Abstract
Chickens were immunized with protein extracts from respectively roasted Virginia and raw Runner peanuts. Immunoglobulins were conveniently extracted from the egg yolk in order to evaluate the immune response. Antibodies towards roasted peanuts were subsequently used in a competitive indirect immunoassay for the quantification of peanut proteins in foods. Various assay parameters were proven to have a significant influence on the assay sensitivity. Peanut proteins could be detected in foods at least down to a 1 ppm level. The isolated immunoglobulins proved to be very specific towards peanut proteins. Immune reaction of the antibodies varied, however, depending upon the peanut variety and the processing history of the peanuts themselves. In addition, experiments on spiked food matrices showed that careful selection of the extraction buffer is of prime importance as well. The developed immunoassay can be used as a semi-quantitative tool to confirm the presence or absence of a group of important allergic proteins in foods.
Introduction
Allergies can be considered as individual adverse reactions to foods, provoked by an abnormal response of one's immune system towards a particular food protein (Taylor & Hefle Citation2001, Taylor & Lehrer Citation1996). Many foods and food proteins are reported to induce such a food sensitivity, but it is accepted that particularly eight foods are responsible for the majority of the reported allergy cases (peanut, soy, egg, tree nuts, fish, crustaceans, wheat and milk) (Hefle et al. Citation1996). Food allergies represent a considerable health problem in industrialized areas because of the relatively high number of reported cases (2% adult population up to 8% in children; Sampson Citation1999, Ortolani et al. Citation2001, Sicherer et al. Citation2003). Clinical symptoms of an allergic attack after ingestion of the offending food include digestive and respiratory symptoms and skin reactions (Sampson Citation1999). Of these ‘big eight’ food allergens, particularly peanut allergy should be considered with much interest because of the reported life-threatening consequences due to an anaphylactic shock. Peanut is accounted to be responsible for more then 50% of the food allergy-induced fatalities (Bock et al. 2000). Moreover, peanut allergy seems to be a lifelong problem in contrast to some other allergies (Bock & Atkins Citation1989, Tariq et al. Citation1996). Although several peanut proteins have been identified to induce allergic reactions, particularly Ara h1 (Burks et al. Citation1991) and Ara h2 (Burks et al. Citation1992) are considered as major allergens. These proteins of, respectively, the viciline and conglutinine family, are major proteins in peanuts and have been studied in detail.
In order to avoid an allergic attack, a patient should avoid ingestion of food containing the offending proteins. Due to the evermore increasing complexity of food production and food formulation, it is very difficult for the consumer to decide whether a food may contain the offending allergen. In accordance with the Codex Alimentarius Commission's (Citation1999) recommendations, the European Commission issued a proposal to amend the European Food Labelling Directive 2000/13/EC (EC Citation2000, Poms et al. Citation2004). The proposal abolishes the ‘25% rule’ which specifies that for some foods it is not obligatory to label the ingredients that make up less than 25% of the final food product. Consequently, all ingredients intentionally added will have to be included on the label's ingredients list (Poms et al. Citation2004). Particular attention is paid to the labelling of the eight allergens mentioned above in addition to some other relevant European allergic foods such as celery. Because of these evolutions, the food industry is confronted with the need for reliable analytical methods that are able to detect and quantify particular allergens in food. Various techniques have been used to achieve this goal for a variety of important allergens as recently reviewed by Poms et al. (Citation2004). Most frequently, immuno-based techniques are used and in particular the enzyme-linked immunosorbent assay has gained a lot of attention throughout the years. Therefore it is not surprising that various enzyme linked immunosorbent assay (ELISA) methods have been described already for the detection of peanut proteins and that even several kits have been placed on the market (Poms et al. Citation2004). Most of these assays, however, are making use of mammal poly- or monoclonal antibodies. As an alternative source for antibody production however, chickens are reported to be very useful and promising host animals. This is because the chicken antibodies (IgY antibodies) can be isolated very conveniently out of the egg yolk. Moreover, because of the daily egg production, a nearly infinite source of antibodies is obtained (De Meulenaer & Huyghebaert Citation2001). In the present paper, the usefulness of chickens in the production of peanut protein specific chicken egg yolk immunoglobulins (IgY) was evaluated. Moreover, the isolated antibodies were used in an indirect competitive ELISA in order to allow quantification of peanut proteins in aqueous solutions and foods. Parameters influencing assay performance were studied in detail.
Materials and methods
Reagents and buffers
Sodium hydrogen carbonate and technical hexane were obtained from Chem-Lab, Belgium. Anhydrous disodium carbonate, sodium chloride, hydrochloric acid 25% and tri-sodium citrate dihydrate were purchased from UCB, Belgium. Potassium dihydrogen phosphate, disodium hydrogen phosphate dodecahydrate, sodium tetraborate decahydrate, sodium fluoride, cupper sulphate dodecahydrate, disodium ethylenediaminetetraacetate (EDTA), calcium lactate and ammonium sulphate were from Acros Organics, USA. Bovine serum albumin (BSA, fraction V, 96%), Freund's incomplete adjuvant, Freund's complete adjuvant, 2, 2′-azino-bis (3-ethylbenzthiazoline-6 sulfonic acid) (ABTS), Tris, Tricine and Tween 20 were from Sigma Chemical, USA. Hydrogen peroxide 30%, orthophenylenediamine (OPD), Folin Ciocalteu reagent, Mish indicator, mercaptoethanol and potassium chloride were from Merck, Germany. Horseradish peroxidase (HRP) conjugated rabbit anti chicken IgG was from ICN Biomedicals Inc., USA. All these reagents were of analytical grade unless otherwise mentioned.
Potassium caseinate was a gift of Rovita, Germany. All peanut and other nut samples were generous gifts from MD Menken, Belgium. Other protein isolates were obtained from various companies. Foods which were spiked with peanut protein isolate were obtained from local retail shops and their labels were checked for any declared presence of peanut proteins. All foods were considered to be free from peanut protein contamination.
Protein extraction buffer 1 consisted of a 20mM NaH2PO4 in 1M NaCl pH 7 solution (Yeung & Collins Citation1996); extraction buffer 2 (Keck-Gassenmeier et al. Citation1999, Mills et al. Citation1997) was a 200 mM NaCl, 50 mM Tris buffer, pH 8.2; extraction buffer 3 (Holzhauzer & Vieths Citation1999) was composed of 8mM Tris, 25 mM Tricine and 2M calciumlactate and the pH was adjusted to 8.6; extraction buffer 4 was similar as buffer 4, unless the pH was adjusted to 7.4; extraction buffer 5 consisted of phosphate buffered saline (PBS), extraction buffers 6 and 7 (Poms, personal communication) were 15 mM borax solutions with respectively a pH of 7 and 9.
PBS (pH 7.4) consisted of 0.135 M NaCl, 1.5 mM KH2PO4, 8 mM Na2HPO4. 12H2O, and 2.7 mM KCl. The coating buffer (pH 9.6) was a 15 mM Na2CO3 and a 35 mM NaHCO3 solution. The dilution buffer (PBS-Tween 20) consisted PBS with 0.05% (v/v) Tween 20. The wash solution was 0.05% (v/v) Tween 20 solution in 0.15 M NaCl. The blocking solution was PBS with 3% (w/v) K-caseinate.
If OPD was used as a substrate, substrate buffer (pH 5.0) was a 40 mM citric acid and 35 mM Na2HPO4. 12H2O solution. The substrate solution consisted of 40 mg of OPD in 100 mL of substrate buffer to which just before use 5 mL of 0.03% (v/v) H2O2 was added. The stop solution was 2.5 M HCl. If ABTS was used as a chromogen, substrate buffer (pH 4.0) consisted of 0.05 M tri-sodium citrate in water. Substrate solution consisted of 30 mg ABTS in 100 mL of substrate buffer to which just before use 5 mL of 6% (v/v) H2O2 was added. Stop solution was a 0.1 M NaF, 0.008 M NaOH and 0.001 M Na2EDTA solution.
Protein isolation and characterization
A peanut protein extract was obtained according to the protocol described earlier by Yeung and Collins (Citation1996). Briefly, 100 g of ground peanut were defatted with four times 100 mL technical hexane. After evaporation at ambient temperature of the solvent during 24 h, 10 g of defatted peanut flour was mixed with 100 mL of extraction buffer 1. This mixture was centrifuged for 30 min at 4°C and 20000 g. The clear supernatant was dialysed (Spectrapor Membrane Tubing, MW cut-off 12,000–14,000, Polylab Belgium) using the extraction buffer, for 48 h at 4°C. Finally the protein solution was filtered over a 0.45 µm membrane filter (Supor®-40, 25 mm, Pall Gelman Laboratory, Michigan, USA). Isolated proteins were characterized using SDS-PAGE using the Pharmacia Phast system, according to the manufacturer's instructions.
The same protocol was followed for the preparation of protein isolates from the other nuts samples analysed.
Protein quantification
Crude protein content was determined using the Kjeldahl procedure and applying a conversion factor of 6.25 for all products analysed. For routine analysis, a modified Lowry method as described by Schacterle and Pollack (Citation1973) was used, using bovine serum albumin as a standard and the Kjeldahl method as a reference.
Chicken immunization and immunoglobulin isolation
For every immunization experiment, three Isa Brown chickens of 43 weeks old were injected intramuscularly with 1 mL of a 1:1 (v/v) mixture of Freund's complete adjuvant and PBS containing 500 µg of protein. One series of chickens were injected with a protein extract obtained from raw peanut, type Runner USA (chicken 1–3). The second series of three chickens were injected with a protein extract obtained from roasted peanuts, type Virginia USA (chicken 4–6).
After 20 days, a supplementary injection of 1 mL of a 1:1 (v/v) mixture of Freund's incomplete adjuvant and PBS containing 500 µg of the respective protein isolates was given. Afterwards boaster injections of 500 µg of the corresponding immunizing antigen preparations in PBS were administered respectively 36 and 65 days after the initial injection.
Eggs were collected daily and individually identified. The immunoglobulins were isolated from the egg yolk using a modified aqueous dilution method described by Akita and Nakai (Citation1992). Briefly, v mL of egg yolk was separated from the egg and diluted with 8×v mL of water and pH was set with 1 N HCl between 5.0 and 5.2. After 16 h incubation at 4°C and centrifugation (10,000 g, 1 h, 4°C), the supernatant was filtered. After addition of 72 g of ammonium sulphate and about 170 mL of water in order to achieve a 60% saturated ammonium sulphate solution, the mixture was incubated for 1 h at room temperature (RT). After centrifugation (10,000 g, 20 min, RT), the residue was dissolved in a 19% (w/v) sodium sulphate solution and subsequently incubated for 20 min at RT. Again the solution was centrifuged (2000 g, 20 min, RT) and the residue was dissolved in a 14% (w/v) sodium sulphate solution. After 20 min of incubation at RT and centrifugation (2000 g, 20 min, RT) the residue was dissolved in v/6 mL PBS and stored in small aliquots at −18°C. For further use they were diluted from this final solution.
Immunoassays
General conditions
In this paper, two assays were evaluated depending upon the chicken antibodies used. Antibodies from chickens 1–3, were used in the so-called Runner raw peanut assay, while antibodies from chickens 4–6 were used in the Virginia roasted peanut assay. When relevant, specific differences between these two assays will be mentioned, whereas otherwise, the same protocol as mentioned below is followed for both assays.
Ninety-six well F 96 Maxisorp Nunc immuno plates from Nunc (Denmark) were coated with coating solution (12.5 µg peanut protein/mL coating buffer, 100 µL/well, raw Runner peanut extract for Runner raw peanut assay and roasted Virginia peanut extract for Virginia roasted peanut assay) by overnight incubation at 4°C in the dark. Plates were washed three times (200 µL wash solution/well) and blocked (200 µL blocking solution/well) for 2 h at RT, in the dark. Afterwards the plates were washed twice as previously. In order to study the immune response of the chickens or to obtain competition curves, primary antibodies were added as described elsewhere. Afterwards, the plates are washed as described above (three times).
For the detection reaction, the HRP-conjugated secondary antibody was added (100 µL/well, 3.4 µg/mL dilution buffer). After 1 h incubation at 37°C and washing of the plates (three times), 100 µL/well of substrate solution was added followed by an additional incubation at 37°C for 1 h. Finally 25 µL/well of the appropriate stop solution was added before measuring the absorbance at the appropriate wavelength (for OPD 492 nm; for ABTS 405 nm) using a Titertek multiskan plus MK II (USA). Absorbances were corrected for blanc readings obtained by using immunoglobulins isolated from the eggs of non-immunized chickens. These conditions were followed unless otherwise stated. All results are the average of four readings.
Indirect ELISA
In order to study the immune response of the chickens, the appropriate primary antibody dilution was added to the coated wells (100 µL/well in dilution buffer, for Runner raw peanut assay, IgY isolates from chickens 1–3, for Virginia roasted peanut assay; IgY isolates from chickens 4–6) and the plates were incubated for 1 h at 37°C. OPD was used as a chromogen.
Indirect competitive ELISA
For the competition step, 50 µL of the appropriate diluted protein solution in distilled water and 50 µL of the primary antibody solution were added to each well. Primary antibodies (for Runner raw peanut assay, IgY isolates from chickens 1–3, for Virginia roasted peanut assay; IgY isolates from chickens 4–6) were diluted 1/5000 in a buffer prepared by the addition of 1.42 mL 4M NaCl to 7.58 mL PBS containing 0.3% BSA. To this dilution is referred as the competition buffer. The plates were incubated for 1 h at 37°C. ABTS was used as a chromogen. These conditions were followed unless otherwise stated.
pH studies
The amount of NaOH (0.1 N) or HCl (0.1 N) added to the competition buffer was adjusted together with the amount NaCl 4 M and PBS in such a way that the desired pH was reached keeping the ionic strength constant. pH studies were carried out in the 2–12 range.
Ionic strength studies
The amount of 4 M NaCl added to the competition buffer was adjusted in order to vary its ionic strength. The amount of PBS was reduced accordingly, keeping the primary antibody concentration constant for all experiments. If necessary the addition of NaCl solution was replaced by the addition of water. The ionic strength was calculated using the following formula:
Cross reactivity studies using the Virginia roasted peanut assay
Competitive assays using the appropriate coating antigen and IgY isolates were performed using various protein isolates from peanuts, nuts and other important food proteins in order to determine their respective I50 values (µg/ml). I50 is the concentration of the analyte at which half of the maximal signal intensity is reached. Cross reactivity was calculated as:
Cross reactivity of the Runner raw peanut assay was evaluated in a comparable manner using the corresponding immune reagents. In these assays, OPD was used as a chromogen.
Boiling experiments
Peanut protein solutions (10 mg/mL, 1ml in PBS) were put into test tubes, which were closed and subsequently put in boiling water for the appropriate time. Afterwards, the test tubes were immediately put in ice water and diluted with PBS to a final volume of 10 mL. From this solution, dilutions were prepared in PBS which were applied in the indirect competitive ELISA as described in order to calculate the cross reactivity of the obtained protein solution towards the reference protein of the assay.
Extraction experiments
Extraction experiments were based on the procedure described by Yeung and Collins (Citation1996). Briefly the food samples of interest were ground into a fine powder from which a 5g subsample was put into a 50 mL volumetric flask. After spiking the sample at the appropriate level with proteins extracted from roasted Virginia peanuts, the studied extraction buffers were added and the flask was shaken vigorously and incubated for 1 h at room temperature, turning the flask upside down every 10 min. Subsequently, the mixture was centrifuged for 10 min (1500 g) and the supernatant was applied in the assay as described.
Data processing
Competition curves were obtained in quadruplate. When required, curves were normalized by expressing the experimental absorbance levels (B) as (B/B0), where B0 is the absorbance in absence of analyte. Absolute or normalized signals were fitted to a four parameter logistic equation using a commercial software package (SPSS 10.0).
Results and discussion
Chicken immunization experiments
Two protein extracts were selected for the immunization of the chickens, respectively from raw Runner and roasted Virginia peanuts. Proteins extracts were characterized by SDS-PAGE. As can be observed from , protein profiles were quite similar, despite the fact that quantitative differences were observed. In addition, some supplementary protein extracts were prepared from other raw and roasted peanut varieties. Protein profiles seemed to be dependent upon the peanut variety and upon the processing, confirming previously reported data (Beyer et al. Citation2001, Maleki et al. Citation2000, Basha & Young Citation1985). It is obvious, however, that in all samples and especially those used as immunizing antigens, proteins with a molecular weight corresponding to the major allergens present in peanut are present (Ara h1 64 kDa, Ara h2 17 kDa).
Table I. Protein profiles (SDS-PAGE) and cross reactivities of protein extracts from various roasted and raw peanuts in a Virginia roasted peanut assay (Virginia roasted peanuts cross reactivity = 100%).
For each protein extract, three chickens were immunized (chicken 1–3: raw Runner; chicken 4–5: roasted Virginia), but for each group one chicken abandoned egg production shortly after the start of the immunization procedure, probably because of their relatively elevated age (43 weeks). Response of the chickens towards the immunization procedure was evaluated by using an indirect ELISA as described. Immune response started after about 20 days and maximal titers were observed just before the first boaster injection. As can be observed from , titers typically higher then 1/100,000 are observed for most chickens. Despite the boaster injections, immune response decreased afterwards, but the isolated immunoglobulins were still useful for further experiments. As can be observed from , one of the chickens immunized with the proteins extracted from the roasted peanuts, showed significant lower titers. Previously, however, Yeung and Collins (Citation1996) immunized rabbits with proteins extracted from respectively roasted, denatured and raw peanuts. In their study, proteins from roasted peanuts were the most effective immunogens. Surprisingly, significant titers were obtained only within 5–6 months after the initial injection in their study.
Figure 1. Response of chickens immunized with peanut proteins isolated out of respectively raw Runner (1–3) and roasted Virginia peanuts (4–6) on the specified day following the first immunization. (⋄ = chicken 1, day 34; • = chicken 3, day 34,× = chicken 5, day 34, □ = chicken 6, day 34; ♦ = chicken 1, day 7; response was normalized to the maximal absorbance observed, B0, which equalled 1.49).
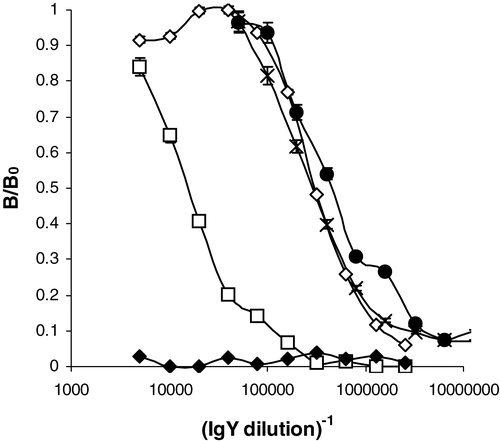
To the authors’ knowledge, this is the second reported successful chicken immunization with peanut proteins (Blais & Philippe Citation2000). In their paper, a comparable immunization procedure was followed, but no data with regard to the immune response as a function of time neither with regard to the kind of protein extracts used were specified. It should be noted as well that the use of chicken antibodies in the analysis of food allergens is not so well documented compared to the use of other antibodies, despite of the ease of immunization and antibody isolation and purification. In addition, large quantities of antibodies can be collected because of the daily egg production and the relatively high concentration of antibodies in the egg yolk (De Meulenaer & Huyghebaert Citation2001).
In this study, preference was given to use a mixture of peanut proteins instead of using a purified preparation of a single peanut protein, such as Ara h1. Although using this approach will not enable specific detection of a particular allergic protein, some specific advantages should be considered in order to enable detection of the hidden presence of peanut proteins in foods using the antibodies produced. First of all, by the detection of a mixture of proteins, sensitivity could probably be higher, since although Ara h1 is a major protein in peanuts, other proteins are present in even higher concentrations, depending upon the variety and the processing conditions (see ). Secondly, it should be realized that other major allergens are present as well. It would be a tedious task to develop assays for every allergic protein present in peanuts. Therefore it seems more practical to measure the peanut protein content of a particular food, instead of the concentration of a particular allergen. Obviously, if peanut proteins are present, it seems likely that peanut allergens are present as well. Thirdly it could be possible that due to chemical reactions involving proteins (e.g. denaturation, cross linking), immune recognition between the antigen and the produced antibodies is altered (Koch et al. Citation2003). In a ‘worst case’ situation immune recognition would totally disappear, giving rise to a false negative results in the assay. In the case of monoclonal antibodies towards one particular peanut protein, this scenario could be rather dramatic. The chance that such a scenario occurs, applying a mixture of polyclonal antibodies towards a mixture of proteins, is probably much lower.
Optimization of the competitive indirect assay
The isolated antibodies from the chickens were evaluated in a competitive indirect ELISA. Therefore in the first place the parameters having an influence on the assay performance were evaluated.
pH studies
Because the molecules of interest are proteins, their charge and consequently their interactions with antibodies may be influenced by the pH of the competition buffer. Therefore, for both antibodies isolated, the influence of this parameter on the assay performance was evaluated, keeping other factors such as the ionic strength constant. Only in the pH range 6–10, useful competition curves could be obtained. From the results summarized in , it can be concluded that especially the Virginia toasted peanut assay proved to be dependent upon the pH.
Figure 2. The influence of the pH of the competition buffer on the I50 (closed symbols) and B0/B0, max (open symbols). (a) Runner raw peanut assay (B0, max equalled 0.52). (b) Virginia roasted peanut assay. (B0, max equalled 0.27).
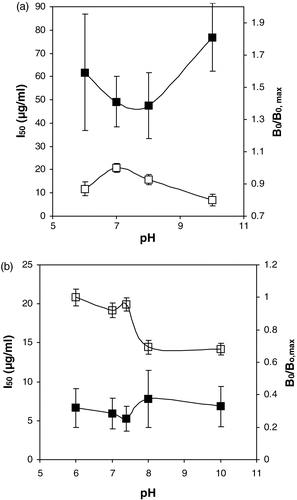
The binding between the chicken immunoglobulins and the coated roasted peanuts in absence of competitive binding proteins, as assessed by the B0 value, seems to be influenced in a significant manner (30% less binding) if the pH is increased from 7–10. For the Runner raw peanut assay this effect is much less pronounced and shows moreover a different pattern as a function of the pH. These data did not correlate with the solubility of these proteins as a function of the pH, which were similar for both of the protein isolates (results not shown). In a previous study, however, using chicken immunoglobulins towards bisphenol A (De Meulenaer et al. Citation2002), no significant influence of the pH on the assay performance was observed. Of course, charge interactions between antibodies and the target molecules are more likely to be influenced by the pH if the latter are proteins as well. It remains unclear whether the observed differences between Runner raw and Virginia roasted peanut assays dependence upon the pH, can be attributed to the merely qualitative differences in protein pattern of the two isolates (). It should not be excluded however that the charge characteristics of the peanut protein may be changed as well due to the chemical reactions involved in the roasting process (Beyer et al. Citation2001, Maleki et al. Citation2000, Basha & Young Citation1985). In this regard, it is worthwhile mentioning that the allergenicity or other properties of peanut proteins are reported to be influenced by the roasting process, confirming the involvement of chemical changes in the proteins as a result of this process (Maleki et al. Citation2003, Chung et al. Citation2003, Beyer et al. Citation2001, Maleki et al. Citation2000).
From the data in , it is obvious that the roasted Virginia peanut protein assay proved to be more sensitive compared to the raw Runner peanut protein assay as well, as revealed by the difference in I50 values.
Ionic strength studies
As indicated in , ionic strength of the competition buffer was of importance as well with regard to the performance of both assays. An increase of the ionic strength resulted in both cases in a decreased interaction between the antibodies and the coated antigens in absence of free antigen. In parallel, increased ionic strength resulted in increased I50 levels.
Figure 3. The influence of the ionic strength of the competition buffer on the I50 (closed symbols) and B0/B0, max (open symbols). (a) Runner raw peanut assay (B0, max equalled 0.60). (b) Virginia roasted peanut assay. (B0, max equalled 0.42). It should be noted that the reported ionic strength values represent the final ionic strength during the competition step, i.e. a ½ dilution with an aqueous protein solution is taken into account.
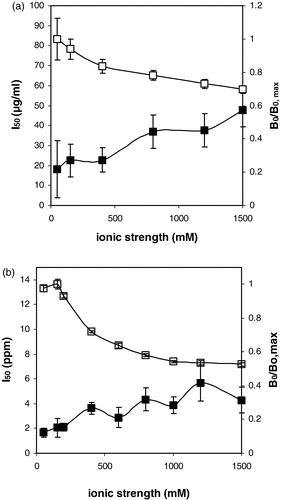
The ionic strength of the competition buffer should not be too low as well, since it is obvious from that the repeatability of the assay deteriorates in these conditions, particularly for the raw peanut protein assay. Similar observations were made using both chicken and mammalian antibodies towards small organic molecules (Abad & Montoya Citation1997, De Meulenaer et al. Citation2002). Presumably, the ionic strength influences the interaction between antibodies and both hydrophobic and hydrophilic target molecules. For proteins of course, these observations were to be expected because of the well-known influence of ionic strength on the electrophilic behaviour of charged particles (De Meulenaer et al. Citation2001). These results indicate that care should be taken in the ionic load of a food sample extract, as revealed already before in other immunoassays (De Meulenaer et al. Citation2002) or in the selection of an appropriate extraction buffer.
Other relevant parameters
In order to obtain good assay characteristics of a competitive ELISA, limiting concentrations of immunoreagents are required. Therefore the effect of the antibody and the HRP-labelled antibody concentration in combination with the incubation time of the competition step and the detection reaction were evaluated as well for respectively the raw and roasted peanut assay. In summary, lowering the concentration of the primary antibody and the HRP-labelled antibody resulted both in lower maximal absorbances and lower I50 values. Similar observations were made if the incubation times were restricted.
In order to shorten the total analysis time, attempts were undertaken to shorten the blocking step (2 h in initial procedure). Lowering the blocking time, however, resulted in an increase of the blanc readings from 0.1–0.2 relative absorbance units. Therefore, the concentration of the caseinate proteins in the blocking buffer was increased from 3–4.5% and 6% w/v. Blanc readings were at both caseinate concentrations comparable with those obtained using the initial blocking conditions, even if the blocking time was reduced to 30 min. At the highest caseinate concentration tested, however, a significant decrease of the maximal absorbance value was observed (0.1 relative units), compared to the other blocking solutions at 3% and 4.5% w/v. Probably due to the higher amount of absorbed caseinate proteins, the binding between the immunoreagents is affected. Therefore, the caseinate concentration of the blocking solution was changed from 3 to 4.5% w/v, enabling as well a reduction of the total assay time with 90 min without affecting the final assay characteristics.
During the competition step, surface-active agents are frequently added in order to reduce non-specific interactions. Therefore, the following surfactants were evaluated: Bovine serum albumin (0.03 and 0.1% w/v), gelatine (0.1% w/v), potassium caseinate (0.1% w/v) and Tween 20 (0.1%, w/v). No significant differences in assay performance were detected, for both assays. This is in contrast to previously reported data on assays for carbaryl (Abad & Montoya Citation1997) and bisphenol A (De Meulenaer et al. Citation2002) using respectively rabbit and chicken polyclonal antibodies. It remains unclear if these different observations are due to the totally different character of the target molecules studied.
The use of OPD as an alternative chromogen instead of ABTS was evaluated as well. Previously, preference was given in our laboratories to the use of ABTS because of the significant improvement on assay sensitivity (De Meulenaer et al. Citation2002), despite of the fact that maximal absorbance levels decreased in an important extent. In the current Virginia roasted peanut proteins assay however, no such an improvement was observed (I50 (OPD) 0.22 µg/ml±0.05; I50 (ABTS) 0.24 µg/ml±0.04). Therefore preference was given to use OPD as a chromogen instead, because of the significantly higher maximal absorbance values observed.
Finally the influence of the citrate concentration in the substrate buffer was investigated using OPD as a chromogen. In previous work in our laboratories (De Meulenaer Citation2002) dealing with the development of an immunoassay for bisphenol A analysis, citrate concentration of the substrate buffer influenced absorbance levels of the final assay if ABTS was used as a substrate. Lower citrate concentrations resulted in higher absorbance levels. In addition, however, it seemed that absorbance readings without adding the secondary antibody-peroxydase complexes increased as well. Presumably, citrate prevented spontaneous oxidation of the chromogen by the hydrogen peroxide. For OPD, an increase of the citrate concentration from 38 mM to 100 mM resulted in a nearly linear decrease of the maximal absorbance from 0.7 to 0.2, without affecting the blanc readings. These observations are surprising, considering the fact that in literature different citrate concentrations are being used for both ABTS and OPD (Catty & Raykundalia Citation1989, Saunders Citation1979).
If OPD is used as a substrate however, it seems relevant to stress to take care of using the appropriate substrate concentration. Absorbance levels in the absence of peanut proteins seemed to be highly depended upon the peroxide concentration used, for both assays considered. As indicated in , at low substrate concentrations, low maximal absorbance levels are observed, as could be expected. At much higher substrate concentrations, such as used by Catty and Raykundalia (Citation1989) and Porstmann et al. (Citation1981), net absorbance levels were very low as well.
Figure 4. Influence of substrate concentration (hydrogen peroxide) in the substrate solution on the maximal absorbance in the Virginia roasted peanut assay using OPD as a chromogen.
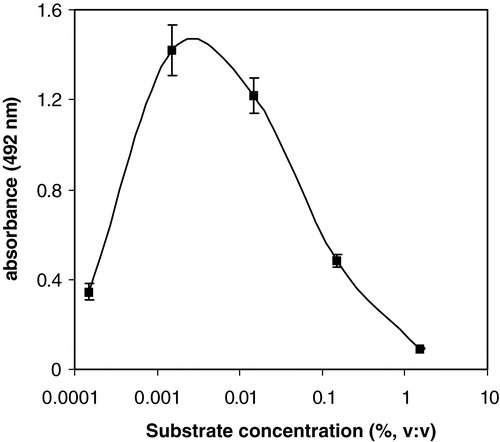
Finally, if OPD was used as a chromogen, a final substrate concentration of 0.0015% (v:v) was selected. For ABTS a continuous increase in signal intensity was observed until the substrate concentration amounted 0.05% (v:v). In contrast to OPD however, no decrease in signal intensity was observed if higher substrate concentrations were applied (up to 0.6% v:v, not shown). No effect of the substrate concentration on the blanc reading (without enzyme-secondary antibody complex) could be observed either, in the concentration range tested. The deviating behaviour for OPD can be partially explained by the high blanc readings which were observed using the high substrate concentrations reported. Since the overall absorbance levels (not corrected for the blanc) in these experimental conditions were also lower then those observed with the optimal substrate concentration, this could not be the only explanation. Possibly, the chromogen dependent formation of an inactive substrate-enzyme complex at these high substrate concentrations could be the cause (Porstmann et al. Citation1981). Because of the chromogen dependent character of this inactivation, the observed difference between OPD and ABTS could be explained as well. Curiously, to the authors’ knowledge no other references confirming these findings could be found in literature.
In conclusion, various factors were proven to be of influence on the assay performance. An indirect competitive enzyme-linked immunosorbent assay for respectively raw and roasted peanuts using chicken immunoglobulins was developed. The Virginia roasted peanut assay seemed to be more sensitive (I50 0.66±0.03 µg/ml) compared to the Runner raw peanut assay (I50 12.8±1.1 µg/ml). Considering a likely 1/10 dilution of a food sample upon extraction, especially the roasted peanut assay was considered to be promising since detection levels as low as 1 ppm on product basis should be feasible. This sensitivity is comparable with the ones obtained in previously developed and commercial assays (Hefle et al. Citation1994, Yeung & Collins Citation1996, Holzhauser & Vieths Citation1999, Pomes et al. Citation2003, Newsome & Abbott Citation1999, Poms et al. Citation2004, Koch et al. Citation2003). Considering moreover the low threshold doses reported to trigger a mild reaction in peanut allergic persons, these detection levels can be considered to be useful as well from a clinical perspective (Hourihane et al. Citation1997, Wensing et al. Citation2002, Bindslev-Jensen et al. Citation2002). Since especially the Virginia roasted peanut assay showed promising results, this particular assay was further studied in detail for its applicability to the analysis of foods.
Roasted peanut proteins assay specificity
The specificity of the optimized Virgin roasted peanut assay was evaluated for proteins isolated from real nuts, other major food proteins and different roasted and raw peanut varieties as well. Results are summarized in (peanut varieties), II (real nut proteins) and III (other food proteins). In parallel, protein composition using SDS-PAGE was investigated as well. Cross reactivity of the optimized Runner raw peanut assay towards proteins extracted from the roasted Virginia peanuts amounted 42%.
Considering the data from and , it seems that the produced antibodies showed low to very low cross reactivity towards proteins from real nuts and other major food proteins, including other important allergens.
Table II. Protein profiles (SDS-PAGE) and cross reactivities of protein extracts from various nuts in a Virginia roasted peanut assay (Virginia roasted peanuts cross reactivity = 100%)
Table III. Protein profiles (SDS-PAGE) and cross reactivities of protein extracts from various major food proteins nuts in a Virginia roasted peanut assay (Virginia roasted peanuts cross reactivity = 100%)
From however, it can be observed that the immune reaction depended upon the peanut variety, even if the proteins were extracted from roasted peanuts. The effect of the roasting process seemed to be different for every peanut variety. One of the protein extracts from raw peanuts (Runner) showed relatively low cross reactivity. According to the authors’ knowledge, reports paying attention to the varying immune reactivity of peanut proteins from different varieties in enzyme-linked immunosorbent assays are very scarce as are the data illustrating the influencing of the roasting process. Recently Koch et al. (Citation2003) compared various commercial kits for the detection of peanut proteins in foods spiked with raw and roasted Runner peanuts. Recovered peanut protein concentrations depended from kit to kit, indicating that the various antibodies used showed a different immune response to the peanut proteins examined. Correspondingly, it is not surprising that in our study, the antibodies showed a different immune response towards protein extracts from different peanut varieties. Moreover, Koch et al. (Citation2003) revealed that the recovered amount of peanut protein depended upon the intensity of the roasting process, which again confirms our findings reported in .
In order to further investigate the differences observed between the various peanut varieties and between the proteins extracted from raw and roasted peanuts, some preliminary experiments considering the effect of boiling the peanut proteins were conducted. Results are summarized in .
Table IV. Effect of heating peanut protein extracts on cross reactivity in respectively raw Runner peanut (untreated raw Runner peanut proteins cross reactivity = 100%) and roasted Virginia peanut (untreated roasted Virginia proteins cross reactivity = 100%) assay
As can be observed, short boiling of raw Runner peanut proteins did slightly decrease cross reactivity in the Runner raw peanut assay (relative loss of 20%). In the Virginia roasted peanut assay, a stronger decrease of cross reactivity towards heated proteins extracted from respectively raw Runner (relative loss of 90%) and roasted Virginia peanuts (relative loss of 80%) was observed. Increasing the heat treatment, resulted in more pronounced reductions in cross reactivity in the Virginia roasted peanut assay for both the raw Runner (relative loss of 97%) and roasted Virginia peanut proteins (relative loss of 99%). Also boiling of the proteins extracted out of raw and roasted Argentina peanuts resulted in serious losses in cross reactivity. These data show that boiling a protein extract does not compensate for the differences observed in cross reactivity in the developed assay between proteins extracted from raw or roasted peanuts. Moreover, it indicates that processing of the food containing the peanut proteins could affect in a high extent the quantification of the peanut proteins, as reported before by Koch et al. (Citation2003). Considering the reported heat stability of the allergic properties of peanuts, these observations are quite important to be considered, since although the proteins are not detectable in the assay, they still may exhibit their allergic properties.
These and previously reported results (Koch et al. Citation2003) show that immuno assays for proteins in general and peanut proteins in particular should merely be considered as semi-quantitative instead of having a real quantitative value. This is supported by the observations that proteins from different peanut varieties may induce different immune reactions in the assay developed. In addition, roasting or boiling the peanuts may affect the immune response as well. These aspects are of particular importance with regard to the specification of tolerable levels of peanut proteins in peanut-free foods.
Evaluation of appropriate extraction buffers
Various extraction buffers compositions have been used to determine peanut proteins in foods (Yeung & Collins Citation1996, Keck-Gassenmeier et al. Citation1999, Mills et al. Citation1997, Holzhauser & Vieths Citation1999, Poms, personal communication). Because of the previously observed results with regard to the competition buffers characteristics on assay performance, it was worthwhile to verify whether the extraction buffers used would have a similar influence on the assay performance as could be expected from the results mentioned above. Therefore, dilution series of peanut proteins were made in the respective extraction buffers and subsequently the inhibition curves characteristics were compared with the reference (dilution in water). Results are summarized in .
Table V. Maximal absorbances of the competition curves obtained in the Virginia roasted peanut assay as a function of the dilution buffer
Only the maximal absorbance values are reported, because I50 values did not significantly vary for all the buffers used (on average about 1µg/ml). Deviating maximal absorbance values from the reference, however, could lead to false positive samples if calibration and extraction is performed with different buffers. From the results obtained, observations reported in and are confirmed. Increase of the ionic strength and pH levels exceeding 7 both resulted in a decreased maximal absorbance. This experiment underlines the importance of proper calibration of the assay in this regard that the dilution buffer used for calibration should have a comparable composition as the extraction buffer used. Alternatively, the extract may be diluted in a particular manner, but this will affect the sensitivity of the assay.
The next step consisted of evaluating the buffers capacity to extract peanut proteins from spiked food samples. Since extraction buffers 4–6 gave merely similar calibration curves, they were all compared for extraction of peanut proteins out several spiked foodstuffs. Results in indicate that only minor differences between the three extraction buffers tested can be distinguished.
Table VI. Competition curves characteristics obtained after extraction of several spiked food samples using the Virginia roasted peanut assay, using several extraction buffers
Presumably, the extraction characteristics of all buffers were comparable and therefore for the further experiments it was decided to continue working only with extraction buffer 4, which was phosphate buffered saline. Again some supplementary matrices were investigated, including chocolate-containing biscuits. Results are summarized in .
Table VII. Competition curves characteristics obtained after extraction of several spiked food samples using the Virginia roasted peanut assay
For the chocolate-containing products, results are comparable to those reported in . For corn and rice based products however, quite big differences in especially the maximal absorbance values were observed. These results were quite unexpected and in disagreement with those obtained in . Because of the limited aqueous solubility of the proteins present in these foods tested, the decreased maximal absorbance values cannot be due to a non-specific binding of competing proteins to the antibodies. No explanation for the observed matrix effect could be found. These results indicate that care should be given to the proper calibration of the presented assay in various matrices in order to avoid a possible risk for false positive results caused by unresolved matrix effects.
Conclusions
This study indicated that chickens are a useful alternative for the production of suitable immunglobulins for the detection of peanut protein in foods. These IgY antibodies can be used in an indirect competitive ELISA in order to detect undeclared peanut proteins in foods. Care, however, should be taken with regard to the interpretation of the results of the assay. More precisely, the assay should merely be considered as semi-quantitatively because the immunoglobulins reaction towards peanut proteins depended upon the peanut variety and the peanut processing history.
Acknowledgments
This paper is dedicated to Prof. Emeritus Andre Huyghebaert, who guided the corresponding author throughout several years in the fascinating world of food chemistry and stimulated him to start working in the field of immunchemical analytical methods.
References
- Abad , A and Montoya , A . 1997 . Development of an enzyme-linked immunosorbent assay to carbaryl. 2. Assay optimization and application to the analysis of water samples . J Agric Food Chem , 45 : 1495 – 1501 .
- Akita , EM and Nakai , S . 1992 . Immunologlobulins from egg yolk: Isolation and purification . J Food Sci , 57 : 629 – 634 .
- Basha , SM and Young , CT . 1985 . Changes in the polypeptide composition of peanut (Arachis hypogaea L.) seed during oil roasting . J Agric Food Chem , 33 : 350 – 354 .
- Beyer , K , Morrow , E , Li , X-M , Bardina , L , Bannon , GA , Burks , AW and Sampson , HA . 2001 . Effects of cooking methods on peanut allergenicity . J Allergy Clin Immunol , 107 : 1077 – 1081 .
- Bindslev-Jensen , C , Briggs , D and Osterballe , M . 2002 . Can we determine a threshold level for allergenic foods by statistical analysis of published data in the literature? . Allergy , 57 : 741 – 746 .
- Blais , BW and Phillippe , LM . 2000 . A Cloth-based enzyme immunoassay for detection of peanut proteins in foods . Food Agric Immunol , 12 : 243 – 248 .
- Bock , SA , Munoz-Furlong , A and Sampson , HA . 2001 . Fatalities due to anaphylactic reactions to foods . J Allergy Clin Immunol , 107 : 191 – 193 .
- Bock , SA and Atkins , FM . 1989 . The natural history of peanut allergy . J Allergy Clin Immunol , 83 : 900 – 904 .
- Burks , AW , Williams , LW , Connaughton , C , Cockrell , G , O'Brien , TJ and Helm , RM . 1992 . Identification and characterization of a second major peanut allergen, Ara h II, with use of the sera of patients with atopic dermatitis and positive peanut challenges . J Allergy Clin Immunol , 90 : 962 – 969 .
- Burks , AW , Williams , LW , Helm , RM , Connaughton , C , Cockrell , G and O'Brien , T . 1991 . Identification of a major peanut allergen, Ara h I, in patients with atopic dermatitis and positive peanut challenges . J Allergy Clin Immunol , 88 : 172 – 179 .
- Catty , D and Raykundalia , C . 1989 . ELISA and related enzyme immunoassays: Antibodies, a practical approach , 97 – 154 . Oxford , , UK : IRL Press .
- Chung , SY , Butts , CL , Maleki , SJ and Champagne , ET . 2003 . Linking peanut allergenicity to the process of maturation, curing and roasting . J Agric Food Chem , 51 : 4273 – 4277 .
- Codex Alimentarius Commission . 1999 . Food labelling-complete texts . Joint FDA/WHO Food Standards Programme ( Rome , FAO/WHO ) .
- De Meulenaer B 2002 . Chemical interactions between packaging materials and foodstuffs . PhD Thesis, Ghent University Belgium .
- De Meulenaer , B , Baert , K , Lanckriet , H , van Hoed , V and Huyghebaert , A . 2002 . Development of an enzyme-linked immunosorbent assay for bisphenol A using chicken immunoglobulins . J Agric Food Chem , 50 : 5273 – 5282 .
- De Meulenaer B , van der Meeren P , Vanderdeelen J . 2001 . Electrophoretic studies of liposomes, Interfacial electrokinetics and electrophoresis . Delgado A , editor . New York : Marcel Dekker .
- De Meulenaer , B and Huyghebaert , A . 2001 . Isolation and purification of chicken egg yolk immunoglobulins: A review . Food Agric Immunol , 13 : 275 – 288 .
- EC 2000 . Food Labelling Directive . Official Journal , L 109 .
- Hefle , SL , Bush , RK , Yuyinger , JW and Chu , FS . 1994 . A sandwich enzyme-linked immunosorbent assay (ELISA) for the quantification of selected peanut proteins in foods . J Food Protection , 57 : 419 – 423 .
- Hefle , SL , Nordlee , J and Taylor , S . 1996 . Allergenic foods . Crit Rev Food Sci Nutrition , 36 : 69 – 90 .
- Holzhauser , T and Vieths , S . 1999 . Indirect competitive ELISA for determination of traces of peanut (Arachis hypogaea L.) protein in complex food matrices . J Agric Food Chem , 47 : 603 – 611 .
- Hourihane , JO'B , Kilburn , SA , Nordlee , JA , Hefle , SL , Taylor , SL and Warner , JO . 1997 . An evaluation of the sensitivity of subjects with peanut allergy to very low doses of peanut protein: A randomised, double-blind, placebocontrolled food challenge study . J Allergy Clin Immunol , 100 : 596 – 600 .
- Keck-Gassenmeier , B , Bénet , S , Rosa , C and Hischenhuber , C . 1999 . Determination of peanut traces in food by a commercially-available ELISA test . Food Agric Immunol , 11 : 243 – 250 .
- Koch , P , Schäppi , GF , Poms , RE , Wüthrich , B , Anklam , E and Battaglia , R . 2003 . Comparison of commercially available ELISA kits with human sera-based detection methods for peanut allergens in foods . Food Addit Contam , 9 : 797 – 803 .
- Maleki , S , Chung , S-Y , Champagne , ET and Raufman , J-P . 2000 . The effects of roasting on the allergic properties of peanut proteins . J Allergy Clin Immunol , 106 : 763 – 768 .
- Maleki SJ , Viquez O , Jacks T , Dodo H , Champagne ET , Chung SY , Landry SJ . 2003 . The major peanut allergen, Ara h2, functions as a trypsin inhibitor and roasting enhances this function . J Allergy Clin Immunol 112 : 130 – 135 .
- Mills , ENC , Potts , A , Plumb , GW , Lambert , N and Morgan , MRA . 1997 . Development of a rapid dipstick immunoassay for the detection of peanut contamination in food . Food Agric Immunol , 9 : 37 – 50 .
- Newsome , WH and Abbott , M . 1999 . An immunoaffinity column for the determination of peanut proteins in chocolate . J AOAC Int , 82 : 666 – 668 .
- Ortolani , C , Ispano , M , Scibilia , J and Pastorello , EA . 2001 . Introducing chemists to food allergy . Allergy , 56 ( Suppl. 67 ) : 5 – 8 .
- Pomes , A , Helm , RM , Bannon , GA , Burks , AW , Tsay , A and Chapman , MD . 2003 . Monitoring peanut allergen in food products by measuring Ara h . J Allergy Clin Immunol , 111 : 640 – 645 .
- Poms , RE , Klein , CL and Anklam , E . 2004 . Methods for allergen analysis in food: A review . Food Addit Contam , 21 : 1 – 31 .
- Porstmann , B , Porstmann , T , Gaede , D , Nugel , E and Egger , E . 1981 . Temperature dependent rise in activity of horseradish peroxidase caused by non-ionic detergents and its use in enzyme-immunoassay . Clinica Chimica Acta , 109 : 175 – 181 .
- Sampson , HA . 1999 . Food allergy. Part 1: Immunopathogenesis and clincial disorders . J Allergy Clin Immunol , 103 : 717 – 728 .
- Saunders , GC . 1979 . The art of solid-phase enzyme immunoassay including selected protocols. Immunoassays in the clinical laboratory , 99 – 118 . New York : Wiley-Liss Inc .
- Schacterle , GR and Pollack , RL . 1973 . A simplified method for the quantitative assay of small amounts of proteins in biological material . Analyt Biochem , 51 : 654 – 655 .
- Sicherer , SH , Munhoz-Furlong , A , Murphy , R , Wood , RA and Sampson , HA . 2003 . Symposium: Pediatric food allergy . Pediatrics , 111 : 1591 – 1594 .
- Tariq , SM , Stevens , M , Matthews , S , Ridout , S , Twiselton , R and Hide , DW . 1996 . Cohort study of peanut and tree nut sensitisation by age of 4 years . BMJ , 315 : 514 – 517 .
- Taylor , S and Hefle , SL . 2001 . Food allergies and other food sensitivities . Food Technol , 55 : 68 – 79 .
- Taylor , S and Lehrer , SB . 1996 . Principles and characteristics of food allergens . Crit Rev Food Sci Nutrition , 36 : 91 – 118 .
- Wensing , M , Penninks , AH , Hefle , SL , Koppelman , SJ , Bruinzeel-Koomen , CA and Knulst , AC . 2002 . The distribution of individual threshold doses eliciting allergic reactions in a population with peanut allergy . J Allergy Clin Immunol , 110 : 915 – 920 .
- Yeung , JM and Collins , PG . 1996 . Enzyme immunoassay for determination of peanut proteins in food products . J AOAC Int , 79 : 1411 – 1416 .