays Df (transfer zone for foragers) and Ds (transfer zone for storers), each of length d (). Every patch of Df is associated with a single patch on Ds. Each simulated honeybee i belongs to one of two cohorts: it is either a forager bee f or a storer bee s. The set of forager bees is defined as H and is of size Nh. The set of storer bees is defined as S and is of size Ns. At time step t, an agent i is in state state i (t), one of the states defined in (for foragers) or (for storers). The duration dur i (t, state) is the length of time that an agent i has been in the state (state) at time step t. The value of nearby i, k (t) is defined as TRUE if agent i is on Df and if agent k is on the associated patch on Ds at time step t; else it is defined as FALSE. The value of is_in_hive i (t) is defined as TRUE if at time step t agent i∈S or if state i (t)∈{‘searching-for-storer’, ‘unloading-to-storer’, ‘waiting-for-flight’}; else it is defined as FALSE. If an agent i receives nectar from an agent j, or agent i unloads nectar to j, then transferpartner i (t)=j until the nectar transfer is finished. After finishing the nectar transfer, transferpartner i (t) is set to { }. The value of position i (t)= ‘fp’ as long the honeybee is on a flower patch (fp), and position i (t)= ‘nfp’ if the bee is on a non-flower patch (nfp).
The amount of nectar uploaded by a hungry bee from the honeycomb is defined as Fh. The amount of nectar uploaded by a forager bee before leaving the colony on a foraging flight is defined as F fo. The duration Tf is defined as the time a bee needs to fly the distance between the hive and the source it is visiting, thus it is directly related to the distance between the colony and the nectar source. The duration Th is defined as the time a bee spends after unloading until it leaves the hive on another foraging flight. The duration Ts is defined as the time a storer bee needs to store a nectar load in the honeycombs. The metabolic rate of an agent during a time interval depends on the state the agent is in during that interval.
2.3 Metabolism and crop loads of bees
The bees in our model always have one of the two activity levels: a high activity level, associated with all flying activities, and a low activity level, associated with all other activities. In our model, forager bees fly after leaving the hive ‘on-way-to-flower’, on the way back to the colony (on-way-home), or when flying from one flower to another (search-for-flower). Storer bees stay inside the colony at all times so their activity level is always low.
These activity levels are used in the calculations of the metabolic rates. The equations used were taken from Seeley Citation(1995). The metabolic rate mr
i
(t) of an agent i at time step t is calculated from its weight W
i
(t) and the sugar concentration of the nectar it has consumed, NC (see ), by EquationEquation (1):
Table 3. Fixed variables used in the model.
The maximum crop load C
max i
of an agent i is calculated using a normally distributed random function with a mean of 45.9 μl and a standard deviation of 8.7 μl; these values were taken from Huang and Seeley Citation(2003). The crop load level at which the bee is saturated (and stops uploading from a flower or from another bee) is Cf
i
, and the crop load level at which the bee stops a transfer is Ce. These variables are modelled by EquationEquations (3)–Equation(5)
:
2.4 The state automaton and the behaviour of an agent
We implemented our agents as finite-state automatons (foragers depicted in ; transition probabilities listed in ). Each agent is associated with exactly one behavioural state in every timestep t. Each behavioural state is correlated with a characteristic behavioural pattern.
A forager bee flying to a nectar source is in state ‘on-way-to-flower’. In this state, the forager bee has a high metabolic rate. When the bee arrives at the meadow after a defined amount of time (Tf, for values see ), it lands with a certain probability per time step (p
ff) on a flower and starts to drink nectar (‘drink-on-flower’). In this state, the forager bee has a low metabolic rate. When the flower is empty, the bee starts to fly randomly around in the meadow to search for a new flower (‘search-for-flower’). In this state, the forager bee has a high metabolic rate again. As soon as the bee decides to return to the colony, it switches to the state ‘on-way-home’. The bee decides to return to the colony if it is fully loaded or if just enough nectar is left in its crop to make it back to the colony. In addition, a return can be triggered by a random function with the probability of 0.01 per timestep. After some time (for values see ), it reaches the colony and tries to find a storer bee (‘searching-for-storer’). In nature, when a forager returns from a foraging trip, it tries to find a storer bee in the transfer zone near the hive entrance to which it can unload the nectar. We implemented this in our model by allowing the forager bees and the storer bees to change their positions randomly left and right on the transfer zones. When a waiting storer and a searching forager are located on contiguous transfer zone patches, the transfer is initiated: the forager bee switches to the state ‘unloading-to-storer’ and the storer bee switches to the state ‘loading-from-forager’ (, ). If the transfer is stopped because the storer bee is fully loaded, the forager bee starts to search for a new storer bee to unload to and switches back to the state ‘searching-for-storer’. In nature, a transfer between forager and storer may also be stopped because of jostling in the transfer zone. To incorporate this fact into our model, we implemented a 5% chance of breaking the transfer per timestep, even if the storer is not fully loaded. As soon as the forager bee is empty, it switches to the state ‘waiting-for-flight’, which represents the delay between finishing the nectar transfer and flying out again. After that time delay (for values see ), the forager bee switches to the state ‘on-way-to-flower’ again.
To implement a basic worker allocation mechanism influencing foraging behaviour, we calculated the total number of dance rounds performed for each source at a given time t. The number of dance rounds NDR
i
(m, t) performed by a forager i for a source m is calculated by the function dancerounds(quality(m, t)) as described in Frisch Citation(1965) and Seeley Citation(1995): the dancerounds scale linearly with the quality of the source, which is the ratio between the energetic gain and the costs of the foraging trip to that source m. Foragers leaving the colony decide to fly to one of the two sources and the probability for each source is calculated according to the ratio of dance rounds performed for that source. The function f_dances(t) depicts the probability (which scales linear to the ratio of total number of dancerounds for each source, divided by the sum of all performed dancerounds) of a bee to fly from one source to the other, whereby
A storer bee (, ) in the state ‘searching-for-forager’ moves randomly left and right within the transfer zone. If a storer bee in this state and a forager bee in the state ‘searching-for-storer’ are located on contiguous transfer zone patches, the storer bee switches to the state ‘loading-from-forager’, whereas the forager bee switches to the state ‘unloading-to-storer’. If the transfer is finished by the forager bee, because the forager bee is empty, the storer bee switches back to the state ‘searching-for-forager’. As described above, we assigned a 5% chance of breaking a transfer, even if the forager is not empty, to model possible jostling in the transfer zone. The storer bee switches in the state ‘storing-nectar’ as soon as it is fully loaded. The bee remains in this state for a given amount of time steps (for values see ). Before the storer switches back to the state ‘searching-for-forager’, its crop load is set to Ce i .
In the simulation runs reported below, we used a set of fixed global parameters. The values of these parameters are given in . The numbers of foragers and storers used in our simulation are comparable with those in a small bee colony of about 2000 individuals, which is approximately the size of many of the observation colonies used in empirical experiments. The value of Tf is calculated for a distance of 500 m between the colony and the flower patches.
3. The reliability of the model
3.1 Depicting and evaluating a single foraging cycle
To check the reliability of our model, we simulated several experiments which were described in the literature and compared our results with the published empirical data (Schmidt-Hempel et al. Citation1985; Huang and Seeley Citation2003). The first picture of the dynamics of crop loads of foraging honeybees was published in Schmidt-Hempel et al. Citation(1985). This picture was highly idealised, but it is the only projection so far that incorporates all relevant phases of a honeybee's foraging trip and its relevant metabolic expenditures. Such data are very hard to obtain empirically from real bees, because such a measurement would require measuring the weight of the bee at many points in time during the foraging trip without disturbing the bee's foraging behaviour. Nevertheless, the knowledge of the dynamics of crop volumes of honeybee foragers during the foraging flight is crucial for understanding the economics of foraging strategies. This underscores the importance of using a tailored mathematical model such as ours.
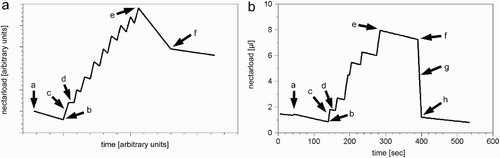
We compared the data published on the dynamics of the crop loads of foragers (Schmidt-Hempel et al. Citation1985) with the results from our model (). The decrease of nectar in the crop of a forager after leaving the colony (‘a’ in ) and before reaching meadow m (‘b’ in ) is caused by the forager bee metabolising mr
i
(t) at every time step t while flying. The duration of drinking nectar depends on the upload rate F
fl and the amount of nectar Ma
m
available in the meadow (‘c’ in ). The forager bee starts to search for a new flower after drinking (‘d’ in ). The duration of this search depends on p
ff and on the density of flowers Md
m
within the meadow. The amount of nectar used for these searching flights is in turn influenced by the metabolic rate mr
i
(t). The duration of the flight back to the colony (‘e’ and ‘f’ in ) depends on Tf. Upon returning to the hive (‘f’ in ), the forager bee searches for a storer bee and unloads the nectar to it (‘g’ in ). Please notice that the unloading event (‘g’) is missing in the graph redrawn by Schmidt-Hempel et al. Citation(1985) (), because unloading events are not discussed and are therefore not depicted. The duration of unloading depends on F
bb. If the forager bee is nearly empty , it stays in the colony for Th time steps.
Figure 4. Dynamics of the crop load of a forager bee during one foraging cycle as predicted by our simulation (B) and as depicted in published idealised data (Schmidt-Hempel et al. Citation1985) (A). The forager leaves the colony (a) to fly to the nectar source, lands on a flower (b), uploads the nectar (c), flies to the next flower (d), and so on. After some time the forager stops flying from flower to flower and starts to fly back to the colony (e). As soon as the forager reaches the colony (f), it unloads most of its nectar to a waiting storer bee ((g) missing in A), and then stays in the colony for Th time steps (h). The graph predicted by our model shows a foraging cycle under conditions of low nectar flow with Ma m =1 μl. The bee stops foraging before it is fully loaded (C i (t)<Ce i ).
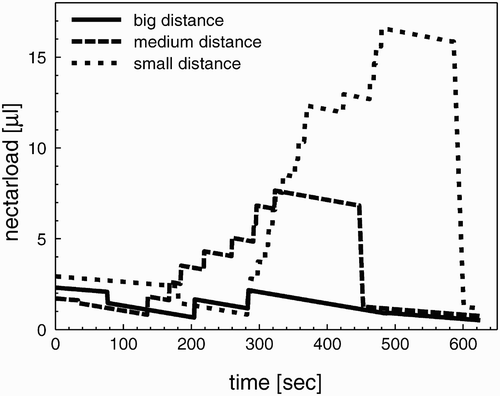
3.2 Influence of flower-to-flower distances on the dynamics of a single foraging cycle
We compared the dynamics of the crop load of forager bees in environments with differing inter-flower distances to investigate the influence of the mean flower-to-flower distance on the dynamics of foraging flights. In an environment with big flower-to-flower distances (, solid line), the bees use all the collected nectar immediately for the flight to the next flower, and this way they return to the colony without any nectar gain. In environments of medium flower-to-flower distances (, wide dashed line), the bees are able to gather nectar and return to the colony with a positive net nectar budget. In an environment with small flower-to-flower distances (, fine dashed line), this effect even increases and enables the bees to return to the colony with more nectar.
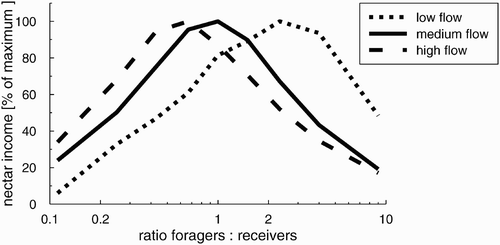
3.3 Influence of forager-to-receiver ratio on the foraging successes of the colony
To investigate how different forager-to-receiver ratios influence nectar income of a colony, we performed several simulation runs with varying forager-to-receiver ratios. We kept the total numbers of bees in the colony constant in these simulation runs. It showed that, depending on the nectar flow in the environment, the optimal forager-to-receiver ratio changed ().
3.4 Comparing the nectar loads of returning foragers
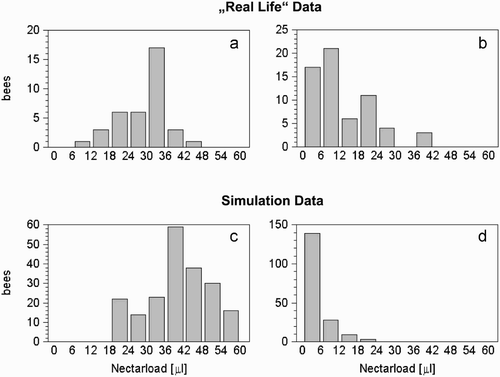
We compared the nectar loads of returning foragers under different environmental conditions generated by our model with those of real honeybees (Huang and Seeley Citation2003) under comparable conditions (). In our model as well as in nature, foragers return with very small amounts of nectar under conditions of low nectar flow. Under conditions of high nectar flow, the forager bees return almost fully loaded.
Figure 7. Crop loads of returning forager bees found in nature (A, B) and in our model (C, D). Under conditions of high nectar flow in the environment, most forager bees return to the hive with full or nearly full loads (A, C). Under conditions of low nectar flow, forager bees return with very little nectar (B, D). Graphs A and B are redrawn from (Huang and Seeley Citation2003). The larger loads in C compared with A, as well as the low variances in the crop loads of the foragers (D) compared with C are attributable to the idealised environmental situation in our simulation: all simulated flights were direct, with no wind and an entirely homogeneous environment.
4. Results
4.1 Multiple unloadings as channel of information
The goal of our simulation experiments was to investigate whether bees foraging on one source can detect changes in the nectar flow of another source without visiting that source themselves. To answer this question, we started the simulation with two sources having the same low nectar flow (Ma m =1 μl). After 90 min (=5400 steps), we changed the flow of one nectar source in the environment by increasing the available amount of nectar per flower (Ma m =50 μl). After another 90 min, we decreased the nectar flow of that source back to its initial low level. The number of flowers, the distance from flower to flower, and the simulated sugar concentration were kept constant during the whole experiment.
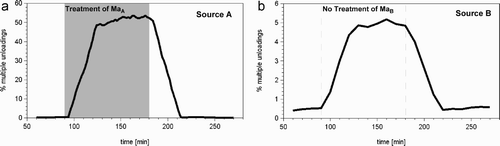
For the bees flying to the treated source (source A), there was a significant increase in the occurrence of multiple unloadings (). If a returning forager bee cannot unload the full nectar load to the first storer, due to limited space in this storer bee (Huang and Seeley Citation2003), double unloadings or even multiple unloadings occur. This happens more often in times of high nectar flow than in times of low nectar flow. In times of high nectar flow, the percentage of multiple unloadings experienced by the bees flying to the treated source (source A) increased 100-fold, from a level of 0.5% to ∼ 50% (). This increase in the frequency of multiple unloadings was caused by a much higher arrival rate of fully loaded forager bees returning to the colony. We also found a somewhat smaller increase in multiple unloadings for those bees that were visiting source B, which was the one that did not experience any treatment. For those bees, the increase in the rate of multiple unloadings was about 10-fold, from 0.5% to ∼ 5% (). This increase in the frequency of multiple unloads on the untreated source was caused by the high number of almost-filled storer bees, which were still waiting near the hive's entrance. This high number in turn was due to the high percentage of fully filled foragers returning from the treated source. This means that one out of 20 bees visiting source B was likely to detect evidence of the change in nectar flow on another source they had not visited. Thus, the relative frequency of multiple unloadings was a narrow channel of information transfer between the two groups of foraging bees.
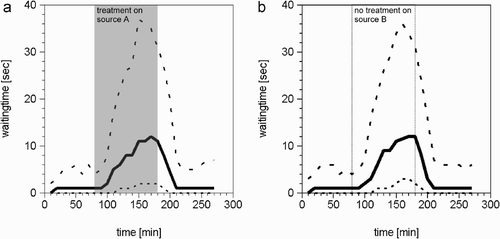
Figure 8. Changes in the percentage of multiple unloads in the group of bees flying to the source with a temporarily increased rate of nectar flow (source A) and in the group of bees flying to the source that had a constant low rate of nectar flow (source B). The grey area shows the time during which source A was treated (=high nectar flow).
4.2 Waiting time in the hive as channel of information
We found a massive (>10-fold) increase in the in-hive search time experienced by the bees foraging on the source that was treated (). When both sources had a low nectar flow, a returning forager bee had to wait approximately one time step before it found a receptive storer bee. When we changed the nectar flow of source A to high flow, we found that the search time experienced by the bees returning from the untreated source also increased more than 10-fold (). This means that, on average, every bee had to search about 10 times longer for a storer bee in times of a high nectar flow on one source, compared with times of low nectar flow on both sources. This shows that the waiting time experienced by an individual forager bee in the hive provides a channel of indirect communication between the two groups of foragers.
Figure 9. Changes in the duration of the searching-for-storer-period experienced by bees foraging on the treated source (A), and those foraging on the untreated source (B). During the period of high nectar flow on the treated source (90–180 min) there was a more than 10-fold longer searching time experienced by both groups of foragers, those foraging on the treated source and those foraging on the untreated source. The bold line indicates the median, the dashed lines first and third quartiles.
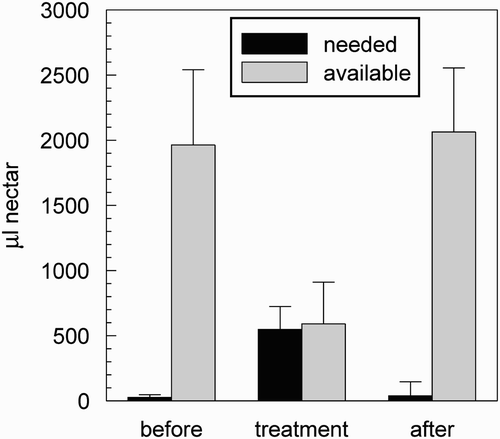
Under conditions of low nectar flow (before and after treatment), in the storer bees, only a small crop space is needed (), because the foragers return with small amounts of nectar to the colony (). During that time, a large crop space is available (), because many storers wait near the hive's entrance. On the other hand, under conditions of average higher nectar flow (during treatment, ), more crop space is needed, because foragers return with much larger crop loads (). This leads to less available crop space in the storer bees as a delayed consequence. Thus, returning forager bees have to search longer (on average) to unload the collected nectar ().
Figure 10. Differences between the crop space needed for unloading by returning foragers, and the average available crop space in storer bees per time step, in our simulations. In the experimental phase before treatment (two columns on the left), when only small amounts of nectar are brought into the colony by foragers, many storers are waiting near the hive's entrance. During the treatment (two middle columns), much larger amounts of nectar are brought into the colony by foragers, and less crop space in storers is available. After the treatment (two columns on the right), the amounts of needed and available crop space return to former levels.
4.3 Application of the model
The results shown above inspired us to use our model to analyse whether or not individual forager bees are predicted to have enough information available to optimise their foraging decisions in favour of the colony by social learning. We assumed that foragers can modulate their dance behaviour (waggle dances) in response to changes in queuing delays or to a sudden increase in the frequency of multiple unloadings. Section 4.2 suggests that queuing delays pose a better source of information than the occurrence of multiple unloadings (Section 4.1); thus we concentrated on this channel of information. Studies (Seeley Citation1995) showed that bees are able to modulate their dancing behaviour in response to environmental changes. For example, waggle dances, which recruit additional foragers to the same source that is exploited by the dancing bee (Farina and Nunez Citation1993), are performed with lower probability and with shorter duration after the nectar stores of the colony increased. Other studies showed that the probability of waggle dancing is also individually modulated in case of a numerical forager-receiver-mismatch (Seeley Citation1995). Our model predicted sudden increases in multiple unloadings and queuing delays in response to changes in the nectar flow at flower patches that are not visited by the dancing bee, thus they can represent an indirect flow of information between cohorts of foragers foraging on different flower patches. If a forager bee takes advantage of this information, then the most likely adaptation of behaviour is the dance length and the frequency of waggle dances. To test our hypothesis, we altered our model to investigate the following question: does a decreased dancing duration in response to a sudden decrease of a forager's own nectar source (compared with all other sources) change the net colony nectar gain? To answer this question, we implemented an additional function into our model, which is used to modulate the (formerly fixed) waggle-dance durations:
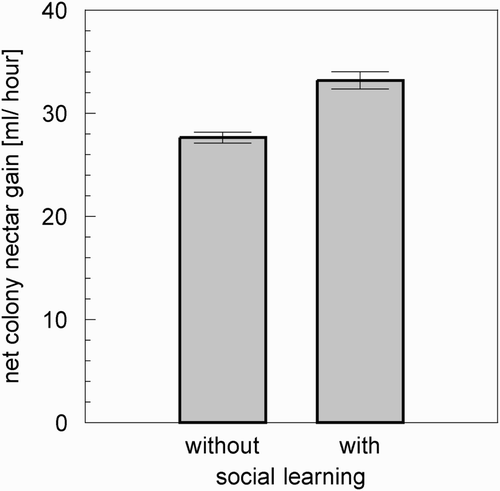
Figure 11. Comparison of the net nectar gain of a colony of 2000 bees (500 forager bees, 500 storer bees, and 1000 additional colony-bees). The colony that used the social learning gained about 20.1% more nectar than a colony without social learning. (Bars indicate mean values, whiskers indicate standard deviation. n=10.)
5. Discussion
Using the proposed model, we investigated pathways of information about the environmental situation in a highly complex social system: the honeybee colony. The results predicted by our model were successfully compared with data from the literature (Schmidt-Hempel et al. Citation1985; Huang and Seeley Citation2003).
We demonstrated that novel communication channels exist in the honeybee society, which have the potential to communicate environmental conditions among groups of foragers visiting different nectar sources. These forager-to-forager communication channels differ from the known pathways of information inside a honeybee colony (Frisch Citation1965; Seeley Citation1995), which are dances. As our model demonstrates, these additional communication channels emerge from side-effects of other, already documented communication channels.
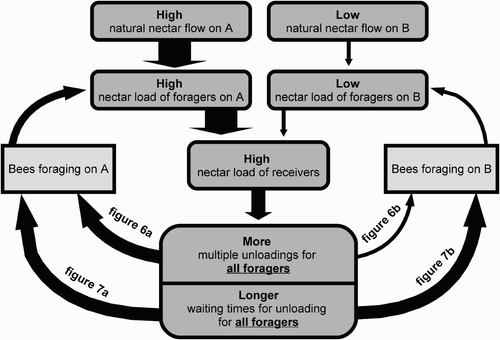
Our studies showed that individual foraging bees have enough information available to be able to detect changes in nectar flow on food sources that they themselves are not currently visiting. They can acquire this information indirectly via the cohort of storer bees, using two channels of communication: (1) a change in the percentage of multiple unloadings experienced by the forager bees and (2) a change in their searching time for storer bees. The pathways of the flow of direct and indirect information about the current status of the environment available to foraging bees are summarised in . Although it is well known that queuing delays are used by bees to balance the storing workforce according to the foraging activity, the potential aspects of queuing delays in indirect forager-to-forager communication have never been described so far. Without a mathematical individual-based model, such indirect social modulations as indirect forager-to-forager communication are hard to discover and to describe. It is not individual adaptation that allows the colony to pass over the information from one forager cohort to another. In contrast, the information is passed (and averaged) from foragers to storers and then back to other foragers. Thus we conclude that any adaptation of the colony that arises from exploitation of these mechanisms can be best called ‘social adaptation’ or ‘social learning’.
Figure 12. Scheme of the flow of information about the environmental situation between two groups of foragers via the nectar unloading mechanism. Under conditions of the coexistence of a source with high nectar flow and a source of low nectar flow, the resulting high loads of the nectar receivers lead to a high waiting time experienced by both the foragers returning from the source with the high nectar flow, and the foragers returning from the source with the low nectar flow. The arrows in the diagram symbolise causal influences.
The transfer of information between single bees as well as between groups of bees is very important in a decentralised system like a honeybee colony. Honeybees have to optimise their foraging decisions without any central processing unit. The amount and the quality of information available to each single forager bee is of great importance in this decision-making process. In nature, bees foraging on a source of low nectar flow might abandon this source with a higher probability as soon as they indirectly perceive a source of higher nectar flow in the environment.
In recent years, several models of honeybee foraging behaviour have been developed. Higginson and Gilbert Citation(2004) created a model of the changes in inflorescence acceptance under conditions of increasing wing damage (with age) of a forager bee. The influence of partial nectar loads on the emergence of multiple unloadings was investigated by Gregson, Hart, Holcombe, and Ratnieks Citation(2003). The advantages and disadvantages of different foraging strategies under different environmental conditions were discussed by Anderson Citation(2001). A very detailed model of the foraging mechanism and decision-making was described in DeVries Citation(1998) and Biesmeijer (2002). In Bartholdi, Seeley, Tovey, and Vant Citation(1993), the mechanisms of forager allocation among different flower patches in the environment are described. In Seeley Citation(1994), Anderson and Ratnieks Citation(1999), and Ratnieks and Anderson Citation(1999), the queuing delay in a honeybee colony is investigated using a highly simplified, individual-based model. Using a run model in combination with empirical experiments, Seeley and Tovey Citation(1994) showed the correlation between waiting time and workload balancing in a honeybee colony. The empirical results about decision-making between two artificial nectar feeders of Huang and Seeley Citation(2003) were implemented in a very detailed individual-based model by Schmickl and Crailsheim Citation(2004), Thenius, Schmickl, and Crailsheim Citation(2006), Schmickl, Thenius, and Crailsheim Citation(2005), and Thenius, Schmickl, and Crailsheim Citation(2005).
Compared with those models, our model includes a more natural environment: scattered nectar sources with variable distances between flowers. By implementing these environmental factors, we are able to predict the dynamics of a single forager's crop load during a foraging flight (). This is especially important, because the resulting distribution of crop loads of returning forager bees predicted by our model is comparable with that found in real honeybees (). This makes the dynamics of nectar influx into the colony under different environmental conditions () more natural than in other models of honeybee foraging behaviour. A comparison between the dynamics of a single forager's crop load during a foraging flight as predicted by our simulation () and data from Schmidt-Hempel et al. Citation(1985) () shows that our scattered nectar sources lead to crop load dynamics that are more natural than depicted there. No unloading occurs in the results of Schmidt-Hempel et al. Citation(1985) (), and in addition, a homogeneous distribution of flowers is assumed by these authors, which leads to an artificial rhythmical increase of nectar in the crops of the foragers ().
An important advantage of our model is the implementation of the metabolism of honeybees, which is crucial for understanding honeybee foraging in an environment with scattered nectar sources. Another advantage is that we consider the spatial dynamics of bee-to-bee interactions in the hive, which is especially important for modelling queuing delays for foragers and storers. In case of a mismatch between the number of foragers and receivers in the colony, long queuing delays arise, resulting in a lower efficiency of the colony (). We found a clear optimum at a forager-to-receiver ratio of 0.6. This optimum strongly depends on the time length of the foraging cycle and the time length of the storage cycle and on the crop filling of the returning foragers. The mean time of a foraging trip was calculated by considering the bee flight speed, distances, and time spent on drinking nectar (values taken from the literature (Perez and Farina Citation2004)). The storage cycle period was also taken from the literature (Seeley Citation1995). The third important factor, that is the crop filling of returning foragers, is one of the striking results that we derived from our model. Thus, the clear optimum shown () depends significantly on the fact that our model implements the foraging trips of honeybees in much more detail than other ‘honeybee foraging models’ did so far. The distribution of crop loads of returning foragers in nature is significantly different from the distribution found in experiments, where artificial feeders were used to feed the bees up to the saturation point. With our individual-based model, we could show that the question of ‘optimal’ forager-to-receiver ratios cannot be answered without considering the typical dynamics of honeybee foraging trips in nature.
We tested our assumptions concerning queuing delays as a possible pathway to communicate environmental changes between distinct cohorts of foragers. Our simulations showed that social leaning can enable a honeybee colony to increase the nectar-collecting efficiency (). The ability of a colony to increase its net nectar gain under conditions of environmental changes is based on the changes of individual forager behaviour. The feasibility of such behavioral changes of single individuals, having only local perception available, in a colony without any central processing unit, can only be guaranteed by a reliable social network, offering information about the environmental situation. In this case, the communicated information is offered to the single individual forager by the duration of experienced waiting times ().
We plan to perform further simulation studies under conditions of differing distances to the sources. Also, we plan to use the model to focus on different strategies that forager bees use to decide whether to stay on the same food source or return to the colony.
Our model is a useful tool to investigate internal mechanisms that regulate foraging of honeybee colonies and to study the potential pathways of information flow in such a decentralised insect society.
Acknowledgements
This study was partially supported by the following research grants: EU-IST FET project I-Swarm, no. 507006, the research grant ‘Temperature-induced aggregation of young honeybees’, no. P19478-B16, the EU FP7 project REPLICATOR, no. 216240, and the EU FP7 project SYMBRION, no. 216342.
References
- Anderson , C. and Ratnieks , F. L.W. 1999 . Task Partitioning in Insect Societies: I. Effect of Colony Size on Queueing Delay and Colony Ergonomic Efficiency . The American Naturalist , 154 : 521 – 535 .
- Anderson , C. and Ratnieks , F. L.W. 1999 . Worker Allocation in Insect Societies: Coordination of Nectar Foragers and Nectar Receivers in Honey Bee (Apis mellifera) Colonies . Behavioral Ecolology and Sociobiology , 46 : 73 – 81 .
- Anderson , C. 2001 . The Adaptive Value of Inactive Foragers and the Scout-Recruit System in Honey Bee (Apis mellifera) Colonies . Behavioral Ecology , 12 : 111 – 119 .
- Bartholdi , J. J. , Seeley , T. D. , Tovey , C. A. and Vante , J. H.V. 1993 . The Pattern and Effectiveness of Forager Allocation among Flower Patches by Honey Bee Colonies . Journal of Theoretical Biology , 160 : 23 – 40 .
- Biesmeijer , J. C. and DeVries , H. 2001 . Exploration and Exploitation of Food Sources by Social Insect Colonies: a Revision of the Scout-Recruit Concept . Behavioral Ecolology and Sociobiology , 49 : 89 – 99 .
- Bonabeau , E. , Dorigo , M. and Theraulaz , G. 1999 . Swarm Intelligence – From Natural to Artificial Systems , Oxford : Oxford University Press .
- DeGrandi-Hoffman , G. and Hagler , J. 2000 . The Flow of Incoming Nectar Through a Honey Bee (Apis mellifera L) Colony as Revealed by a Protein Marker . Insect Sociaux , 47 : 302 – 306 .
- DeVries , H. and Biesmeijer , J. C. 1998 . Modelling Collective Foraging by Means of Individual Behaviour Rules in Honey-Bees . Behavioral Ecology and Sociobiology , 44 : 109 – 124 .
- DeGrandi-Hoffman , G. and Hagler , J. 2002 . Self-Organization in Collective Honeybee Foraging: Emergence of Symmetry Breaking, Cross Inhibition and Equal Harvest-Rate Distribution . Behavioral Ecology and Sociobiology, , 51 : 557 – 569 .
- Farina , W. and Nunez , J. 1993 . Trophallaxis in Honey Bees: Transfer Delay and Daily Modulation . Animal Behaviour , 45 : 1227 – 1231 .
- Frisch , K. V. 1965 . Tanzsprache und Orientierung der Bienen , Berlin, Heidelberg, NY : Springer Verlag .
- Gmeinbauer , R. and Crailsheim , K. 1993 . Glucose Utilization during Flight of Honeybees (Apis mellifera) Workers, Drones and Queens . Journal of Insect Physiology , 39 : 959 – 967 .
- Gregson , A. M. , Hart , A. G. , Holcombe , M. and Ratnieks , F. L.W. 2003 . Partial Nectar Loads as a Cause of Multiple Nectar Transfer in the Honey Bee (Apis mellifera): a Simulation Model . Journal of Theoretical Biology , 222 : 1 – 8 .
- Hart , A. G. and Ratnieks , F. 2001 . Why Do Honey-Bee (Apis mellifera) Foragers Transfer Nectar to Several Receivers? Information Improvement Through Multiple Sampling in a Biological System . Behavioral Ecology and Sociobiology , 49 : 244 – 250 .
- Higginson , A. D. and Gilbert , F. Paying for Nectar with Wingbeats: a New Model of Honeybee Foraging . Proceedings of the Royal Society of London, Series B (Biological Science) , Vol. 271 , pp. 2595 – 2603 .
- Hrassnigg , N. and Crailsheim , K. 1999 . Metabolic Rates and Metabolic Power of Honeybees in Tethered Flight Related to Temperature and Drag (Hymenoptera: Apidae) . General Entomology , 24 : 23 – 30 .
- Huang , M. and Seeley , T. A. 2003 . Multiple Unloadings by Nectar Foragers in Honey Bees: a Matter of Information Improvement or Crop Fullness? . Insectes Sociaux , 50 : 1 – 10 .
- Kennedy , J. and Eberhart , R. C. 2001 . Swarm Intelligence , San Francisco, , USA : Morgan Kaufmann .
- Perez , N. and Farina , W. 2004 . Nectar-receiver Behavior in Relation to the Reward Rate Experienced by Foraging Honeybees . Behavioral Ecology and Sociobiology , 55 : 574 – 582 .
- Ratnieks , F. and Anderson , C. 1999 . Task Partitioning in Insect Societies II. Use of Queuing Delay Information in Recruitment . The American Naturalist , 154 : 536 – 548 .
- Schmickl , T. and Crailsheim , K. 2004 . Costs of Environmental Fluctuations and Benefits of Dynamic Decentralised Foraging Decisions in Honey Bees . Adaptive Behavior , 12 : 263 – 277 .
- Schmickl , T. , Thenius , R. and Crailsheim , K. Simulating Swarm Intelligence in Honey Bees: Foraging in Differently Fluctuating Environments . Proceedings of the Genetic and Evolutionary Computing Conference (GECCO) . June 25–29 . pp. 273 – 274 . Washington, DC ACM 1-59593-010-8/05/0006
- Schmidt-Hempel , P. , Kacelnik , A. and Houston , A. 1985 . Honeybees Maximize Efficiency by not Filling their Crop . Behavioral Ecology and Sociobiology , 17 : 61 – 66 .
- Seeley , T. D. and Tovey , C. 1994 . Why Search Time to Find a Food-Storer Bee Accurately Indicates the Relative Rates of Nectar Collecting and Nectar Processing in Honey Bee Colonies . Animal Behavior , 47 : 311 – 316 .
- Seeley , T. D. 1994 . Honey Bee Foragers as Sensory Units of the Colonies . Behavioral Ecology and Sociobiology , 34 : 51 – 62 .
- Seeley , T. D. 1995 . The Wisdom of the Hive , Cambridge, MA, London, , England : Harvard University Press .
- Seeley , T. D. , Camazine , S. and Sneyd , J. 1991 . Collective Decision-Making in Honey Bees: How Colonies Choose Among Nectar Sources . Behavioral Ecology and Sociobiology , 28 : 277 – 290 .
- Thenius , R. , Schmickl , T. and Crailsheim , K. 2006 . “ Economic Optimisation in Honeybees: Adaptive Behaviour of a Superorganism ” . In From Animal to Animats 9 (SAB 2006), Lecture Notes in Artificial Intelligence 4095 , Edited by: Nolfi , S. , Baldassarre , G. , Calabretta , R. , Hallam , J. C.T. , Marocco , D. , Meyer , J.-A. , Miglino , O. and Parisi , D. 725 – 737 . Berlin Heidelberg : Springer Verlag .
- Thenius , R. , Schmickl , T. and Crailsheim , K. 2005 . “ The ‘Dance or Work’ Problem: Why Do not all Honeybees Dance with Maximum Intensity ” . In CEEMAS 2005. Lecture Notes in Artificial Intelligence (LNAI) 3690 , Edited by: Pechaucek , M. , Petta , P. and Varga , L. Z. 246 – 255 . Berlin Heidelberg : Springer-Verlag .
- Wilensky , U. 1999 . “ NetLogo ” . Evanston, IL : Center for Connected Learning and Computer-Based Modeling, Northwestern University . http://ccl.northwestern.edu/netlogo/