?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
We present a system-level connectionist model of pupil control that includes brain regions believed to influence the size of the pupil. It includes parts of the sympathetic and parasympathetic nervous system together with the hypothalamus, amygdala, locus coeruleus, and cerebellum. Computer simulations show that the model is able to reproduce a number of important aspects of how the pupil reacts to different stimuli: (1) It reproduces the characteristic shape and latency of the light-reflex. (2) It elicits pupil dilation as a response to novel stimuli. (3) It produces pupil dilation when shown emotionally charged stimuli, and can be trained to respond to initially neutral stimuli through classical conditioning. (4) The model can learn to expect light changes for particular stimuli, such as images of the sun, and produces a “light-response” to such stimuli even when there is no change in light intensity. (5) It also reproduces the fear-inhibited light reflex effect where reactions to light increase is weaker after presentation of a conditioned stimulus that predicts punishment.
1. Introduction
The dilation and contraction of the pupil of the eye has been extensively studied for the last 50 years and it has been established that in addition to being influenced by light, a surprising number of brain processes influence the size of the pupil. This includes general arousal, the circadian rhythm, the perception of emotional stimuli, novelty detection and even cognitive processes, including memory operations (Goldwater, Citation1972; Laeng, Sirois, & Gredebäck, Citation2012; Pamplona, Oliveira, & Baranoski, Citation2009; Sirois & Brisson, Citation2014; Tryon, Citation1975).
The largest change in pupil dimeter is caused by light changes that elicit the pupil light reflex (Ellis, Citation1981; Hess & Polt, Citation1960; Heller, Perry, Jewett, & Levine, Citation1990; Hess & Polt, Citation1964; Woodhouse & Campbell, Citation1975). Although the pupil diameter can vary between 2 and 8 mm (Pamplona et al., Citation2009; Watson & Yellott, Citation2012), in normal light conditions it is around 3 mm (Wyatt, Citation1995), and a flashing bright light will give a contraction of 0.2–2.5 mm depending of the light intensity (Ellis, Citation1981). Ellis (Citation1981) reported a latency of the light reflex at a minimum 220 ms and suggested that this was mainly due to slow muscle constriction. After constriction is complete, the pupil will dilate again and return to its initial size. Interestingly, the contraction of the pupil is approximately three times faster than the subsequent dilation. A higher light intensity reduces the latency of pupil contraction and the maximum constriction velocity increases with increasing stimulus intensity (Ellis, Citation1981; Pamplona et al., Citation2009).
Emotion is also an important regulator of pupil size. For example, the pupil reacts to images of emotional facial expressions (Kret, Roelofs, Stekelenburg, & de Gelder, Citation2013). This may play a role in social interaction. It has recently been shown that we can automatically mimic the pupil dilations of others and that this leads to increased trust (Kret, Fischer, & Dreu, Citation2015).
In a classic study, subjects were presented with pictures of semi-nude adults (Hess & Polt, Citation1960). The results showed that pictures of the opposite sex led to a pupil dilation of 20%. However, later studies have found pupil dilation to nudity irrespective of the sex (Aboyoun, Dabbs, & James, Citation1998). It has been suggested that novelty as well as nudity played a role in generating these reactions.
The pupil reacts to both unconditioned and conditioned stimuli. After fear conditioning, the presentation of the conditioned stimulus will increase the pupil diameter in a similar way to the unconditioned stimulus. However, the conditioned stimulus has the additional property that it will attenuate the light-reflex (Bitsios, Szabadi, & Bradshaw, Citation1996; Bitsios, Philpott, Langley, Bradshaw, & Szabadi, Citation1999), something that does not happen after presentation of the unconditioned stimulus. It has been shown that such a modulation of the light reflex can also be produced by showing emotionally charged images, whether they are pleasant or unpleasant (Henderson, Bradley, & Lang, Citation2014), suggesting that this is an arousal effect.
Pupil size is also influenced by cognitive processing. Cognitive reactions are generally smaller than the light response and are typically in the order of 0.5 mm (Beatty & Lucero-Wagoner, Citation2000). For example, when subjects were asked to multiply two numbers, the complexity of the multiplication was reflected in pupil size (Ahern & Beatty, Citation1979; Hess & Polt, Citation1964). Pupil dilation is present in memory encoding and recall) and is also influenced by memory load (Beatty & Kahneman, Citation1966). The intensity of processing is another factor (Just & Carpenter, Citation1993). In a mixed listening task, people that gave up often had smaller pupil dilation while particiapants that performed well on the task had larger pupil dilation (Zekveld & Kramer, Citation2014).
In an interesting experiment, Binda, Pereverzeva, & Murray (Citation2013) presented greyscale images in four categories: (1) photographs of the sun, (2) uniform luminance pictures of each sun image, (3) phase scrambled images of the sun, and (4) photographs of the moon with a mean luminance matched to the photographs of the sun. They found significant constriction of the pupil to the sun photographs. The experiment illustrates that the pupil response is sensitive to the identity of the stimulus used. It is possible that the pupillary response to the sun pictures resulted from a conditioned light-avoidance behaviour (Binda et al., Citation2013), but as the authors note, experiments with conditioning of the pupil response have been inconclusive in humans (Lennartz & Weinberger, Citation1992). Some of the earliest experiments failed in various ways and it was believed that pupillary responses could not be directly conditioned. Some of the successful experiments used shock as a US and it is possible that the pupil dilation found was part of the autonomous response to anticipated fear rather than true pupil conditioning (Gerall, Sampson, & Boslov, Citation1957; Gerall & Woodward, Citation1958; Young, Citation1965). However, direct conditioning of pupillary responses have been demonstrated in cats and dogs (Cassady, Citation1996; Harlow & Stagner, Citation1933; Lennartz & Weinberger, Citation1992).
Despite its importance as an indicator of different brain processes, there have been very little interest in including pupil control in computational models of cognitive processes. Several models of pupil control exist, but most of them only address the detailed properties of the light reflex (Longtin & Milton, Citation1989; Pamplona et al., Citation2009; Semmlow & Chen, Citation1977). Furthermore, models that include other types of stimuli do not usually take the relevant neurophysiology into account (Korn & Bach, Citation2016). There is thus a need for a computational model that can reproduce a wider range of phenomena while being based on the structure of the relevant brain structures. Our goal here is to develop such a model that can be used both to explain properties of the pupillary response in different conditions and as a component of a larger brain model, where it can link cognitive processes with functions of the peripheral nervous system. Using a system-level approach, we aim for a structural model that includes the relevant brain areas and where each component is of the minimal complexity necessary to reproduce the desired pupil responses. The main objective is to show that a system-level model that includes the brain regions believed to influence pupil size can reproduce a wide range of results. The next section reviews the main brain regions involved, while Section 3 presents the details of the model. We test the model on a number of critical experiments in Section 4 and subsequently discuss the results inSection 5.
2. The neurophysiology of pupil control
In this section we present a short overview of the brain regions that influence the pupil. There are many anatomical differences between species. However, we will not address these here.
The pupil is controlled by two sets of muscles. The pupillary sphincter is responsible for constriction of the pupil and is located in a ring along the pupil opening. The pupillary dilator is located around the sphincter and will make the pupil dilate. Together, these two sets of muscles control the opening of the pupil.
The control of the constriction of the pupil is part of the parasympathetic systems. The pupillary sphincter receives signals from the Edinger–Westphal nucleus (EW) through the Ciliary ganglion (CG). EW, in turn, receives its primary input from the pretectal area (PTA) that receives input from the retinal ganglion cells. This results in a loop where increased light stimulation of the retina will produce constriction of the pupil. Both the PTA and EW are paired structures. The left and right PTA receive signals from the corresponding visual fields of both eyes, thus adding up light responses from both eyes. Each PTA subsequently projects to both sides of EW producing a second step of summation where both sides react to light from both eyes. Consequently, light from either eye will let the pupils of both eyes constrict, and as expected, stimulation of the PTA results in constriction of the pupil (Reiner, Karten, Gamlin, & Erichsen, Citation1983).
Although EW has traditionally been seen as a unitary structure, it has been shown that it consists of two distinct sets of cells. Only the preganglion part of the nucleus (EWpg) is involved in pupillary control while the centrally projecting (EWcp) is involved in sympathetic and stress-related functions (Kozicz et al., Citation2011).
The control of dilation of the pupil is part of the sympathetic system. The pupillary dilator receives signals from the hypothalamus through two synapses. The first can be found in the ciliospinal centre of Budge, which is located in the intermediolateral cell column (IML) of the spinal cord, between the 8th cervical segment and the 2nd thoracic segment. The second synapse occurs in the superior cervical sympathetic ganglion (SCG). The axons from the SCG follows the carotoid artery to the trigeminal and on through the CG without synapsing there until it reaches the pupillary dilator.
In cats, it has been found that the cerebellum (CB) receives input from EWpg (Hultborn, Mori, & Tsukahara, Citation1978; Røste & Dietrichs, Citation1988; Sugimoto, Itoh, & Mizuno, Citation1978) as well as projecting back (Cohen, Chambers, & Sprague, Citation1958; Hultborn, Mori, & Tsukahara, Citation1978). Lesions of parts of CB in cats influence the pupillary light reflex by reducing its frequency response (Ijichi, Kiyohara, Hosoba, & Tsukahara, Citation1977). Furthermore, electrical stimulation of the cerebellar nuclei produce pupillary dilation (Hultborn et al., Citation1978).
The Locus coeruleus (LC) is involved in arousal and stress, including the processing of noxious stimuli. LC can influence pupillary constriction both by inhibition of the parasympathetic system and through sympathetic excitation (Szabadi & Szabadi, Citation2012).
The amygdala (AMY) also has indirect access to EWpg through its influence on LC (Szabadi & Szabadi, Citation2012). Through fear conditioning, a stimulus can acquire the ability to modulate the light reflex. Koikegami & Yoshida (Citation1953) showed that stimulation of AMY induced pupillary dilation in the cat. This effect can either be produced through AMY activation of the hypothalamus or through excitation of LC.
In addition, several parts of the hypothalamus, like the Ventrolateral Preoptic Nucleus (VPLO), which is involved in sleep regulation, and the suprachiasmatic nucleus (SCN) and the dorsomedial nucleus (DMH), that are involved in circadian rhythms, can influence the pupil through LC. There is also a light controlled path from the retina to the SCN that inhibits the paraventricular nucleus (PVN), which in turn connects with the sympathetic system. We do not address the effects of these hypothalamic nuclei here.
3. Model
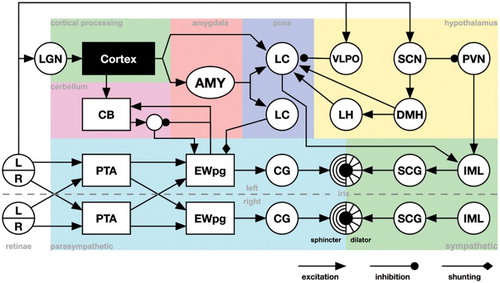
The overall structure of the model can be seen in Figure . It consists of a number of nuclei and some larger brain regions including CB and AMY. The cortex, specifically the visual system, is modelled as a black box that detects different image stimuli and is not further described here. The connectivity of LC and hypothalamus closely follows that described by Szabadi & Szabadi (Citation2012). The goal of the model is to look at qualitative system properties of a network model where each component has the minimal properties necessary to reproduce the different phenomena described above.
Figure 1. Model of the pupil control network. L/R: Left and right visual fields of the retinas, PTA: Pretectal area. EWpg: Edinger–Westphal nucleus, preganglion portion, CG: ciliar gangion, SCG: superior cervial ganglion, IML: intermediolateral column of the spinal cord, CB: cerebellum, LH: lateral hypothalamus, DMH: dorsomedial hypothalamus, VLPO: ventrolateral preoptic nucleus, SCN: suprachiasmatic nucleus, PVN: paraventricular nuclues. Except for the parasympathetic and sympathetic portion of the model, we do not separate the left and right parts of the included structures.
The activity of each nucleus x is modelled using the following equation, where are the excitatory inputs,
are direct inhibitory inputs and S is shunting inhibition.
(1)
(1) α is the resting level, β and γ are scaling factors for the excitation and inhibition, respectively, Unless otherwise stated, these are set to,
and
, where N and M are the number of excitatory and inhibitory connections to a nucleus respectively. This makes the response of the nucleus independent of its number of input connections. The excitatory weights
are set to 1 for all non-plastic connections, that is, everywhere except for AMY and CB. The output from the nucleus is calculated as
(2)
(2) where φ is a scaling factor set to
.
For both AMY and CB we use Equation (Equation1(1)
(1) ) in combination with a minimal classical conditioning model using the delta rule (Rescorla & Wagner, Citation1972; Widrow & Hoff, Citation1960). This model adapts the weights
in Equation (Equation1
(1)
(1) ) according to a US input when
,
(3)
(3)
In the minimal CB model, the current output from EWpg is subtracted before it is allowed to influence EWpg (see Figure ). This creates a negative feedback loop that makes CB adjust the activity level of EWpg when it deviates from expected levels.
In addition, we assume that there is a delay τ in each connection. This delay is set to 20 ms for all connections except the Cortex to AMY connection, were a delay of s is used to set the optimal inter-stimulus interval for classical conditioning.
4. Evaluation of the model
The model was implemented in the Ikaros system (Balkenius, Morén, Johansson, & Johnsson, Citation2010). We conducted five simulations with the implemented model to test how well it would reproduce some aspects of the pupil response in different situations. The aim was to investigate if the model would qualitatively reproduce different phenomena. We did not attempt to tweak the parameters to fit one particular experiment. Instead, parameters were set to sensible default values. In most cases, the parameters were set to 1 (see Appendix). The learning rates for AMY and CB were set to require a small number of presentations for the learning to converge. These do not otherwise influence the qualitative results. Finally, the parameter β for LC was set lower to model a slower response of this nucleus compared to the other nuclei in the model. The exact value of the parameter is not critical for the results, excepts that it needs to be well below 1. All simulations were run at a temporal resolution of 50 Hz.
The different stimuli used in the simulations are shown in Figure . They consisted of images of pixel resolution. The brightness of the stimuli ranges from 0% to 100%. We do not attempt to scale these intensities to any real light measurements, but for comparison with empirical results we assume that the scaling is logarithmic.
Figure 2. The stimuli used for the simulations. The top row consists of different symbols, all 50% grey except the second sun symbol which is 75% grey. The happy and sad faces were assigned emotional values of 1, 0.5, −0.5 and −1 respectively. All other stimuli were neutral. The second row consists of grey scale images from 0% to 100% white.

The visual categorisation of the stimuli are not part of the model and can be considered a black box that produces three types of outputs (Figure ): the identity of the stimulus, its novelty and emotional value (through the connections to AMY and LC).
4.1. Simulation 1: pupil light reflex
In simulation 1, we investigated the shape of the pupil light reflex. The model was first dark adapted and then presented with brief light stimuli during 200 ms followed by a 10 s dark interval. The light intensity varied from 10% to 100% light (see Figure ). Although there were no parameter to specifically set the time course of contraction and dilation, the model produces the characteristic shape of the response where initial contraction is approximately three times as fast compared to the following dilation (Figure (A)). This result critically depends on the activation function used (Equation (Equation2(2)
(2) )).
Figure 3. Results of simulation 1. ( A) The light reflex for different light intensities. L: light stimulus. ( B) The response latency for different light intensities. ( C) Reflex magnitude as a function of light intensity.
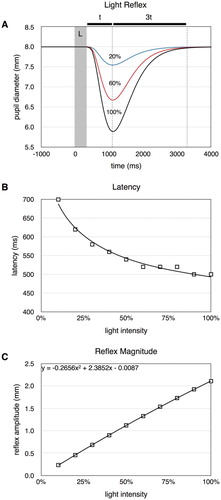
The latency of the response was defined as the interval from stimulus on-set to when the constriction was at least 0.1 mm. This produced the type of power relationship between latency and light intensity seen in empirical data (Figure (B)). We also recorded the peak response amplitude for the different stimuli. The relationship is approximately linear but with a slight negative quadratic effects (Figure (C)), similar to what was found in experimental studies (Ellis, Citation1981).
4.2. Simulation 2: responses to novelty
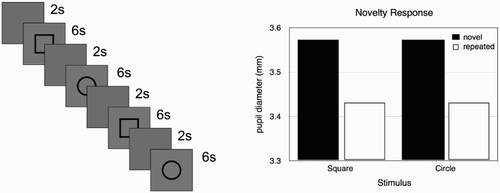
In simulation 2, we looked at how the model responded to novel stimuli compared to already seen stimuli. After adapting the model to a background light intensity of 50%, it was presented with a circle and a square stimulus two times (Figure (A)). We measured the average pupil size after each presentation and, as can be seen in Figure (B), the pupil dilation was larger for the first presentation. This shows that the model accurately predicts that the pupil will dilate to novel stimuli (Aboyoun et al., Citation1998).
4.3. Simulation 3: responses to emotional stimuli
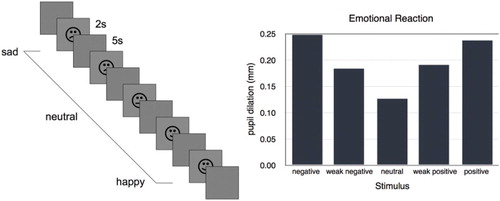
Simulation 3 tested the responses to different emotional stimuli using the sequence of stimuli shown in Figure (A). As expected, the pupil reacted according to the emotionality and novelty of the stimulus (Figure 5(B), Hess & Polt, Citation1960). The stronger positive and negative emotions resulted in similar pupil dilations while the two weaker positive and negative stimuli gave slightly lower responses. Since the novel stimulus was presented for the first time, that too resulted in a small dilation of the pupil (Aboyoun et al., Citation1998).
4.4. Simulation 4: prediction
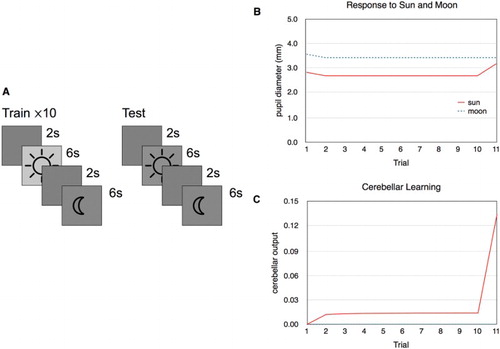
Simulation 4 was a simplified version of the experiment where responses to images of the sun were tested (Binda et al., Citation2013). The model was first trained on repeated presentations of the sun and the moon (Figure (A)). The sun stimulus always had a relatively brighter background. This was intended to represent the experience of the sun usually being bright. The model was subsequently tested on the sun and the moon stimuli, but with equal 50% brightness. The results of the simulation are shown in Figure (B) and (C). There is an initial dilation caused by the two stimuli because of their novelty followed by the responses to the brightness of the sun and the moon. As expected, the pupil diameter decreased as a response to the sun stimulus. In the last trial, there is a clear pupillary reaction to the sun although it is now presented with identical brightness to the moon. This is the result of the cerebellar learning mechanism that has associated the sun stimulus with increased brightness (Figure (C)) and influences EWpg to produce a constriction of the pupil.
Figure 6. ( A) Stimulus sequences used in simulation 4. ( B) Results of simulation 4. The pupil diameter for each stimulus presentation. The first trial results in larger dilation because of the novelty of the two stimuli. In trial 11, the sun stimulus is darker than before, but the pupil still contracts as a result of the expected brightness for this pattern, although not as much as for the brighter sun image presented before. ( C) The output from CB for each trial. When the sun stimulus is darker than expected (trial 11), the response increases to compensate and generates a light response to the sun stimulus even though its brightness is identical to the moon stimulus that does not generate any reaction.
4.5. Simulation 5: fear-inhibited light reflex
In simulation 5 we addressed the inhibition of the light reflex that occurs after aversive conditioning (Bitsios et al., Citation1996). The training and test sequences are shown in Figure (A). We first ran five conditioning trials where a neutral conditioned stimulus (CS, a circle) was followed by an aversive unconditioned stimulus (US, an angry face). We subsequently tested the responses of the model in three conditions. First we looked for the anticipatory fear response (CS Only). Then we tested whether the light reflex would be attenuated following the conditioned stimulus (Fear). Finally, we measured the amplitude of the light reflex on its own (Neutral).
Figure 7. (A) Stimulus sequences used in simulation 5. (B) The pupil diameter over time for CS Only (upper), fear (middle), and neutral (lower) conditions. (C) The light reflex was attenuated in the fear condition compared to the neutral. (D) The initial pupil diameter was higher in the neutral condition compared to the fear condition.
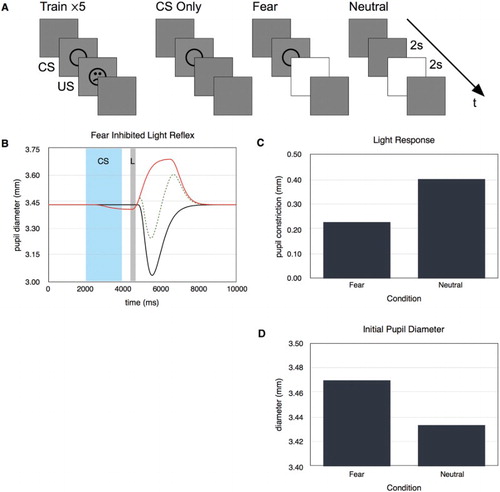
Figure (B) shows the pupil diameter over time for the three test conditions. There is a clear anticipatory respons to the CS only stimulus. The amplitude of the light response in the fear condition is lower than in the neutral condition showing that the model replicates the fear-inhibited light reflex (Figure (B)). In addition, the initial diameter for the fear condition is larger than for the neutral condition. This effect depends on the timing of the light reflex because the initially larger pupil is the result of the anticipatory fear response.
5. Discussion
We have presented a system-level computational model of pupil control. The model reproduces a number of phenomena seen in empirical studies.
In simulation 1, we showed that the model reproduces the relative duration of constriction and dilation as well as the shape of the latency curve (Ellis, Citation1981; Heller et al., Citation1990; Hess & Polt, Citation1960, Citation1964; Woodhouse & Campbell, Citation1975). The latency found in simulation 1 was higher than what is typically seen in experimental work. This is partly the results of the threshold used to decide that the response had started at 0.1 mm. This relatively high threshold was necessary to get reliable measurements because of the temporal resolution of the simulation. Using a finer resolution and a lower threshold would result in a lower latency. However, the critical aspect is the shape of the latency function that would not change by such an operation.
We did not attempt to reproduce the exact shape of the empirical light reflex amplitude, but this could easily be done adjusting the sensitivity on the sensory or motor side to reproduce the desired response function. In fact, the implementation allows for any such function to be included on the sensory side and many models are available (e.g. Korn & Bach, Citation2016; Longtin & Milton, Citation1989; Pamplona et al., Citation2009; Usui & Stark, Citation1982; Watson & Yellott, Citation2012). However, since we are interested in the qualitative properties of the system, we did not include it here.
The most surprising result is the shape of the light reflex that is similar to the empirical shape despite any attempt to fit the parameters. This shape, where the initial constriction is approximately three times as fast as the following dilation, is the result of two factors. The first is the activation function used. The second is the sequence of nuclei from PTA to CG that gradually transform the shape of the reflex. The shape of the light reflex is thus a true system property in this model.
The pupillary responses to novel and emotional stimuli was expected as these signals were generated in the black-box cortex model and sent to the models of AMY and LC. The simulations show that these signals can be handled by the model and are transformed into pupillary reactions of appropriate sizes. It is interesting to note that both positive and negative stimuli are handled in the same way. It is the intensity of the emotion and not its polarity that determines the response.
Conditioning of the pupillary response is a complex area and different experiments appear to contradict each other. In simulation 4 we demonstrated that by including a cerebellar learning component, the model can learn to associate a stimulus with a pupillary response. This ability depends on the loop between EWpg and CB. This is the most speculative part of the model as these connections have not been established in humans. There is however evidence for this loop in other species, such as cat (see Section 2).
It has been suggested that the fear-inhibited light response could be a potential laboratory model of human anxiety (Bitsios et al., Citation1996). Although pupil size is influenced by any stimulus, only a cue associated with punishment decreases the light response. Interestingly, while anticipation of fear reduces the light response, it increases the startle reflex (Bitsios et al., Citation1999). Simulation 5 addressed this phenomenon and combined most of the components of the model. First, the model is conditioned to expect a punishing stimulus after the presentation of a neutral conditioned stimulus. This illustrates the operation of the simplified amygdala model and its interaction with LC as well as its influence on both the sympathetic and parasympathetic parts of the model. The anticipatory fear response is able to attenuate the light response through the shunting inhibition from LC to EWpg. This effect is counteracted by the excitation of the sympathetic pathway that will initially make the pupil dilate. This leads to the initially larger diameter of the pupil.
This general design is ubiquitous throughout the peripheral nervous system, and the proposed model can potentially be used as a starting point for understanding the how the sympathetic and parasympathetic nervous systems maintain an equilibrium that is shifted as a response to various stimuli. An interesting aspect of this shift in equilibrium is that it is not simply the results of the difference between two signals. Instead, the sympathetic system is excited while the parasympathetic system is modulated by a change in LC activation. To this effect, the model suggests that EWpg is modulated through shunting inhibition from the LC. This is consistent with the fact that the LC-EWpg interaction is mediated by a α2-adrenoceptor which is known to have a shunting function.
The proposed model illustrates the power of system-level models of the brain in that it is able to reproduce a number of different results based on the structure of the model. It shows that it is not necessary to include the finer details of the neural processing in each nucleus to model many reactions of the pupil. Instead, the qualitative effects are given directly by the structure of the interconnected components. No parameter tuning was necessary. This can be contrasted with black-box models that tries to characterise the system without regard for the underlying neural structure (Longtin & Milton, Citation1989; Pamplona et al., Citation2009; Semmlow & Chen, Citation1977). In this case, the main aim of the model is usually to optimise parameters to make the model fit as well as possible to data. While the black-box approach has been very successful in analysing the dynamic control properties of the system, it does not explain how each of the involved nuclei contribute to the control. Furthermore, a black-box model is not usually suitable as a component of a larger system-level brain model.
This is, to our knowledge, the first system-level model of the pupil control system in the brain, and as such may offer a template for how to model the peripheral nervous system. In the future, we want to include the model presented here as a part of more complex cognitive models. We also want to extend the model with a more advanced model of LC that includes its tonic and phasic modes of operation (Aston-Jones & Cohen, Citation2005). In addition, we will also extend the model with more detailed models of the different hypothalamic nuclei, specifically in relation to circadian rhythms.
Disclosure statement
No potential conflict of interest was reported by the authors.
ORCID
Birger Johansson http://orcid.org/0000-0002-9834-4279
Christian Balkenius http://orcid.org/0000-0002-1478-6329
Additional information
Funding
References
- Aboyoun D. C., Dabbs J., & James M. (1998). The Hess pupil dilation findings: Sex or novelty? Social Behavior & Personality: An International Journal, 26(4), 415–420. doi: 10.2224/sbp.1998.26.4.415
- Ahern S., & Beatty J. (1979). Pupillary responses during information processing vary with scholastic aptitude test scores. Science, 205(4412), 1289–1292. doi: 10.1126/science.472746
- Aston-Jones G., & Cohen J. D. (2005). An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annual Review of Neuroscience, 28, 403–450. doi: 10.1146/annurev.neuro.28.061604.135709
- Balkenius C., Morén J., Johansson B., & Johnsson M. (2010). Ikaros: Building cognitive models for robots ikaros: Building cognitive models for robots. Advanced Engineering Informatics, 24(1), 40–48. doi: 10.1016/j.aei.2009.08.003
- Beatty J., & Kahneman D. (1966). Pupillary changes in two memory tasks pupillary changes in two memory tasks. Psychonomic Science, 5(10), 371–372. doi: 10.3758/BF03328444
- Beatty, J., & Lucero-Wagoner, B. (2000). The pupillary system. In J. T. Cacioppo, L. G. Tassinary, & G. Berntson (Eds.) Handbook of psychophysiology (pp. 142–162). Cambridge, MA: Cambridge University Press.
- Binda P., Pereverzeva M., & Murray S. O. (2013). Pupil constrictions to photographs of the sun pupil constrictions to photographs of the sun. Journal of Vision, 13(6), 8–8. doi: 10.1167/13.6.8
- Bitsios P., Philpott A., Langley R. W., Bradshaw C. M., & Szabadi E. (1999). Comparison of the effects of diazepam on the fear-potentiated startle reflex and the fear-inhibited light reflex in man. Journal of Psychopharmacology (Oxford), 13(3), 226–234. doi: 10.1177/026988119901300303
- Bitsios P., Szabadi E., & Bradshaw C. M. (1996). The inhibition of the pupillary light reflex by the threat of an electric shock: A potential laboratory model of human anxiety. Journal of Psychopharmacology, 10(4), 279–287. doi: 10.1177/026988119601000404
- Cassady J. M. (1996). Increased firing of neurons in the posterior hypothalamus which precede classically conditioned pupillary dilations. Behavioural Brain Research, 80(1–2), 111–121. doi: 10.1016/0166-4328(96)00026-5
- Cohen D., Chambers W. W., & Sprague J. M. (1958). Experimental study of the efferent projections from the cerebellar nuclei to the brainstem of the cat. The Journal of Comparative Neurology, 109(2), 233–259. doi: 10.1002/cne.901090207
- Ellis C. J. (1981). The pupillary light reflex in normal subjects. British Journal of Ophthalmology, 65(11), 754–759. doi: 10.1136/bjo.65.11.754
- Gerall A. A., Sampson P. B., & Boslov G. L. (1957). Classical conditioning of human pupillary dilation. Journal of Experimental Psychology, 54(6), 467–474. doi: 10.1037/h0041227
- Gerall A. A., & Woodward J. K. (1958). Conditioning of the human pupillary dilation response as a function of the CS-UCS interval. Journal of Experimental Psychology, 55(5), 501–507. doi: 10.1037/h0041138
- Goldwater B. C. (1972). Psychological significance of pupillary movements psychological significance of pupillary movements. Psychological Bulletin, 77(5), 340–355. doi: 10.1037/h0032456
- Harlow H. F., & Stagner R. (1933). Effect of complete striate muscle paralysis upon the learning process. Journal of Experimental Psychology, 16(2), 283–294. doi: 10.1037/h0069776
- Heller P. H., Perry F., Jewett D. L., & Levine J. D. (1990). Autonomic components of the human pupillary light reflex. Investigative Ophthalmology & Visual Science, 31(1), 156–162.
- Henderson R. R., Bradley M. M., & Lang P. J. (2014). Modulation of the initial light reflex during affective picture viewing. Psychophysiol, 51(9), 815–818. doi: 10.1111/psyp.12236
- Hess E. H., & Polt J. M. (1960). Pupil size as related to interest value of visual stimuli. Science, 132(3423), 349–350. doi: 10.1126/science.132.3423.349
- Hess E. H., & Polt J. M. (1964). Pupil size in relation to mental activity during simple problem-solving. Science, 143(3611), 1190–1192. doi: 10.1126/science.143.3611.1190
- Hultborn H., Mori K., & Tsukahara N. (1978). Cerebellar influence on parasympathetic neurones innervating intra-ocular muscles. Brain Research, 159(2), 269–278. doi: 10.1016/0006-8993(78)90534-6
- Ijichi Y., Kiyohara T., Hosoba M., & Tsukahara N. (1977). The cerebellar control of the pupillary light reflex in the cat. Brain Research, 128(1), 69–79. doi: 10.1016/0006-8993(77)90236-0
- Just M. A., & Carpenter P. A. (1993). The intensity dimension of thought: Pupillometric indices of sentence processing. Canadian Journal of Experimental Psychology/Revue canadienne de psychologie expérimentale, 47(2), 310–339. doi: 10.1037/h0078820
- Koikegami H., & Yoshida K. (1953). Pupillary dilatation induced by stimulation of amygdaloid nuclei. Psychiatry and Clinical Neurosciences, 7(2), 109–126. doi: 10.1111/j.1440-1819.1953.tb00600.x
- Korn C. W., & Bach D. R. (2016). A solid frame for the window on cognition: Modeling event-related pupil responses. Journal of Vision, 16(3), 28. doi: 10.1167/16.3.28
- Kozicz T., Bittencourt J. C., May P. J., Reiner A., Gamlin P. D., Palkovits M., & Ryabinin A. E. (2011). The Edinger-Westphal nucleus: A historical, structural, and functional perspective on a dichotomous terminology. Journal of Comparative Neurology, 519(8), 1413–1434. doi: 10.1002/cne.22580
- Kret M. E., Fischer A. H., & Dreu C. K. W. D. (2015). Pupil mimicry correlates with trust in in-group partners with dilating pupils. Psychological Science, 26(9), 1401–1410. doi: 10.1177/0956797615588306
- Kret M. E., Roelofs K., Stekelenburg J., & de Gelder B. (2013). Emotional signals from faces, bodies and scenes influence observers' face expressions, fixations and pupil-size. Frontiers in Human Neuroscience, 7, 810. doi: 10.3389/fnhum.2013.00810
- Laeng B., Sirois S., & Gredebäck G. (2012). Pupillometry a window to the preconscious? Perspectives on Psychological Science, 7(1), 18–27. doi: 10.1177/1745691611427305
- Lennartz R. C., & Weinberger N. M. (1992). Analysis of response systems in Pavlovian conditioning reveals rapidly versus slowly acquired conditioned responses: Support for two factors, implications for behavior and neurobiology. Psychobiology, 20(2), 93–119. doi: 10.3758/BF03327169
- Longtin A., & Milton J. G. (1989). Modelling autonomous oscillations in the human pupil light reflex using non-linear delay-differential equations. Bulletin of Mathematical Biology, 51(5), 605–624. doi: 10.1007/BF02459969
- Pamplona V. F., Oliveira M. M., & Baranoski G. V. G. (2009). Photorealistic models for pupil light reflex and iridal pattern deformation. ACM Transactions on Graphics, 28(4), 1–12. doi: 10.1145/1559755.1559763
- Reiner A., Karten H. J., Gamlin P. D. R., & Erichsen J. T. (1983). Parasympathetic ocular control – functional subdivisions and circuitry of the avian nucleus of Edinger-Westphal. Trends in Neurosciences, 6, 140–145. doi: 10.1016/0166-2236(83)90068-1
- Rescorla R. A., & Wagner A. W. (1972). A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and nonreinforcement. In A. H. Black & W. F. Prokasy (Eds.), Classical conditioning II: Current research and theory (pp. 64–99). New York, NY: Appleton-Century-Crofts.
- Røste G. K., & Dietrichs E. (1988). Cerebellar cortical and nuclear afferents from the Edinger-Westphal nucleus in the cat. Anatomy and Embryology, 178(1), 59–65. doi: 10.1007/BF00305015
- Semmlow J. L., & Chen D. C. (1977). A simulation model of the human pupil light reflex. Mathematical Biosciences, 33(1), 5–24. doi: 10.1016/0025-5564(77)90060-8
- Sirois S., & Brisson J. (2014). Pupillometry. WIREs Cognitive Science, 5(6), 679–692. doi: 10.1002/wcs.1323
- Sugimoto T., Itoh K., & Mizuno N. (1978). Direct projections from the Ediger-Westphal nucleus to the cerebellum and spinal cord in the cat: An HRP study. Neuroscience Letters, 9(1), 17–22. doi: 10.1016/0304-3940(78)90041-1
- Szabadi E., & Szabadi E. (2012). Modulation of physiological reflexes by pain: Role of the locus coeruleus. Frontiers in Integrative Neuroscience, 6, 94. doi: 10.3389/fnint.2012.00094
- Tryon W. W. (1975). Pupillometry: A survey of sources of variation. Psychophysiology, 12(1), 90–93. doi: 10.1111/j.1469-8986.1975.tb03068.x
- Usui S., & Stark L. (1982). A model for nonlinear stochastic behavior of the pupil. Biological Cybernetics, 45(1), 13–21. doi: 10.1007/BF00387209
- Watson A. B., & Yellott J. I. (2012). A unified formula for light-adapted pupil size. Journal of Vision, 12(10), 12–12. doi: 10.1167/12.10.12
- Widrow B., & Hoff M. E. (1960/1988). Adaptive switching circuits. In J. A. Anderson & E. Rosenfeld (Eds.), Neurocomputing: Foundations of research (pp. 123–134). Cambridge, MA: MIT Press.
- Woodhouse J. M., & Campbell F. W. (1975). The role of the pupil light reflex in aiding adaptation to the dark. Vision Research, 15(6), 649–653. doi: 10.1016/0042-6989(75)90279-5
- Wyatt H. J. (1995). The form of the human pupil. Vision Research, 35(14), 2021–2036. doi: 10.1016/0042-6989(94)00268-Q
- Young F. (1965). Classical conditioning of autonomic functions. In W. F. Prokasy (Ed.), Classical conditioning: A symposium (pp. 358–377). New York: Appleton-Century-Crofts.
- Zekveld A., & Kramer S. (2014). Cognitive processing load across a wide range of listening conditions: Insights from pupillometry. Psychophysiology, 51(3), 277–284. doi: 10.1111/psyp.12151
Appendix
The constants for the different nuclei used in the simulations are presented in Table . The learning rate λ was set to 0.05 for AMY and 0.1 for CB. These parameters are not critical for the results as long as the acquisition is completed before testing.