?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Breast cancer (BC) masses and microcalcification are nonlinear with complex dynamics due to which radiologists fail to properly diagnose breast cancer. In this paper, we used a hybrid features extracting approach based on texture, morphological, Scale Invariant Feature Transform (SIFT), Gray Level Co-occurrence Matrix (GLCM), entropy, Elliptic Fourier Descriptors (EFDs), RICA, and sparse filtering methods. Various machine learning techniques have been employed to detect breast cancer, viz. Support Vector Machines (SVM), Decision Trees (DT), k-Nearest Neighbour, and Naïve Bayes classifiers. The RICA-based feature set using SVM RBF has resulted in total accuracy of (94.88%), and ROC AUC = 0.9914. The hybrid features using RICA have been computed with other combinatorial logics. Moreover, the highest performance to detect BC based on the fusion of features was obtained with RICA with Textural features using SVM Gaussian kernel and yielded a total accuracy of (97.55%), and ROC AUC = 0.9976. The hybrid features with RICA were found to yield the highest detection performance. It is revealed that the new feature-extracting approach can be useful for the early detection of breast cancer by physicians to decrease the overall mortality rate. The methods will be very useful for treatment modification to achieve better clinical outcomes.
Abbreviations
| Artificial intelligence | = | (AI) |
| Abnormality detection classifier | = | (ADC) |
| Micro calcification cluster | = | (MCC) |
| Discrete wavelet transform | = | (DWT) |
| Reconstruction Independent Component Analysis | = | (RICA) |
| Computer aided diagnostic | = | (CAD) |
| Bayesian Networks | = | (BNs) |
| Support Vector Machine | = | (SVM) |
| Artificial Neural Networks | = | (ANNs) |
| K-nearest Neighbours | = | (kNN) |
| Scale invariant Fourier transform | = | (SIFT) |
| Elliptic Fourier descriptors | = | (EFDs) |
| Machine-learning | = | (ML) |
| Convolutional neural network | = | (CNN) |
| Cross validation | = | (CV) |
| Receiver operating characteristic | = | (ROC) |
| Positive predictive value | = | (PPV) |
| Negative predictive value | = | (NPV) |
Introduction
Breast cancer (BC) is the leading cause of cancer across the world. Recently, the occurrence rate of breast cancer is 23% among all other types of diagnosed cancer cases which affect 1.6 million women worldwide (Dheeba et al., Citation2014; Forouzanfar et al., Citation2011; Jemal et al., Citation1999). More significantly, patients suffering from breast cancer are diagnosed one out of three (Desantis et al., Citation2016). In 2016, deaths due to breast cancer were 29% in the United States. In the US, women have a 12% chance of developing breast cancer in their lifetime at any stage. There is a 2.6% chance of a woman dying due to breast cancer, which accounts for one in thirty-eight women. BC in Asian and African countries has a higher incidence rate than in European and North American countries. The BC in Pakistan is very aggressive and has reflected a higher rate than the neighbouring countries (B. & Shankar, Citation2017). This risk of BC spread in the families due to heritable characters. The large volume of mammograms and potentially missed diagnoses are major burdens to radiologists. The ability to detect cancers reliably and automatically on screening mammograms has the potential to significantly improve radiologist workflow and diagnosis accuracy.
Using artificial intelligence (AI) methods, researchers are trying to optimise and develop some novel algorithms for mammogram interpretation to enhance breast cancer detection by reducing false positive rates (Houssami et al., Citation2017; Trister et al., Citation2017). These applications of AI in the diagnosis of BC extend to interpreting the pathology and imaging modalities. For example, AI is used to identify metastatic breast cancer in whole images of sentinel lymph node biopsies (Litjens et al., Citation2016). Traditional computer-aided diagnostics (CAD) improves significantly cost-effective and screening performance mostly because of the low specificity (Lehman et al., Citation2015) which precludes their use as a standalone for reading mammograms. In recent years, researchers developed methods based on deep learning methods, and few of them showed attractive results upon comparing with earlier techniques used by radiologists, but in homogenous and very limited scenarios (Becker et al., Citation2017; Kooi et al., Citation2017).
In the past few years, CAD tools were being employed for image analysis. There are several approaches that have been used in combination with physical examination, imaging and biopsy (Ardakani et al., Citation2015). Ultrasound and mammography are among the imaging techniques for detecting breast cancer for which the X-rays are used to produce an image of the breast. The trained radiologists read the mammograms to detect breast cancer. An effective screening process depends on the explanation of the radiologist (Sprague et al., Citation2016). The CAD helps the radiologist identify suspicious regions. Cancer detection can be further improved by combining mammography with CAD (Metz, Citation1986). In younger women, cancerous masses and micro-calcifications are hidden in dense breast tissue which makes both detection and diagnosis more intricate and complex (Motakis et al., Citation2009).
The treatment response of BC can be improved by early detection and diagnosis. A combination of methods can be utilised including physical examination, imaging and biopsy to detect breast cancer (Ardakani et al., Citation2015). The imaging methods include ultrasound and mammography to detect breast cancer. X-rays are used to create an image from the breast known as mammograms. Expert radiologists can predict breast cancer. The process of screening is based on the radiologists’ expert opinion (Sprague et al., Citation2016). To confirm breast cancer, the biopsy is utilised which has physical and psychological impact on the patient and is an invasive surgical operation. To avoid this, the researchers are developing CAD systems (Acharya et al., Citation2017; Dheeba et al., Citation2014) by identifying ultrasound and clinical features (Zhang et al., Citation2015), hybrid features paradigm using data mining classification techniques (Acharya et al., Citation2017), medical imagining and CAD (Sathish, Kamath, Rajagopal, & Prasad, Citation2016), and breast magnetic resonance imaging (Machida et al., Citation2017) providing stable detection rates.
The research reveals that radiologists miss up to 30% of BC which depends on the breast density (Kolb et al., Citation2002). The micro-calcifications and masses are the two main indicators to evaluate the BC. The detection based on masses is more challenging not only due to the shape, size and large variations but also due to poor contrast (Cheng et al., Citation2006). The experience, training and subject experts help the concerned radiologist to read mammograms properly. There is a variation in the judgment of about 65-75% even by the trained experts (Skaane & Engedal, Citation1998). Thus, CAD can be more reliable to improve early breast cancer detection systems. The research also shows that about 65-90% of biopsies of the suspected cancer turned to benign. The accuracy can be improved with the combination of methods including CAD, machine learning and expert knowledge. The detection performance without CAD was obtained below 80% and with CAD, it was above 90% (Doi et al., Citation1996). CAD can automatically identify the area of abnormal contrast, calling the radiologist towards suspicious regions, thus mammograms with computer-aided diagnosis (CAD) will improve the detection of cancer (Dheeba et al., Citation2014). The cancer masses and micro-calcifications in many cases are hidden in the intense breast tissues, especially in younger women, that will become complex to detect and diagnose cancer (Dheeba et al., Citation2014). Features extraction is an important step to detect any pathologies from physiological and neurophysiological systems. Researchers in the past utilised various feature extraction methods such as to detect colon cancer, and researchers (S. Rathore et al., Citation2012, Citation2014) extracted the hybrid and geometric features.
Recently, (Dhahri et al., Citation2019) applied genetic algorithms to improve the classification performance of breast cancer mammograms. Moreover, (Gupta et al., Citation2022) employed machine learning methods to improve the detection of Alzheimer’s disease. More recently, (Tian & Zhang, Citation2022) applied the boosted decision tree to improve the diagnostic capability of breast cancer. Likewise, (Nassif et al., Citation2022) applied artificial intelligence (AI) methods to improve the prediction of breast cancer. CoroNet (Mobark et al., Citation2022) was a CNN-based approach that relied on the Xception architecture, which was pre-trained on the ImageNet dataset with fine-tuning using mammograms with alteration in the final layers being modified for the number of classes in the target dataset. Zhang et al. (Zhang et al., Citation2019) introduced Anomaly Separation Network (ASN) to generate candidate microcalcification (µCs) carrying two major components: an encoder-decoder network to learn the image reconstruction mapping and a t-test loss function to separate the distributions of the reconstruction residuals of µCs from normal tissues. Similarly, Chakravarthy and Rajaguru (S R & Rajaguru, Citation2021) introduced a method with deep learning and Extreme Learning Machine (ELM) for feature extraction and classification of breast cancer. They used Sine-Cosine Crow-Search Optimisation Algorithm (SC-CSOA) for improving the ELM’s classification performance. Falconí et al. (Falconi et al., Citation2019) used a transfer learning-based approach for the malignancy classification of breast abnormality. They used NasNet and MobileNet models and compared the performance with InceptionV3 and Resnet50. Similarly, Khamparia et al. (Khamparia et al., Citation2021) modified VGG (MVGG) model and experimented with 2D and 3D images of mammograms. The proposed hybrid transfer learning model (a fusion of MVGG and ImageNet) provided outclass results. Li et al. (Li et al., Citation2020) used DCNN for the mass classification task of predicting malignant mass, benign mass, and normal tissue using DBT and FFDM. They modelled the DCNNs as a three-class classification problem by including the normal tissue as a third class. Recently, Malebary and Hashmi (Malebary & Hashmi, Citation2021) introduced a Breast Mass Classification (BMC) system based on a hybrid of k-means clustering, long short-term memory network, and random forest, to categorise the breast mass as benign, malignant, and normal. Similarly, Salma and Aly (Salama & Aly, Citation2021) used a pre-trained modified U-Net model, and different deep learning models viz. InceptionV3, DenseNet121, ResNet50, VGG16 and Mobile-NetV2 for segmentation and classification of breast mass respectively.
The machine learning classification task merely dependent on the choice of hand-crafted features extraction approach, which is still a challenging task in predicting the imaging pathologies (Hussain et al., Citation2020; Hussain, Saeed, et al., Citation2019; Saroja & Selwin Mich Priyadharson, Citation2018). The breast calcifications dynamics are highly nonlinear which requires more robust features to capture these dynamics. In the past, researchers employed limited feature extraction methods using texture and morphological feature sets (Hussain, Aziz, et al., Citation2018). There are several limitations to choosing fewer features as they may not capture most of the prominent hidden information, which can be helpful to improve the detection accuracy of breast cancer. Thus, we considered multiple aspects of breast masses and microcalcification properties to capture the textural, spatial, geometric, scaling, rotation, complex and non-stationarity properties along with the sparsity, and orthogonality information. This reduced computational cost to deal with big images by estimating whitening etc. to achieve state-of-the-art performance. We extracted textural, morphological, entropy-based and wavelet-based features, EFDs, scale SIFT, Reconstruction Independent Component Analysis (RICA) and Sparse filter features. The RICA features (Hussain, Almaraashi, et al., Citation2021; Lei et al., Citation2016; Zhu et al., Citation2018) among all feature categories yielded higher detection performance, thus, we proposed RICA-based hybrid feature approach by considering most of the important feature to unfold the hidden dynamics present in the breast cancer.
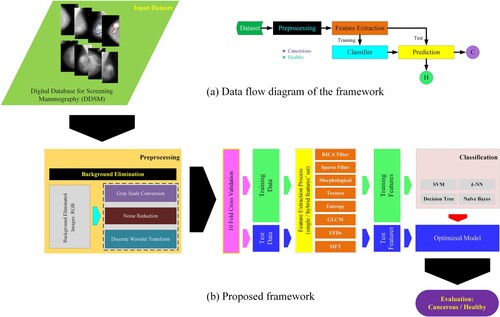
The current study is divided into different steps as reflected in the schematic diagram in Figure . We first took breast mammograms from Digital Database for Screening Mammography (DDMS) database (Zhang, Tomuro, Furst, & Stan Raicu, Citation2009). In the second step, we applied some pre-processing techniques such as RGB to grey-level conversion. Third, we extracted the hybrid features, which is the most important step in our study. Researchers are trying to compute the most relevant features from mammograms to improve the detection performance. However, the detection performance is merely based on the most relevant features extracted from the imaging database. The main objective of this study is to increase the breast cancer prediction performance by extracting robust multimodal features by considering different breast mammography properties such as spatiotemporal, shape-based, spectral, nonlinearity, elliptic shape-based and reconstruction ICA and sparsity base features from breast mammograms. The conventional feature extraction methods may be limited to extracting all the desired dynamics from breast cancer mammograms. To cope with these limitations, we proposed robust RICA-based hybrid features along with textural, morphological, sparse filter and GLCM which improved the detection accuracy. Fourth, after extracting the hybrid features, we applied the most robust ML techniques viz. support vector machine with polynomial, Gaussian and RBF kernels, k-nearest neighbour (KNN) and decision tree on single and combination of features. Fifth, the testing and training data validation were performed with standard 10-fold cross-validation. Finally, the following parameters were used for measuring the performance in terms of standard performance evaluation measures. The results for each feature are presented in tabulated form, while the features which outperformed, based on a single and combination of features are presented in graphical form. The results for both single and combination of features are presented using ROC curves. We also compared the results with existing similar studies and their methods, feature extraction approaches and performances obtained. The step-by-step procedure is reflected in Figure .
Materials and methods
Dataset
The public dataset provided by the University of South Florida (Heath et al., Citation1998) and used in (Zhang et al., Citation2010) is available at (http://www.eng.usf.edu/cvprg/Mammography/Database.html). The DDMS database contained mammographic images for research purposes. The suspicious regions of interest in the DDMS database were marked by qualified radiologists, and BI-RADS information was also marked for each abnormal region. These studies are divided into categories s e.g. cancer and benign. In this study, a total of 899 mammograms were randomly selected, out of which 500 are normal and 399 are diseased cases.
Feature extraction
This is an important step in machine learning. Feature plays a vital role in image processing. After applying image processing techniques to the captured image, different feature-extracting techniques are applied to obtain the features used in classification. The behaviour of an image can be defined by its features. The feature extraction is a type of dimensionality reduction in image processing. Extracting the most relevant and required information from the data is one of the main objectives of feature extraction (Kumar, Citation2014).
In previous studies, numerous researchers have extracted many features for detecting various imaging pathologies by considering texture, shape-based morphologies, image scaling and rotation changes and complex dynamics using SIFT, morphological, textural, EFDs and some other most relevant features regarding the nature of the problem of interest (Hussain, Ahmed, et al., Citation2018; Hussain, Rathore, et al., Citation2019; N. Rathore et al., 2014).
In this study, we extracted a total of 265 different features such as morphological (14), texture (13), EFDs (04), entropy with complexity, GLCM (22), SIFT (05), wavelet-based (07), Reconstruction Independent Component Analysis (RICA) (100), and Sparse filter (100) from breast cancer images. These features are considered to extract the most valuable and hidden information present in the microcalcifications and masses of breast cancer images to distinguish between malignant and benign cases. The feature extracted developed and employed in our previous studies are detailed in (L. Hussain et al., 2020; Hussain, Citation2018; Hussain, Ahmed, et al., Citation2018; Hussain, Aziz, et al., Citation2019, Citation2021; Hussain, Saeed, et al., Citation2019).
RICA features
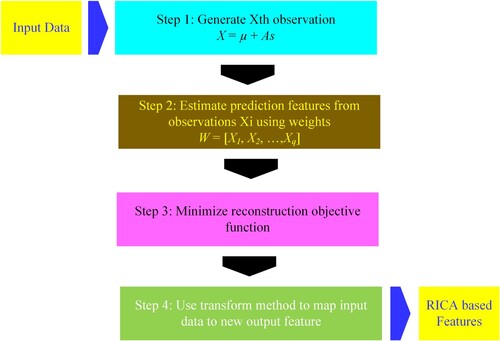
RICA is an unsupervised machine learning algorithm and overcomes the drawbacks raised in ICA. Moreover, the performance achieved using RICA is also better than ICA.
Consider an input of unlabelled data , that denotes optimisation problems of standard ICA to estimate the independent components. Their analysis mathematically can be defined by the following equation (Hyvärinen & Oja, Citation2000; Xiao et al., Citation2015):
(1)
(1)
Where h(.) represents the nonlinear penalty function, is a matrix, the number of rows is denoted by L and I denote the identity matrix. Moreover, to avoid the degenerating of the vectors in X,
is used. Where
denotes the column vector representing a constant length. A following smooth penalty function is used for this purpose (Hyvärinen, Hurri, & Hoyer, Citation2009).
(2)
(2)
The standard ICA has some ortho-normality problems that revoke it from learning completely. Thus, for high dimensional problems, ICA fails to scale. Thus, RICA with soft reconstruction cost is employed to prevent the major drawbacks of ICA. Thus, RICA with the above-mentioned change is then represented by the following mathematical formulation:
(3)
(3)
Here, the parameter λ > 0 shows a tradeoff between reconstruction error sparsity. After swapping the reconstruction penalty with ortho-normality constraints, RICA can even learn on unwhitened data for sparse representation and when X is over-complete. The penalty h produces the sparse representation only and not the invariant itself (Hyvärinen, Hurri, & Hoyer, Citation2009). Thus, RICA swapped it with an additional L2 pooling penalty RICA (Le et al., n.d., 2012) which promotes several pooling features to cluster features together. The sparsity in feature learning is also encouraged using L2 pooling. The L2 pooling is a two-layered network (Boureau, Ponce, & LeCun, Citation2010; LeCun, Citation2012) having square nonlinearity in the first layer
and square root nonlinearity in the second layer
as given by:
(4)
(4)
where
. denotes a row of spatial pooling matrix, H
set to constant weights i.e. 1 for each element in matrix H,
denotes element-wise multiplication and ϵ > 0 is a small constant.
Figure shows step by step procedure for RICA as detailed by: (https://www.mathworks.com/help/stats/feature-extraction.html#bvmxyf6-1).
The RICA algorithm provides major benefi to improve the prediction performance based on:
It reduced the computational cost of optimisation problems by removing the need of using a constrained optimiser. It uses the CG or L-BFGS for faster convergence
RICA is also capable of extraction of overcomplete features
RICA is also less sensitive to whitening and has a low computational cost when dealing with a larger number.
RICA can also handle data with approximate whitening or without whitening
Classification
The process of categorising where objects and ideas are eminent, predicTable and understood. The classifiers are first trained on training examples and then are tested on unseen examples. We applied and optimised the robust machine learning algorithms including Naïve Baye (NB), decision tree (DT), SVM and its kernels. The 10-fold cross validation was utilised for data validation. The performance was measured in terms of different performance evaluation metrics. The details of algorithms, validation methods and performance metrics are detailed in (L. Hussain et al., 2020; Hussain, Citation2018; Hussain, Ahmed, et al., Citation2018; Hussain, Aziz, et al., Citation2019, Citation2021; Hussain, Saeed, et al., Citation2019).
Results
Different classifiers are applied and optimised to detect breast cancer. The performance of these classifiers is evaluated by extracting different features as reflected in Table . The performance of the diagnostic system was obtained using Specificity (Spec.), Sensitivity (Sens.), AUR, FPR, TA, NPV, and PPV, as shown in Table and Figures below:
Table 1. Breast Cancer detection performance utilising single feature with 10-fold CV by employing different machine learning techniques.
Table is based on single feature extraction method, by employing 10-fold cross-validation, SVM polynomial yielded highest performance based on texture features i.e. Sensitivity (92.23%), Specificity (94.60%), PPV (93.16%), NPV (93.85%), TA (93.55%) & ROC AUC (0.9803). Moreover, based on textural features, performance for other classifiers such as SVM Gaussian give TA (92.55%), followed by Decision Tree with TA (87.65%) and Naïve Bayes with TA (84.65%). Likewise, morphological Features yielded the highest performance using the SVM polynomial with TA (88.88%) & ROC AUC (0.9548) followed by SVM Gaussian with TA (87.65%), SVM RBF with TA (87.54%), DT with TA (80.65%) and Bayes with TA (78.98%).
Moreover, SIFT features provided highest performance based on SVM RBF with TA (74.39%), ROC AUC (0.7990) followed by SVM Gaussian with TA (73.68%), ROC AUC (0.7948); DT with TA (74.04%), ROC AUC (0.8039); SVM polynomial with TA (0.6749), ROC AUC (0.5080) and Naïve Bayes with TA (57.54%), ROC AUC (0.5088). Similarly, SVM polynomial produced highest detection based on EFDs features with TA (77.42%), ROC AUC (0.5045) followed by SVM RBF with TA (73.75%), ROC AUC (0.7945); SVM Gaussian with TA (72.75%), ROC AUC (0.7940); Naïve Bayes with TA (55.84%), ROC AUC (0.5045) and DT with TA (47.16%), ROC AUC (05175). Likewise, entropy features delivered the highest performance based on DT with TA (85.65%), ROC AUC (0.9173) followed by SVM Gaussian with TA (85.21%), ROC AUC (0.8857); SVM RBF with TA (84.87%), ROC AUC (0.8779); SVM polynomial with TA (82.42%), ROC AUC (0.5070) and Naïve Bayes with TA (56.06%), ROC AUC (0.5070). The detailed performance evaluation based on other different features and Machine Learning classifiers is shown in Table .
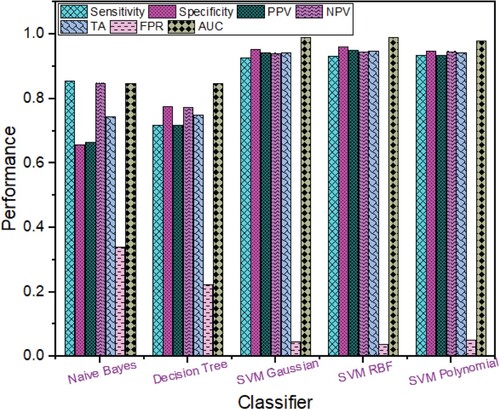
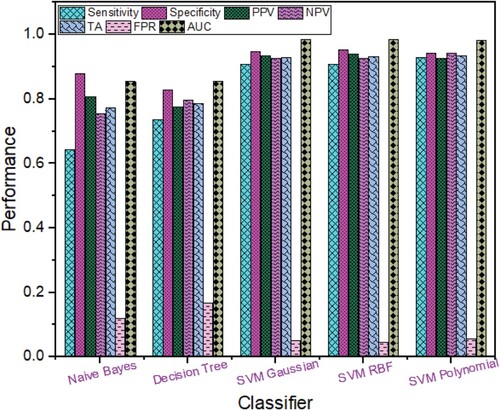
Based on the RICA features extracted from Breast cancer images as shown in Figure , the highest performance was obtained using SVM RBF with TA (94.88%), sensitivity (93.23%), specificity (96.20%), NPV (94.69%), PPV (95.14%), FPR (0.038), ROC AUC (0.9914) followed by SVM Polynomial with TA (94.22%), specificity (94.80%), sensitivity (93.48%), PPV (93.48%), NPV (94.80%), FPR (0.052), ROC AUC (0.9808); SVM Gaussian with TA (94.22%), sensitivity (92.73%), specificity (95.40%), PPV (94.15%), NPV (94.27%), FPR (0.046), ROC AUC (0.9913); Naïve Bayes with TA (74.53%), sensitivity (85.46%), specificity (65.80%), NPV (85.01%), PPV (66.60%), FPR (0.342), ROC AUC (0.8479) and Decision Tree with TA (74.97%), sensitivity (71.68%), specificity (77.60%), PPV (71.86%), NPV (77.45%), FPR (0.224), ROC AUC (0.8479).
Figure 3. Breast Cancer Detection Performance based on Rica Features using different Machine Learning techniques.
Using hybrid Rica fused with texture features, the detection performance is depicted in Figure , the highest SVM Gaussian yielded the highest performance with TA (97.55%), sensitivity (96.49%), specificity (98.40%), PPV (97.96%), NPV (97.23%), FPR (0.016), ROC AUC (0.9976) followed by SVM RBF with TA (97.33%) and ROC AUC (0.9979); SVM Polynomial with TA (97.33%) and ROC AUC (0.9901); Naïve Bayes with TA (89.43%), and ROC AUC (0.9540) and Decision tree with TA (87.65%), and ROC AUC (0.9540).
Figure 4. Breast Cancer Detection Performance based on Rica fused with Texture Features using different Machine Learning techniques.
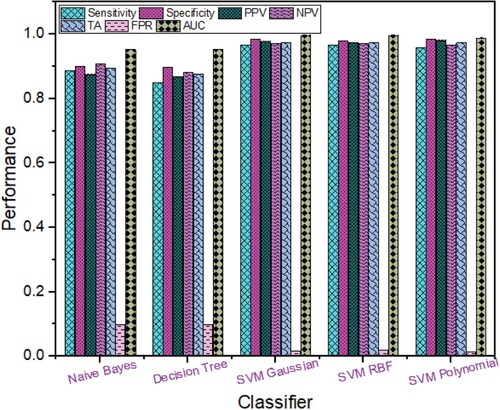
The hybrid Rica hybridised with morphological features performance is reflected in Figure . The highest detection performance was obtained using SVM polynomial with TA (96.22%), sensitivity (95.49%), specificity (96.80%), PPV (95.97%), NPV (96.41%), FPR (0.032), ROC AUC (0.9878) followed by SVM Gaussian with TA (95.88%), ROC AUC (0.9950); SVM RBF with TA (95.66%), ROC AUC (0.9948); Decision Tree with TA (82.76%), ROC AUC (0.8927) and Naïve Bayes with TA (79.87%), ROC AUC (0.8927).
Figure 5. Breast Cancer Detection Performance based on Rica hybridised with Morphological Features using different Machine Learning techniques.
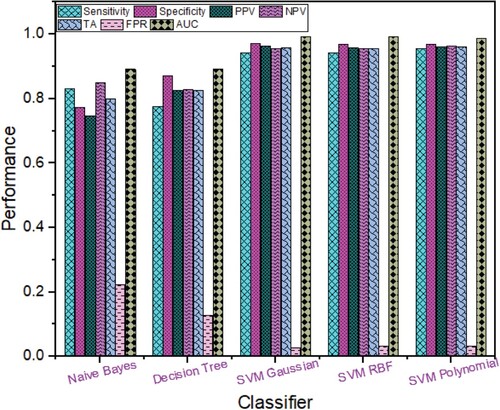
The Breast cancer detection performance using combination of Rica combined with sparse filters is shown in Figure , SVM polynomial provided highest performance with TA (93.66%), sensitivity (92.98%), specificity (94.20%), PPV (92.75%), NPV (94.39%), FPR (0.058), ROC AUC (0.9817) followed by SVM RBF with TA (93.33%) and ROC AUC (0.9863); SVM Gaussian with TA (92.99%) and ROC AUC (0.9858); Decision Tree with TA (78.87%), and ROC AUC (0.8559) and Naïve Bayes with TA (77.42%), sensitivity (64.41%), specificity (87.80%), PPV (80.82%), NPV (75.56%), FPR (0.122), ROC AUC (0.8559).
Figure 6. Breast Cancer Detection Performance based on Rica combined with Sparse Features using different Machine Learning techniques.
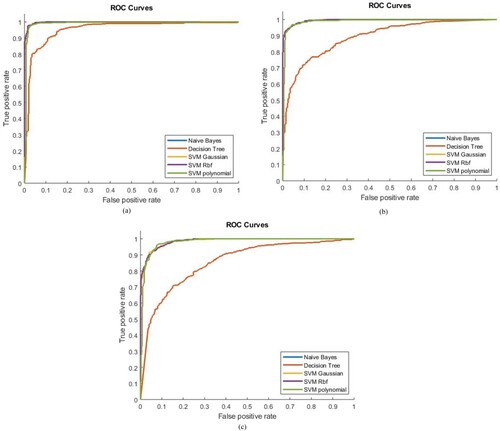
The ROC AUC values with different Machine Learning classifiers by using a single feature are obtained as shown in Figure . The highest separation as shown in the Table is (ROC AUC = 98%) obtained by using SVM RBF, SVM Gaussian and SVM Polynomial classifier with textural features. Decision Tree & Naïve Bayes depicted the highest (ROC AUC = 92.57%) separation with texture features. SVM Gaussian and SVM Polynomial with Morphological features showed the highest separation (ROC AUC = 95%). However, SVM RBF with Morphological features showed the highest separation (ROC AUC = 94%), whereas Decision Tree and Naïve Bayes depicted the highest (ROC AUC = 88.64%) separation with Morphological features. SVM Gaussian and SVM RBF with RICA features showed the highest separation (ROC AUC = 99%). However, SVM Polynomial with RICA features shown highest separation (ROC AUC = 98%), Decision Tree and Naïve Bayes depicted the highest (ROC AUC = 84.79%) separation with RICA features. In Sparse features, SVM Polynomial showed the highest separation (ROC AUC = 91%), SVM Gaussian, SVM RBF, Naïve Bayes and Decision Tree showed separation (ROC AUC = 90%, 89.25%, 82.15%, 82.15%) respectively for Sparse features. In GLCM features, SVM Polynomial showed the highest separation (ROC AUC = 89%), SVM Gaussian, SVM RBF, Naïve Bayes and Decision Tree shown separation (ROC AUC = 84%, 83.6%, 82.15%, 82.81%) respectively for GLCM features.
Figure 7. ROC Analysis based on single feature set using (a) RICA, (b) Sparse, (c) GLCM, (d) Texture, and (e) Morphological.
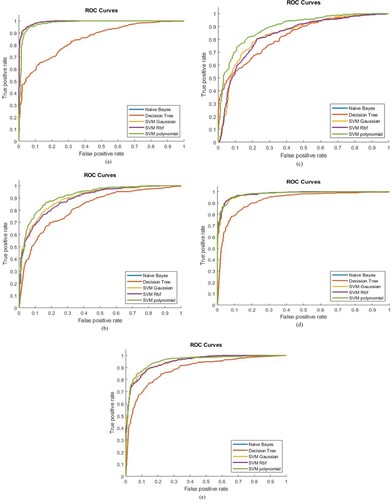
Figure represents the ROC AUC values of hybrid features. The highest separation value of ROC AUC was obtained for the combination of Texture + RICA features by using SVM Gaussian and SVM RBF (ROC AUC = 99.7%) and similarly by using SVM Polynomial (ROC AUC = 99%). However, Decision Tree and Naïve Bayes shown the highest separation (ROC AUC = 95.4%) in the combination of Texture + RICA features. Similarly, in the combination of Morphological + RICA features the maximum separation obtained (ROC AUC = 99.5%, 99.48%) by using SVM RBF and SVM Gaussian, respectively. SVM Polynomial, Naïve Bayes and Decision Tree shown highest separation (ROC AUC = 98.78%, 89.27%, 89.27%) respectively in the combination of Morphological + RICA features. In the combination of Sparse + RICA features the maximum separation shown (ROC AUC = 98.63%, 99.58%) by using SVM RBF and SVM Gaussian, respectively. SVM Polynomial, Naïve Bayes and Decision Trees have shown maximum separation (ROC AUC = 98.17%, 85.59%, 85.59%) respectively in the combination of Sparse + RICA features.
Discussions
Previously researchers utilised few features extraction and ML methods. Park et al. (Park et al., Citation2007) used image processing techniques and algorithms for breast cancer detection and in a few instances, an explanation of its stage so that proper treatment could be given to cancer-affected persons. Digital mammography techniques were also used worldwide for early-stage breast cancer detection but have some negative effects on the human body. Other image techniques, such as Infrared and MRI, were also proposed. A performance comparison of imaging techniques was done and as a result, mammography was included as an accurate imaging technique. In the UK, Ijeacus’s study covered the entropy method effects for the classification of breast cancer subjects present in mammograms based on the concept of Shannon entropy. The proposed system can gain significant results as compared to other variant models (Salem & Bin, Citation2017). Ireaneus et al. proposed a method in which the first step depends on gray-level information of image enhancement and segments of the breast tumors. The morphological features are obtained to categorise the breast tumor and for classification, an SVM classifier is ultimately used. By using this methodology, they achieved a sensitivity of 88.75% (Rejani & Selvi, Citation2009). The precision rate of the proposed system is good for breast cancer (Telagarapu & Poonguzhali, Citation2014). Shewta Kharya et al. This system is used in making expert decisions with a high precision rate. The system was implemented in the countryside and rural areas to act as human diagnostic expertise considering the cure of breast cancer. The system is reliable and user-friendly with an accuracy rate of 93% (Kharya, Citation2014). Venkatesan et al interpreted breast cancer data by applying four algorithms of classification which are j48, Regression Tree as well as Alternating Decision Tree, and Best First Tree. Among all four classifiers, the j48 classifier has an accuracy of 99% (Venkatesan, Citation2015). Sequential minimal optimisation has been the superior classifier in terms of low rate and accuracy (Rathore, Divya, et al., Citation2014). Table reflects the comparison of performance results obtained by different researchers with existing studies.
Table 2. Comparison of results for breast cancer detection with the existing studies.
Table shows the breast cancer detection results with different existing methods utilised. In this study, we computed the classification performance based on diverse single features such as texture, morphological, GLCM, SIFT, EFDs, Sparse and RICA features. We also computed the results based on hybrid features. We then fed these features to robust machine learning algorithms by optimising the hyperparameters using grid search methods and computed the performance based on sensitivity, specificity, accuracy, and AUC.
Machine learning (ML) is a branch of AI that adopts various optimisation, probabilistic and statistical tools to learn from past examples and then apply that prior training to classify new data, and new patterns and trends are then identified and predicted accordingly (Oakden-Rayner et al., Citation2017; Parmar et al., Citation2017). Recognising images is one of the core technologies of ML (Fehr et al., Citation2015). Traditional image recognition methods such as Artificial Neural Networks (ANNs), Support Vector Machines (SVMs), Bayesian Networks (BNs), k-nearest Neighbours (kNN), Decision Trees (DTs) and Adaboost (Fehr et al., Citation2015; Orrù et al., Citation2012) used hand-crafted features such as texture, SIFT, entropy, morphological, EFDs, shape, geometric, density of pixels and off-shelf classifiers. These methods are considered non-deep learning methods. These methods have most widely been used in many applications such as cancer detection, psychiatric diseases, neurodegenerative diseases, and many others (Cruz & Wishart, Citation2006; Doyle et al., Citation2007; Oakden-Rayner et al., Citation2017; Orrù et al., Citation2012; Parmar et al., Citation2017). The main limitations of these non-deep learning methods are that these are dependent on the feature extraction step and it is difficult to find the most relevant and suitable feature for specific image recognition problems. Satisfactory results may not be acquired if these off-shelf features are not effective enough.
In this study, we extracted multimodal features such as texture, GLCM, morphological, sparse, RICA, SIFT, EFDs and entropy to predict breast cancer. We utilised the single and hybrid feature extracting approaches. Based on the single feature extracting strategy, the texture features using SVM polynomial yielded the highest performance with accuracy (99.55%) and AUC (0.9803); morphological features using SVM polynomial with accuracy (88.88%), AUC (0.9548); Sparse filter using SVM polynomial with accuracy (84.65%), AUC (0.9176); GLCM with accuracy (81.20%), AUC (0.8948); SIFT using SVM RBF with accuracy (74.39%), AUC (0.7990); EFDs using SVM polynomial with accuracy (77.42%), entropy using decision tree with accuracy (85.65%), AUC (0.9173) and RICA features using SVM RBF with accuracy (94.88%), AUC (0.9914). By utilising the hybrid features, the highest detection accuracy was yielded using RICA + texture using SVM Gaussian with accuracy (97.55%), AUC (0.9976); RICA + morphological using SVM polynomial with accuracy (96.22%), AUC (0.9878); RICA + sparse using SVM polynomial with accuracy (93.66%), AUC (0.9817).
The results reveal that single RICA features yielded the highest detection performance followed by texture, morphological, entropy features and so on. The combined RICA + texture using SVM Gaussian yielded the highest detection performance. The RICA features provide major benefits to improve detection performance because they reduced the computational cost of optimisation problems by removing the need of using a constrained optimiser. It uses the CG or L-BFGS for faster convergence. Moreover, RICA is also capable of extraction of overcomplete features. Furthermore, RICA is also less sensitive to whitening and has a low computational cost when dealing with a larger number and can also handle data with approximate whitening or without whitening. The SVM using kernel tricks can be used to solve nonlinear problems. The SVM with RBF and Gaussian kernels is capable to solve the more complex and nonlinear problems of radial and Gaussian distributions. The hyperparameters C and gamma have wisely been utilised to provide improved performance.
During this work and study, robust machine learning (ML) classification methods, such as Decision Tree, SVM kernels, and Bayesian approach were employed to classify the malignant and benign cases of breast cancer. Different high-resolution images show higher complexity and nonlinear dynamics which require a multi-dimensional feature extraction strategy to detect breast cancer from an image because numerous variations in shape and size require a multi-dimensional feature extraction strategy to differentiate cancer and normal subjects, effectively. Therefore, different feature-extraction strategies like scale-invariant feature transform (SIFT), elliptical Fourier descriptors (EFDs), Morphology and Texture are used to tackle this problem. To distinguish cancerous from non-cancerous subjects, novel ML classification methods like SVM kernels, Decision Trees, and Bayesian methodology are formed in MATLAB version 2017. After applying the ML classification method, cross-validation (Jack-knife 10-fold) was used to train and test the MR image database. Some measures (specificity, sensitivity, NPV, PPV, FPR and AUC) are used to evaluate the performance. During this work, both single and different combination (hybrid) of feature extraction strategies is used for the evaluation of performance. The higher classification accuracy based on a single feature i.e. texture, morphological, and RICA features were obtained using SVM kernels, but RICA delivered the most promising results compared to other single features. Similarly, in hybrid feature extracting strategies, we used a different combination of features such as RICA + Texture, RICA + Sparse and RICA + Morphological. Texture + RICA gives better results than single features extracting strategies followed by RICA + Morphological and RICA + Sparse using SVM kernels, Decision Tree and Bayesian approach. In the past, few researchers have employed single features-based strategies and some hybrid features to classify cancer and normal subjects. In this study, the reported results revealed that the current features extracting strategy (i.e. RICA with the combination of Texture feature and Morphological feature) has delivered more promising results that are more effective and can play a vital role in detecting breast cancer early and diagnose of breast cancer in detecting sensitivity and specificity to gain higher detection ratio in breast cancer.
In supervised machine learning techniques, computation and extraction of the most relevant features are the most crucial step in relevant imaging problems for improving detection. Breast cancer mammograms contain masses and microcalcifications which contain nonlinear, nonstationary, complex patterns and spatiotemporal dynamical hidden information. For proper investigation and to extricate the hidden information for further diagnosis, we proposed a multimodal feature extracting strategy along with hybrid (combination of features) features from Morphological, Texture, SIFT, Entropy, EFDs, GLCM, RICA and sparse filtering to handle the problem of detection of cancer from an image as a result of the sizable difference in size and shape and these hidden characteristics. We then applied the most robust ML techniques like a k-nearest neighbour, decision tree and SVM with its kernel. We used the Jackknife 10-fold cross-validation approach for testing and training data validation and to avoid underfitting / overfitting the data. The proposed approach outperformed the publicly available dataset. However, there exist some limitations as the dataset contains mammograms and lacks some clinical information. In the future, we will employ these techniques to multi-slice images and longitudinal datasets along with other factors such as severity level, recurrence and progression, and pathological control response.
Conclusion
The dynamics of masses and breast cancer calcification are highly nonlinear and complex dynamics that require more robust features to capture these dynamics. We extracted texture, morphology, entropy based, SIFT and EFDs features along with RICA features for training and validating the ML classifiers. The 10-fold cross-validation was used to train and test the image database. The performance was measured using different performance metrics-based features using both single and combination of features yielded the highest breast cancer detection performance compared to other feature extraction approaches. Based on the results, using RICA can be helpful for the early detection and prediction of breast cancer. This approach was found to be useful to decrease the mortality rate and increase the survival rate, and can be utilised for improved decision-making of the healthcare systems.
Limitations of the work and future directions
The present study was focused to apply machine learning methods with diverse hand-crafted features based approaches. Though researchers are still working on multiple aspects of feature-extracting strategies to improve the classification performance using deep learning algorithms. In this context, a recent light deep learning based architecture has been introduced by Qureshi et al. (Citation2022a) using minimum number of layers for optimized MRI scans with empirically controlled unknown parameters generating dynamic features. The fusion of these features with static features resulted in tumor detection for intra-operative neurosurgery. Similarly, the attention mechanisms are being constantly used to focus the important regions in the image by enhancing the weight of the image location, thereby taking care of the loss of spatial information at the cost of improving the feature information as given by Qureshi et al. (Citation2022b). In the super chain tracker, attention in paired box branch for regression is used for object attention, whereas the identity attention mechanism is used for ID verification. The detections from both is then merged to get the final decision. The mammography images carrying cancerous masses and micro-calcifications region can be detected and segmented using deep learning based transformer attention mechanism. Another future direction is to collect a primary dataset for better BC control, containing the clinical parameters and demographic profiles of the patients as well as pathological control response, survival, and progression of the patients.
Ethical approval and consent to participate
Not Applicable Data were obtained from a publicly available, deidentified dataset. http://www.eng.usf.edu/cvprg/Mammography/Database.html
Availability of supporting data and materials
These data are already available via http://www.eng.usf.edu/cvprg/Mammography/Database.html
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Acharya, U. R., Ng, W. L., Rahmat, K., Sudarshan, V. K., Koh, J. E. W., Tan, J. H., Hagiwara, Y., Yeong, C. H., & Ng, K. H. (2017). Data mining framework for breast lesion classification in shear wave ultrasound: A hybrid feature paradigm. Biomedical Signal Processing and Control, 33(December 2016), 400–410. https://doi.org/10.1016/j.bspc.2016.11.004
- Ardakani, A. A., Gharbali, A., & Mohammadi, A. (2015). Classification of breast tumors using sonographic texture analysis. Journal of Ultrasound in Medicine, 34(2), 225–231. https://doi.org/10.7863/ultra.34.2.225
- B., M., & Shankar, P. (2017). Awareness and screening behaviors of breast cancer among urban women in Mysuru, India- need for breast health education program. International Journal of Community Medicine And Public Health, 4(8), 2967–2972. https://doi.org/10.18203/2394-6040.ijcmph20173354
- Becker, A. S., Marcon, M., Ghafoor, S., Wurnig, M. C., Frauenfelder, T., & Boss, A. (2017). Deep learning in mammography. Investigative Radiology, 52(7), 434–440. https://doi.org/10.1097/RLI.0000000000000358
- Boureau, Y. L., Ponce, J., & LeCun, Y. (2010). A theoretical analysis of feature pooling in visual recognition. Proceedings of the 27th international conference on machine learning (ICML-10) (pp. 111–118).
- Cheng, H. D. D., Shi, X. J. J., Min, R., Hu, L. M. M., Cai, X. P. P., & Du, H. N. N. (2006). Approaches for automated detection and classification of masses in mammograms. Pattern Recognition, 39(4), 646–668. https://doi.org/10.1016/j.patcog.2005.07.006
- Cruz, J. A., & Wishart, D. S. (2006). Applications of machine learning in cancer prediction and prognosis. Cancer Informatics, 2, 117693510600200. https://doi.org/10.1177/117693510600200030
- Desantis, C. E., Fedewa, S. A., Sauer, A. G., Kramer, J. L., Smith, R. A., & Jemal, A. (2016). Breast cancer statistics, 2015 : convergence of incidence rates between black and white women. A Cancer Journal for Clinicians, 66(1), 31–42. https://doi.org/10.3322/caac.21320
- Dhahri, H., Al Maghayreh, E., Mahmood, A., Elkilani, W., & Faisal Nagi, M. (2019). Automated breast cancer diagnosis based on machine learning algorithms. Journal of Healthcare Engineering, 2019, 1–11. https://doi.org/10.1155/2019/4253641
- Dheeba, J., Albert Singh, N., & Tamil Selvi, S. (2014). Computer-aided detection of breast cancer on mammograms: A swarm intelligence optimized wavelet neural network approach. Journal of Biomedical Informatics, 49, 45–52. https://doi.org/10.1016/j.jbi.2014.01.010
- Doi, K., Ph, D., & Rossmann, K. (1996). Computer- aided diagnosis : Potential usefulness in : Diagnostic radiology and telemedicine. Research, Practice, and Opportunities, Proceedings of the National Forum, McLean, VA, USA, 9–13. https://doi.org/10.1109/MTOL.1995.504521
- Doyle, S., Hwang, M., Shah, K., Madabhushi, A., Feldman, M., & Tomaszeweski, J. (2007). Automated grading of prostate cancer using architectural and textural image features. 2007 4th IEEE International Symposium on Biomedical Imaging: From Nano to Macro, 1284–1287. https://doi.org/10.1109/ISBI.2007.357094. 1284 1287 >
- Falconi, L. G., Perez, M., & Aguilar, W. G. (2019). Transfer Learning in Breast Mammogram Abnormalities Classification With Mobilenet and Nasnet. 2019 International Conference on Systems, Signals and Image Processing (IWSSIP), 109–114. https://doi.org/10.1109/IWSSIP.2019.8787295
- Fehr, D., Veeraraghavan, H., Wibmer, A., Gondo, T., Matsumoto, K., Vargas, H. A., Sala, E., Hricak, H., & Deasy, J. O. (2015). Automatic classification of prostate cancer Gleason scores from multiparametric magnetic resonance images. Proceedings of the National Academy of Sciences, 112(46), E6265–E6273. https://doi.org/10.1073/pnas.1505935112
- Forouzanfar, M. H., Foreman, K. J., Delossantos, A. M., Lozano, R., Lopez, A. D., Murray, C. J. L., & Naghavi, M. (2011). Breast and cervical cancer in 187 countries between 1980 and 2010: A systematic analysis. The Lancet, 378(9801), 1461–1484. https://doi.org/10.1016/S0140-6736(11)61351-2
- Gupta, S., Saravanan, V., Choudhury, A., Alqahtani, A., Abonazel, M. R., & Babu, K. S. (2022). Supervised computer-aided diagnosis (CAD) methods for classifying Alzheimer’s disease-based neurodegenerative disorders. Computational and Mathematical Methods in Medicine, 2022, 1–10. https://doi.org/10.1155/2022/9092289
- Heath, M., Kevin, B., Daniel, K., K, P., Richard, M., & Kyong, C. (1998). Current status of the digital database for screening mammography. In N. Karssemeijer, M. Thijssen, J. Hendriks, & L. van Erning (Eds.), Digital mammography (pp. 457–460). Springer.
- Houssami, N., Lee, C. I., Buist, D. S. M., & Tao, D. (2017). Artificial intelligence for breast cancer screening: Opportunity or hype? The Breast, 36, 31–33. https://doi.org/10.1016/j.breast.2017.09.003
- Hyvärinen, A., Hurri, J., & Hoyer, P. O. (2009). Natural image statistics: A probabilistic approach to early computational vision (Vol. 39). Springer Science & Business Media.
- Hussain, L. (2018). Detecting epileptic seizure with different feature extracting strategies using robust machine learning classification techniques by applying advance parameter optimization approach. Cognitive Neurodynamics, 12(3), 271–294. https://doi.org/10.1007/s11571-018-9477-1
- Hussain, L., Ahmed, A., Saeed, S., Rathore, S., Awan, I. A., Shah, S. A., Majid, A., Idris, A., & Awan, A. A. (2018). Prostate cancer detection using machine learning techniques by employing combination of features extracting strategies. Cancer Biomarkers, 21(2), 393–413. https://doi.org/10.3233/CBM-170643
- Hussain, L., Almaraashi, M. S., Aziz, W., Habib, N., & Saif Abbasi, S.-U.-R. (2021). Machine learning-based lungs cancer detection using reconstruction independent component analysis and sparse filter features. Waves in Random and Complex Media, 32, 1–26. https://doi.org/10.1080/17455030.2021.1905912
- Hussain, L., Aziz, W., Alshdadi, A. A. A., Ahmed Nadeem, M. S., Khan, I. R., & Chaudhry, Q.-U.-A. (2019). Analyzing the dynamics of lung cancer imaging data using refined fuzzy entropy methods by extracting different features. IEEE Access, 7, 64704–64721. https://doi.org/10.1109/ACCESS.2019.2917303
- Hussain, L., Aziz, W., Khan, I. R., Alkinani, M. H., & Alowibdi, J. S. (2021). Machine learning based congestive heart failure detection using feature importance ranking of multimodal features. Mathematical Biosciences and Engineering, 18(1), 69–91. https://doi.org/10.3934/mbe.2021004
- Hussain, L., Aziz, W., Saeed, S., Rathore, S., & Rafique, M. (2018). Automated Breast Cancer Detection Using Machine Learning Techniques by Extracting Different Feature Extracting Strategies. 2018 17th IEEE International Conference On Trust, Security And Privacy In Computing And Communications/ 12th IEEE International Conference On Big Data Science And Engineering (TrustCom/BigDataSE), 327–331. https://doi.org/10.1109/TrustCom/BigDataSE.2018.00057
- Hussain, L., Nguyen, T., Li, H., Abbasi, A. A., Lone, K. J., Zhao, Z., ... & Duong, T. Q. (2020). Machine-learning classification of texture features of portable chest X-ray accurately classifies COVID-19 lung infection. BioMedical Engineering OnLine, 19(1), 1–18.
- Hussain, L., Rathore, S., Abbasi, A. A., & Saeed, S. (2019). Automated lung cancer detection based on multimodal features extracting strategy using machine learning techniques. In H. Bosmans, G.-H. Chen, & T. Gilat Schmidt (Eds.), Medical imaging 2019: Physics of medical imaging (Vol. 10948. pp. 64704–64721). SPIE. https://doi.org/10.1117/12.2512059
- Hussain, L., Saeed, S., Awan, I. A., Idris, A., Nadeem, M. S. A. A. A. A. A., Chaudhry, Q.-A., Chaudhary, Q.-A., Chaudhry, Q.-A., & Chaudhary, Q.-A. (2019). Detecting brain tumor using machine learning techniques based on different features extracting strategies. Current Medical Imaging Formerly Current Medical Imaging Reviews, 14(1), 595–606. https://doi.org/10.2174/1573405614666180718123533
- Hyvärinen, A., & Oja, E. (2000). Independent component analysis: Algorithms and applications. Neural Networks, 13(4–5), 411–430. https://doi.org/10.1016/S0893-6080(00)00026-5
- Jemal, A., Bray, F., & Ferlay, J. (1999). Global cancer statistics: 2011. CA: A Cancer Journal for Clinicians, 49(2), 69–90. https://doi.org/10.3322/caac.20107.Available
- Jen, C., & Yu, S. (2015). Expert systems with applications automatic detection of abnormal mammograms in mammographic images. Expert Systems with Applications, 42(6), 3048–3055. https://doi.org/10.1016/j.eswa.2014.11.061
- Khamparia, A., Bharati, S., Podder, P., Gupta, D., Khanna, A., Phung, T. K., & Thanh, D. N. H. (2021). Diagnosis of breast cancer based on modern mammography using hybrid transfer learning. Multidimensional Systems and Signal Processing, 32(2), 747–765. https://doi.org/10.1007/s11045-020-00756-7
- Kharya, S. (2014). Naive Bayes classifiers : A probabilistic detection model for breast cancer. CA: a Cancer Journal for Clinicians, 92(10), 26–31. https://doi.org/10.5120/16045-5206
- Ko, J. M., Nicholas, M. J., Mendel, J. B., & Slanetz, P. J. (2006). Prospective assessment of computer-aided detection in interpretation of screening mammography. American Journal of Roentgenology, 187(6), 1483–1491. https://doi.org/10.2214/AJR.05.1582
- Kolb, T. M., Lichy, J., & Newhouse, J. H. (2002). Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: An analysis of 27,825 patient evaluations. Radiology, 225(1), 165–175. https://doi.org/10.1148/radiol.2251011667
- Kooi, T., Litjens, G., van Ginneken, B., Gubern-Mérida, A., Sánchez, C. I., Mann, R., den Heeten, A., & Karssemeijer, N. (2017). Large scale deep learning for computer aided detection of mammographic lesions. Medical Image Analysis, 35, 303–312. https://doi.org/10.1016/j.media.2016.07.007
- Kumar, G. (2014). A detailed review of feature extraction in image processing systems. 5–12. https://doi.org/10.1109/ACCT.2014.74
- LeCun, Y. (2012). Learning invariant feature hierarchies. In: A. Fusiello, V. Murino, & R. Cucchiara, (Eds.), Computer vision - ECCV 2012. Workshops and Demonstrations. ECCV 2012. Lecture Notes in Computer Science (Vol. 7583). Springer. https://doi.org/10.1007/978-3-642-33863-2_51
- Lehman, C. D., Wellman, R. D., Buist, D. S. M., Kerlikowske, K., Tosteson, A. N. A., & Miglioretti, D. L. (2015). Diagnostic accuracy of digital screening mammography with and without computer-aided detection. JAMA Internal Medicine, 175(11), 1828–1837. https://doi.org/10.1001/jamainternmed.2015.5231
- Lei, Y., Shan, H., Jia, F., & Lin, J. (2016). Reconstruction independent component analysis-based methods for intelligent fault diagnosis. 2016 IEEE 20th International Conference on Computer Supported Cooperative Work in Design (CSCWD), 245–250. https://doi.org/10.1109/CSCWD.2016.7565996
- Li, X., Qin, G., He, Q., Sun, L., Zeng, H., He, Z., Chen, W., Zhen, X., & Zhou, L. (2020). Digital breast tomosynthesis versus digital mammography: Integration of image modalities enhances deep learning-based breast mass classification. European Radiology, 30(2), 778–788. https://doi.org/10.1007/s00330-019-06457-5
- Litjens, G., Sánchez, C. I., Timofeeva, N., Hermsen, M., Nagtegaal, I., Kovacs, I., Hulsbergen - van de Kaa, C., Bult, P., van Ginneken, B., & van der Laak, J. (2016). Deep learning as a tool for increased accuracy and efficiency of histopathological diagnosis. Scientific Reports, 6(1), 1–11. https://doi.org/10.1038/srep26286
- Machida, Y., Shimauchi, A., Kuroki, Y., Tozaki, M., Kato, Y., Hoshi, K., & Fukuma, E. (2017). Single focus on breast magnetic resonance imaging: Diagnosis based on kinetic pattern and patient age. Acta Radiologica, 58(6), 652–659. https://doi.org/10.1177/0284185116668212
- Malebary, S. J., & Hashmi, A. (2021). Automated breast mass classification system using deep learning and ensemble learning in digital mammogram. IEEE Access, 9, 55312–55328. https://doi.org/10.1109/ACCESS.2021.3071297
- Meselhy, M., Faye, I., & Belhaouari, B. (2012). A statistical based feature extraction method for breast cancer diagnosis in digital mammogram using multiresolution representation. Computers in Biology and Medicine, 42(1), 123–128. https://doi.org/10.1016/j.compbiomed.2011.10.016
- Metz, C. E. (1986). ROC methodology in radiologic imaging. Investigative Radiology, 21(9), 720–733. http://www.ncbi.nlm.nih.gov/pubmed/3095258 https://doi.org/10.1097/00004424-198609000-00009
- Mobark, N., Hamad, S., & Rida, S. Z. (2022). Coronet: Deep neural network-based end-to-end training for breast cancer diagnosis. Applied Sciences, 12(14), 7080. https://doi.org/10.3390/app12147080
- Motakis, E., Ivshina, A., & Kuznetsov, V. (2009). Data-driven approach to predict survival of cancer patients. IEEE Engineering in Medicine and Biology Magazine, 28(4), 58–66. https://doi.org/10.1109/MEMB.2009.932937
- Nasir Khan, H., Shahid, A. R., Raza, B., Dar, A. H., & & Alquhayz, H. (2019). Multi-view feature fusion based four views model for mammogram classification using convolutional neural network. IEEE Access, 7, 165724–165733. https://doi.org/10.1109/ACCESS.2019.2953318
- Nassif, A. B., Talib, M. A., Nasir, Q., Afadar, Y., & Elgendy, O. (2022). Breast cancer detection using artificial intelligence techniques: A systematic literature review. Artificial Intelligence in Medicine, 127, Article 102276. https://doi.org/10.1016/j.artmed.2022.102276
- Oakden-Rayner, L., Carneiro, G., Bessen, T., Nascimento, J. C., Bradley, A. P., & Palmer, L. J. (2017). Precision radiology: Predicting longevity using feature engineering and deep learning methods in a radiomics framework. Scientific Reports, 7(1), 1648. https://doi.org/10.1038/s41598-017-01931-w
- Oliver, A., Torrent, A., Lladó, X., Tortajada, M., Tortajada, L., Sentís, M., Freixenet, J., & Zwiggelaar, R. (2012). Knowledge-based systems automatic microcalcification and cluster detection for digital and digitised mammograms. Knowledge-Based Systems, 28, 68–75. https://doi.org/10.1016/j.knosys.2011.11.021
- Orrù, G., Pettersson-Yeo, W., Marquand, A. F., Sartori, G., & Mechelli, A. (2012). Using support vector machine to identify imaging biomarkers of neurological and psychiatric disease: A critical review. Neuroscience & Biobehavioral Reviews, 36(4), 1140–1152. https://doi.org/10.1016/j.neubiorev.2012.01.004
- Park, S., Kong, H.-J., Moon, W. K., & Kim, H. C. (2007). Segmentation of Solid Nodules in Ultrasonographic Breast Image Based on Wavelet Transform. 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, 5649–5652. https://doi.org/10.1109/IEMBS.2007.4353628
- Parmar, C., Bakers, F. C. H., Peters, N. H. G. M., & Beets-, R. G. H. (2017). Deep learning for fully-automated localization and segmentation of rectal cancer on multiparametric. Scientific Reports, 7(1), 1–9. https://doi.org/10.1038/s41598-017-05728-9
- Qian, W., Mao, F., Sun, X., Zhang, Y., Song, D., & Clarke, R. A. (2002). An improved method of region grouping for microcalcification detection in digital mammograms. Computerized Medical Imaging and Graphics, 26(6), 361–368. https://doi.org/10.1016/S0895-6111(02)00045-9
- Qureshi, S. A., Hussain, L., Chaudhary, Q. U., Abbas, S. R., Khan, R. J., Ali, A., & Al-Fuqaha, A. (2022a). Kalman filtering and bipartite matching based super-chained tracker model for online multi object tracking in video sequences. Applied Sciences, 12(19), 9538.
- Qureshi, S. A., Raza, S. E., Hussain, L., Malibari, A. A., Nour, M. K., Rehman, A. U., Al-Wesabi, F. N., & Hilal, A. M. (2022b). Intelligent ultra-light deep learning model for multi-class brain tumor detection. Applied Sciences, 12(8), 3715.
- Rathore, N., Divya, T., & Agarwal, S. (2014). Predicting the survivability of breast cancer patients using ensemble approach. 2014 International Conference on Issues and Challenges in Intelligent Computing Techniques (ICICT), 459–464. https://doi.org/10.1109/ICICICT.2014.6781326
- Rathore, S., Hussain, M., Aksam Iftikhar, M., & Jalil, A. (2014). Ensemble classification of colon biopsy images based on information rich hybrid features. Computers in Biology and Medicine, 47(1), 76–92. https://doi.org/10.1016/j.compbiomed.2013.12.010
- Rathore, S., Iftikhar, A., Ali, A., Hussain, M., & Jalil, A. (2012). Capture largest included circles: An approach for counting red blood cells. Communications in Computer and Information Science, 281, 373–384. https://doi.org/10.1007/978-3-642-28962-0_36
- Rejani, Y. I. A., & Selvi, S. T. (2009). Early detection of breast cancer using SVM classifier technique., 1(3), 127–130. arXiv e-prints(2009): arXiv-0912.
- S R, S. C., & Rajaguru, H. (2021). Deep features with improved extreme learning machine for breast cancer classification. 2021 8th International Conference on Soft Computing & Machine Intelligence (ISCMI), 237–241. https://doi.org/10.1109/ISCMI53840.2021.9654814
- Salama, W. M., & Aly, M. H. (2021). Deep learning in mammography images segmentation and classification: Automated CNN approach. Alexandria Engineering Journal, 60(5), 4701–4709. https://doi.org/10.1016/j.aej.2021.03.048
- Salem, A., & Bin, A. (2017). Breast cancer classification enhancement based on entropy method. August, 7–11. https://doi.org/10.24032/ijeacs/0208/06
- Saroja, B., & Selwin Mich Priyadharson, A. (2018). Colon cancer detection methods-A review.” Annual Research & Review in Biology, 1–16.
- Sathish, D., Kamath, S., Rajagopal, K. V., & Prasad, K. (2016). Medical imaging techniques and computer aided diagnostic approaches for the detection of breast cancer with an emphasis on thermography-a review. International Journal of Medical Engineering and Informatics, 8(3), 275–299. https://doi.org/10.1504/IJMEI.2016.077446
- Skaane, P., & Engedal, K. (1998). Analysis of sonographic features in the differentiation of fibroadenoma and invasive ductal carcinoma. AJR. American Journal of Roentgenology, 170(1), 109–114. https://doi.org/10.2214/ajr.170.1.9423610
- Sprague, B. L., Conant, E. F., Onega, T., Garcia, M. P., Beaber, E. F., Herschorn, S. D., Lehman, C. D., Tosteson, A. N. A., Lacson, R., Schnall, M. D., Kontos, D., Haas, J. S., Weaver, D. L., & Barlow, W. E. (2016). Variation in mammographic breast density assessments among radiologists in clinical practice: A multicenter observational study. Annals of Internal Medicine, 165(7), 457–464. https://doi.org/10.7326/M15-2934
- Telagarapu, P., & Poonguzhali, S. (2014). Analysis of contourlet texture feature extraction to classify the benign and malignant tumors from breast ultrasound images, 6(1), 293–305.
- Tian, J., & Zhang, J. (2022). Breast cancer diagnosis using feature extraction and boosted C5.0 decision tree algorithm with penalty factor. Mathematical Biosciences and Engineering, 19(3), 2193–2205. https://doi.org/10.3934/mbe.2022102
- Trister, A. D., Buist, D. S. M., & Lee, C. I. (2017). Will machine learning Tip the balance in breast cancer screening? JAMA Oncology, 3(11), 1463–1464. https://doi.org/10.1001/jamaoncol.2017.0473
- Venkatesan, E. V. (2015). Performance analysis of decision tree algorithms for breast cancer classiication. December 2016. https://doi.org/10.17485/ijst/2015/v8i
- Verma, B. (2008). Novel network architecture and learning algorithm for the classification of mass abnormalities in digitized mammograms. Artificial Intelligence in Medicine, 42(1), 67–79. https://doi.org/10.1016/j.artmed.2007.09.003
- Vijayarajeswari, R., Parthasarathy, P., Vivekanandan, S., & Basha, A. A. (2019). Classification of mammogram for early detection of breast cancer using SVM classifier and Hough transform. Measurement, 146, 800–805. https://doi.org/10.1016/j.measurement.2019.05.083
- Xiao, Y., Zhu, Z., Zhao, Y., Wei, Y., & Wei, S. (2015). Kernel reconstruction ICA for sparse representation. IEEE Transactions on Neural Networks and Learning Systems, 26(6), 1222–1232. https://doi.org/10.1109/TNNLS.2014.2334711
- Zhang, F., Luo, L., Sun, X., Zhou, Z., Li, X., Yu, Y., & Wang, Y. (2019). Cascaded generative and discriminative learning for microcalcification detection in breast mammograms. 2019 IEEE/CVF Conference on Computer Vision and Pattern Recognition (CVPR), 12(14), 12570–12578. https://doi.org/10.1109/CVPR.2019.01286
- Zhang, L., Li, J., Xiao, Y., Cui, H., Du, G., Wang, Y., Li, Z., Wu, T., Li, X., & Tian, J. (2015). Identifying ultrasound and clinical features of breast cancer molecular subtypes by ensemble decision. Scientific Reports, 5(1), 1–14. https://doi.org/10.1038/srep11085
- Zhang, Y., Tomuro, N., Furst, J., & Stan Raicu, D. (2009, September). Using BI-RADS descriptors and ensemble learning for classifying masses in mammograms. In MICCAI International Workshop on medical content-based retrieval for clinical decision support (pp. 69–76). Springer.
- Zhu, Y., Hu, X., Zhang, Y., & Li, P. (2018). Transfer learning with stacked reconstruction independent component analysis. Knowledge-Based Systems, 152, 100–106. https://doi.org/10.1016/j.knosys.2018.04.010