Abstract
Propolis contains a variety of polyphenolic compounds. We investigated the effect of a water-soluble derivatives of propolis (WSDP) and polyphenolic compounds, components of propolis, on growth of Ehrlich ascites tumour (EAT) in mice. Tumour in peritoneal cavity was produced by 2×106 EAT cells. WSDP and polyphenolic compounds (caffeic acid-CA, caffeic acid phenethyl ester-CAPE and quercetin-QU) were given to mice perorally (po). It was found that the volume of ascitic fluid induced by EAT cells and total number of cells present in the peritoneal cavity was markedly reduced in EAT-bearing mice treated with test components and the survival time of treated mice was prolonged. Inhibition of EAT growth was due to their effect on the immune system of mice. When innate and acquired immune responses were evaluated, a dose-related increase of cytotoxic T-cell, NK and B cells activity was observed in test components-treated mice. Furthermore, exposure of animals to test components increased functional activity of macrophages to produce factors regulating the function of B-, T-, and NK- cells respectively. In conclusion, these findings imply that the antitumour activity of WSDP and polyphenolic compounds of propolis enhanced host resistance in the EAT tumour model, increasing the activities of macrophages, cytotoxic T cells, B cells and NK cells.
Introduction
Polyphenolic compounds are widely distributed in the plant kingdom and display a variety of biological activities, including chemoprevention and growth inhibition of tumours. Chemoprevention is one of the most promising new approaches in cancer research, i.e. the administration of agents to inhibit, delay or reverse the process of carcinogenesis or inhibit proliferation of tumour cell (Heo et al. Citation2001). Compounds classified as blocking agents can prevent, or greatly reduce, the initiation of carcinogenesis, while suppressing agents affect the later stages of the process by reducing cell proliferation. Dietary flavonoids are known to affect proliferation, differentiation, and apoptosis in cancer cells and may play an important role in cancer chemoprevention, especially for cancers of the gastrointestinal tract, because of their direct contact with food (Kuo Citation1996). Many plants have shown various biological activities like immunopotentiating and antitumour activities. Polyphenolic forms, a major part of the dietary antioxidant capacity of fruit and vegetables, honey and propolis, have been identified as chemopreventive agents for major human cancer. Honeybee propolis and its components (caffeic acid, caffeic acid phenethyl ester, artepilin C, quercetin, naringenin, resveratrol, galangin, genistein and other) are of the most promising antitumour agent (Marcucci Citation1995, Galati et al. Citation2000, Femia et al. Citation2001, Luo et al. Citation2001, Kimoto et al. Citation2001, Akao et al. Citation2003, Oršolic et al. Citation2001 Citation2003).
Propolis (bee glue) is the generic name for the resinous substance collected by honeybees from various plant sources and used by bees to seal holes in their honeycombs, smooth out the internal walls, and protect the entrance against intruders (Ghisalberti Citation1979). Propolis is alleged to exhibit a broad spectrum of activities including antibiotic (Grange and Davey Citation1990), anti-inflamation (Wang et al. Citation1993), antioxidant (Scheller et al. Citation1990, Galati et al. Citation2000, Femia et al. Citation2001), anti-viral and tumour cell arrest (Scheller et al. Citation1989, Kawabe et al. Citation2000, Kimoto et al. Citation2001). Recently, several striking reports on immunomodulatory activities of aqueous extracts of propolis have been published (Dimov et al. Citation1992, Orsi et al. Citation2000, Kobayashi et al. Citation2001, Oršolić and Bašic Citation2003). Dimov et al. (Citation1992) showed that a water-soluble extract of propolis (WSDP) increased the protection from gram-negative infections probably via macrophage activation.
According to the literature it seems clear that antibiotic activities, immune modulatory properties as well as anti-inflammatory, wound healing and antitumour effects may be exhibited due to different components of ethanolic or aqueous extracts of propolis. Altogether, at least 200 constituents of propolis are defined as terpenes, various phenylpropane derivatives such as caffeic acid esters, flavonoids, amino acids or various aldehydes and ketones (Walker and Crane Citation1987, Greenaway et al. Citation1991, Bankova et al. Citation1994, Marcucci Citation1995).
Caffeic acid phenethyl ester (CAPE) exhibits differential toxicity to cancer cells versus normal cells (Su et al. Citation1995, Frenkel et al. Citation1993). CAPE was reported to be a lipoxygenase inhibitor with antioxidant properties (SudLina et al. Citation1993). Inhibition of the tumour promoter-mediated oxidative processes by the compound has also been reported in the culture of HeLa cells (Bhimani et al. Citation1993). Recent studies also demonstrate that CAPE and several other caffeic acid esters inhibit azoxymethane-induced colonic preneoplastic lesions and enzyme activities, including ornithine decarboxylase, tyrosine kinase and lipoxygenase, associated with colon carcinogenesis (Rao et al. Citation1992 Citation1995, Frenkel et al. Citation1993). It was also demonstrated that quercetin exhibited a powerful growth-inhibitory activity on tumour cell line (Piantelli et al. Citation1995).
Considering the importance of both innate immunity and acquired immunity in antitumour action(s), it was hypothesized that WSDP by modulating immune reaction in mice influenced host resistance to tumour growth. To delineate the effect of WSDP and polyphenolic compounds of propolis on differential immune functions in vivo, test compounds were administered to mice by gavage and a series of assays related to the cellular or humoral components of the immune reactions were performed and evaluation of their activity on host resistance to tumour cells was detected.
Materials and methods
Mice
Animal studies were carried out according to the guidelines in force in R. Croatia (Law on the Welfare of Animals, N. N. # 19, 1999) and in compliance to the Guide for the Care and Use of Laboratory Animals, DHHS Publ. # (NIH) 86–123. Male and female CBA and Swiss albino inbred mice from our conventional mouse colony were used. In any experiment, mice were of the same sex and were approximately three months old at the initiation of each study.
The animals were kept not more then five to a cage and were maintained on a standard diet (4 RF 21, Mucedola s.r.l., Italy) and given water ad libitum. Experimental groups were composed of 5–13 mice each.
Tumours
Tumours used were a spontaneous mammary carcinoma (MCa) of CBA mice, and a rapidly growing Ehrlich ascites tumour (EAT) of Swiss albino mice. The MCa tumour has been maintained by serial passages in syngeneic mice at 12- or 14-day intervals in a solid form. Tumour is weakly immunogenic for syngeneic recipients as shown by different methods in vivo (Bašic and Varga Citation1979).
Single cell suspension from MCa was prepared as described by Oršolić and Bašic (Citation2003) and inoculated intravenously to mice to produce tumour nodules (metastases). Cells of EAT were used and maintained in male Swiss albino mice through serial intraperitoneal inoculation at seven-day intervals in an ascites form. EAT is immunogenic for syngeneic recipients (Saha and Mondal Citation2000).
EAT cell suspension
After harvesting from peritoneal cavity the viability of cells was determined by trypan blue dye assay and it was more than 90%. The desired concentration of tumour cells (2×106 cells per 0.5 ml) was injected to mice ip.
Water-soluble derivative of propolis (WSDP) treatment
A water-soluble derivative of propolis (WSDP) was prepared by the method described previously (Nikolov et al. Citation1987). Briefly, propolis collected in the hilly area near Zagreb, Croatia, was extracted with 96% ethanol, which was filtered and evaporated to dryness in a vacuum evaporator. The resultant resinous product was added to a stirred solution of 8% L-lysine (Sigma Chemie, Deisenhofen, Germany) and freeze-dried to yield WSDP, a yellow-brown powder. WSDP was stored under sterile conditions at 4°C. Before use WSDP was dissolved in distilled water and given per os (po) to mice at a dose of 50 mg kg−1. Recent analyses by high performance liquid chromatography (HPLC) from our laboratory have shown that our preparations of WSDP speciments from Croatian propolis contained m/V total polyphenols 14.78% (caffeic acid 2.02%, naringenin 2.41%, chrysin 2.45%, pinocembrin 3.06%, galangin 2.12%).
Polyphenolic compounds
Caffeic acid (CA) – 3, 4-dihydroxycinnamic acid was purchased from Aldrich–chemie (Milwaukee, WI, USA). Ca was dissolved in ethanol and further dilutions were made in water. The final concentration of ethanol was less than 0.1% or equal to it and given per os (po) to mice at a dose of 50 mg kg−1.
Quercetin dihydrate (QU) was purchased from Fluka, BioChemica, Switzerland. QU was dissolved in ethanol and further dilutions were made in water. The final concentration of ethanol was less than 0.1% or equal to it and given per os (po) to mice at doses of 50 mg kg−1.
Caffeic acid phenethyl ester (CAPE) was synthesized by esterification of caffeic acid with phenethyl alcohol (molar ratios 1:15) in benzene (refluxing, 3–4 days, water removed by dean-stark trap). Following work-up, excess phenethyl alcohol was removed by Kugelröhr distillation (60°C,(0.1 mm Hg) to give pure CAPE, mp 126–128°C, needles (benzene or H2O), 40% yield. It was shown that properties of natural and synthetic CAPE were identical (Grunberger et al. Citation1988). CAPE was dissolved in ethanol and further dilutions were made in water. The final concentration of ethanol was less than 0.1% or equal to it and given per os (po) to mice at dose of 50 mg kg−1.
Count of the total number of cells present in the peritoneal cavity
Four groups comprising 27 mice were injected ip with 2×106 tumour cells/mouse. The test components were given daily for 7 days starting 2 h prior tumour inoculation, and the daily dose contained 50 mg kg−1 body weight. Seven mice of each experimental group were killed on day 7 after inoculation of EAT cells to determine NK activity of splenocytes. On day 14 after tumour cell inoculation seven mice were killed and 3 ml of saline were inoculated into the abdominal cavity and after gentle agitation of abdominal well, the solution containing peritoneal cells was removed. Total number of cells, differential count of the cells present in the peritoneal cavity, and macrophage spreading were determined. The remaining animals were used for the survival analysis.
Macrophage spreading assay
Functional activity of macrophages in the peritoneal cavity was determined by the spreading technique adapted from Rabinovitch et al. (Citation1977). Thus, 103 cells in 0.1 ml of the cellular suspension obtained in the peritoneal cavity was placed over glass cover slips at room temperature for 15 min. The non-adherent cells were removed by washing with phosphate buffer saline, and the adherent cells were incubated in culture medium 199 containing 10 nM HEPES at 37°C for 1 h. Following this, the culture medium was removed and the cells were fixed with 2.5% glutaraldehyde. Then, the cells were stained with a 5% solution of Giemsa and examined under microscopy where the percentage of spread cells were determined under 40×magnification. Spread cells were those that presented cytoplasmic elongation, while the non-spread cells were rounded (Rabinovitch et al. Citation1977).
Macrophage – HeLa – cells co culture
At the 5th, 10th, 15th and 20th day after treatment with WSDP, CA, or CAPE (50 mg kg−1, once a day, for 3 consecutive days), peritoneal macrophage were collected by a single washing of peritoneal cavity with 5 ml of tissue medium 199 supplemented with 100 µg/ml streptomycin and 100 U/ml penicillin (Flow Lab, UK). Cell suspensions at concentrations of 1×106/ ml were plated into 35 mm wells (Falcon, UK). After culturing for 24 h at 37°C in 5% CO2, macrophage supernatants were collected and stored at −20°C, for measurement of nitric-oxide. Macrophage monolayers were than washed twice with Medium 199 (Imunološki zavod, Zagreb) and 5×104/ wells tumour cells were sided for 24 h. After 6 h, 2.5 µCi [3H] TdR (spec. activity 5 Ci/mM; Sigma, Germany) was added to each well for a period lasting 12–18 h. Cells were collected by trypsinization and the radioactivity determined in a β-scintillation counter (LKB Wallac, Sweden).
NO2 − and NO3 − determination
NO2 − concentrations in the cell-free macrophage supernatants were measured after the conversion of NO3 − to NO2 − by the enzyme nitrate reductase as an indicator of NO production by colorimetric Griess reaction as described by Stuehr and Nathan (Citation1989). Briefly, 50 µl sample aliquots, diluted if needed, were mixed with an equal volume of Griess reagent (1% sulphanilamide) 0.1% naphthylethylene diamine didyprochloride (2% H3PO4) and incubated at room temperature for 10 min. The absorbency at 550 nm was measured in microplate reader (Bio-Tek Instruments, Inc., Burlington, VT, USA). NO2 − was determined using NaNO2 as a standard and double-distilled H2O as a blank. Background NO2 − values of buffers or media were determined in each case and subtracted from the experimental values.
Natural killer (NK) cell activity
The analysis of NK activity of non-adherent spleen cells was performed according to Sato et al. (Citation1992) with few alterations. Excised spleens were pressed on a nylon mesh and converted into a single-cell suspension in RPMI 1640. Cells were layered on a Ficoll-Hypaque gradient (d = 0 1.089 g/ml) and centrifuged. Mononuclear cells thus obtained were washed twice with RPMI 1640 medium supplemented with gentamycin (40 µg/kg), 20 mM Hepes (Sigma Chemical Co.), 2×10−5 M 2-mercaptoethanol (Sigma Chemical Co.), 0.2% sodium bicarbonate, and 10% heat inactivated foetal calf serum. Mononuclear cells resuspended in this complete medium were incubated in a glass Petri dish for 1 h at 37°C to remove adherent cells. Non-adherent cells were collected, washed twice with complete medium, and used as effector cells. These cells were assayed for natural killer activity against murine lymphoma (Yac-1 cells), previous labelled with 51Cr (100 µCi) as sodium chromate. Using ‘U’ bottomed 96-well microplates, the cytotoxic activity was analysed, yielding effector: target (E:T) ratios of 50: 1, 25:1 and 6.25:1 in triplicate. Plates were incubated for 4 h at 37°C under 5% CO2 tension and 100 µl of supernatant of each well was collected for 51Cr-release measurement. Maximum and spontaneous 51Cr-release were determined by incubating tumour cells with detergent (Triton ×-100, diluted 1:2) or medium alone, respectively. Radioactivity was determined in gamma counter (Gamma Nuclear, Hungary) and cytotoxicity was calculated by the formula:% specific lysis=[(cpm of experimental release − cpm of spontaneous release)/(cpm of maximum release − cpm spontaneous release)]×100.
Plaque-forming cells (PFC) assay
A haemolytic assay for the assessment of antibody-producing cells among splenocytes from either normal or the WSDP treated mice (50 mg kg−1) was performed as described by Jerne and Nordin (Citation1963). The suspension of splenocytes (0.025 ml) was mixed with 0.5 ml of agar and 0.5 ml of sheep red blood cells (SRBC), and placed onto agar on a glass slide. After 1 h incubation at 37°C the complement was added, and slides were additionally incubated for 2 h at 37°C. Then the complement was removed by vacuum pump and the PFC were counted on at least three slides per group under the light microscope.
Serum protein determination
Blood samples were obtained from mice by puncture of the axillary vein. Total serum protein levels were determined by the Biuret method (Kenneth and Chavin Citation1975) using a spectrophotometer (UV-160, Shimadzu; Japan) at a wavelength of 546 nm. Estimates of total serum immunoglobulins were performed by semimicroelectrophoresis on gelatinized cellulose acetate strips (Cellogel™, Chemetron, Milano, Italy) as detailed elsewhere (Stato and Kasai Citation1965, Kohn Citation1968).
Lymphocyte phenotyping
Spleen lymphocytes were obtained from spleens mechanically broken up in phosphate buffered saline, Ca2 + and Mg2 + free (PBS). Cell suspensions were filtered through a double layer of sterile gauze, then on a double layer column (wet with PBS) and centrifuged at 400 g at 4°C for 5 min. The pellet was washed and the recovered cells layered on a Ficol-Histopaque gradient (Sigma Chemical Co.) and centrifuged at 700 g for 20 min at 18°C. The ring at the interface was washed three times and the resulting pellet diluted in PBS containing 0.5% BSA and 0.1% NaN3. Labelling with anti-mouse monoclonal antibodies was made in the dark, in ice and lasted 30 min. To the aliquots containing 106 viable cells, anti-mouse mAb, CD4 (0.5 µg) or CD8 (0.1 µg) (Pharmingen, San Diego, CA, USA) were added. Controls were prepared with 106 cells labelled with non-specific IgG2a, k FITC (0.5 µg) or IgG2b, k PE (0.1 µg); a sample containing only cells was used for auto-fluorescence determination. After washing, cells were resuspended in 0.5 ml of medium added with 10% of a solution of 35% formaldehyde. Each analysis consisted of 10,000 events counted.
Statistics
Results are expressed as means±SE obtained from two or three experiments. We tested all data for the assumptions required for parametric testing. Since data did not meet the assumptions, we used Kruskal-Wallis ANOVA test. If the results showed that H 0 can be rejected, we tested the pairs of data with the test for multiple comparisons between the treatments (Siegel and Castellan Citation1988). The level of significance was set to ≥95%. Treatment-dose specific survival curves were calculated according to Kaplan-Meier method, and log rank test was used to compare survival curves using statistical software STATA 7.0 (Stata Press, College station, Texas, USA).
Results
Polyphenolic components from propolis inhibited tumour growth and increased survival time of mice
The effect of WSDP, CA, or qu on the volume of ascitic fluid, total number of cells in peritoneal cavity and the survival time of mice-bearing EAT were studied in mice injected ip with tumour cells. The test components were given po daily for 7 days starting 2 h prior tumour inoculation, and the daily dose contained 50 mg kg−1 body weight.
The volume of ascitic fluid and the total number of EAT cells were significantly reduced in mice protected with WSDP or polyphenolic compounds of propolis (see and ). WSDP was the most potent, inhibiting proliferation of EAT cell for 79%. The effect of test components on the survival rate of mice-bearing EAT cells is shown in . The results demonstrate that WSDP and CA significantly prolonged the lifespan of mice (p=0.0002 or p=0.0068, log rank test) as compared to control. QU, however, was ineffective in this respect (p=0.384).
Figure 1. The Kaplan-Meier survival curves for Swiss albino mice treated with WSDP (A), caffeic acid (B) and quercetin (C). The test components were given po daily for 7 days starting 2 h prior tumour cell inoculation, and the daily dose contained 50 mg kg−1 body weight. Tumour cells (2×106) were injected to mice intraperitoneally; group comprises 10–13 mice each. The results of log rank test show that test components significantly influenced onset of EAT tumour in the mouse (A, p=0.0002; B, p=0.0068). Quercetin (C) did not influenced onset of EAT tumour in the mouse, p=0.384.
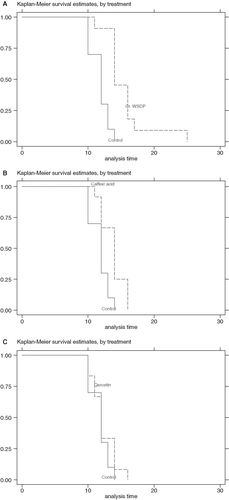
Table I. Number of cells in peritoneal cavity and volume of ascitic fluid in mice-bearing Ehrlich ascites tumour after peroral treatment with WSDP and polyphenolic compounds of propolis.
Macrophage spreading
The functional activity of macrophages was measured by determining the percentage of spreading of macrophages harvested in the peritoneal cavity. The results obtained for the percentage of spreading of macrophages harvested in peritoneal cavity are shown in . The treatment with test components yielded an increase in the functional activity of macrophages in the mice-bearing EAT compared with the control group. It should also be pointed out that in the mice treated with test components there was an increase in the median values of spreading percentage accompanied with an increase cytotoxicity to EAT cells.
Table II. The percentage of macrophages spreading in the peritoneal cavity of the animals on the 14th day of Ehrlich ascites tumour growth after perorally treatment with WSDP and polyphenolic compoundsa of propolis.
Macrophage cytotoxicity and production of NO
Studies revealed that peritoneal macrophages of mice treated with 50 mg kg−1 of WSDP when co-cultured with HeLa cells produced significantly higher amounts of NO (p<0.05) 10 and 15 days after treatment (see ). At the same time the percentage of 3H-TdR incorporation into tumour cells was lower than that in control (). Per os treatment with CAPE elevated the production of NO at days 5 and 10 after treatment, which diminished 15 days following treatment. Incorporation of 3H-TdR into HeLa cells was strongly suppressed at time of elevation of NO production. On the other hand however, in mice treated with CA a significant (p<0.01) drop in production of NO was found throughout the observation period. In contrast, peritoneal macrophages from mice treated with CA expressed very strong cytotoxicity to HeLa cells as compared to control ().
Table III. Incorporation of 3H-TdR into HeLa cells in activated macrophage/HeLa cocultures and NO production by macrophage from untreated and WSDP-, caffeic acid- or CAPE- treated mice.
NK activity
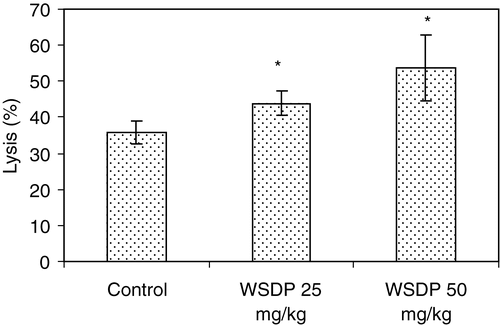
NK cells are an important innate immune mechanism to control tumour growth. We observed that WSDP treatment for 7 days induced an increase in the cytotoxic activity of non-adherent spleen cells as shown in ; results detected at 50:1 (E:T) ratio demonstrated that treatment with WSDP significantly increased NK activity (p<0.05).
Figure 2. Effect of WSDP on natural killer (NK) cell activity in Swiss albino mice. WSDP (25 or 50 mg kg−1) was given po daily for 7 days (last treatment was performed just before sacrifice). Results are expressed as mean (±SE) of percentages of specific lysis. Effector: target ratio = 50:1. Groups comprise 7 mice each. Significantly (p* < 0.05) different from untreated controls.
Cytotoxic T lymphocyte activity
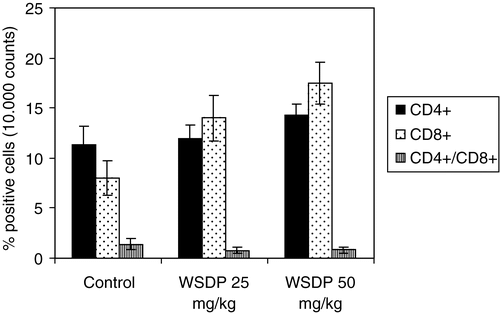
CD8+ T cells play a central role in protection against tumour metastasis and tumour growth. In the study of influence of WSDP on T lymphocyte population, clustering of splenocytes was made by a function of the percentage of cells showing CD4+ and CD8+ antigens. The study performed on mice injected iv with MCa tumour cells and treated with WSDP (25 or 50 mg kg−1 body weight, respectively) on days 2, 7 and 12 after injection of tumour cells showed that WSDP significantly increased the percentage of CD8+ cells, although to a lesser extent, that of CD4+ cells as compared to untreated tumour-bearing mice (see ). However, treatment of tumour-bearing mice with WSDP caused a reduction in the ratio between CD4+ and CD8+ lymphocytes; 1.4±0.5 in control compared to 0.8±0.3 in both groups of the WSDP treated mice, respectively. The elevation of both CD4+ and CD8+ T-cell subsets in tumour-bearing mice after treatment with WSDP showed a dose dependent stimulation by WSDP that lead to progressive reduction of the immuno-index (CD4+/CD8+ ratio) in favour of CD8+ cells.
B lymphocyte activity
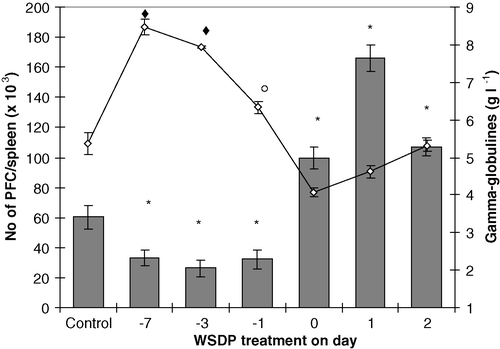
To study the effect of WSDP on the spleen plaque-forming ability, treated mice were immunized with SRBC (4×108, intraperitoneally) at different intervals before or after treatment with WSDP. Four days after immunization the ability of their splenocytes to produce antibodies (plaque-forming cells-PFC) to SRBC was determined. shows that treatment with WSDP did not affect production of PFC when given 7, 3, or 1 day before antigen of SRBC. However, when SRBC were injected into mice at the time of injection of WSDP and 24 or 48 h before WSDP respectively, a significant elevation of PFC was found; the most pronounced effect was achieved in the group of mice receiving SRBC 24 h before WSDP treatment. It is likely that the dose of WSDP plays an important role in PFC formation. When different doses of WSDP were given to mice within 24 h (at 8 h intervals) after injection of SRBC a significant elevation of PFC production was found only in mice receiving 75 mg kg−1 of WSDP (data not shown). It is shown that concentrations of γ-globulins in the serum of mice receiving WSDP (50 mg kg−1 iv) before or after immunization with antigen were different than that in untreated control; especially the levels of γ-globulins. The levels of γ-globulins in WSDP-treated mice were increased (p<0.05) when mice were given WSDP before immunization with antigen; γ-globulin levels were higher when the time between WSDP treatment and antigen administration was longer; treatment with WSDP at the time of antigen injection resulted in the lowest level of γ-globulins.
Figure 4. Mean (±SE) numbers of splenic PFC and serum γ-globulins in CBA mice injected iv (50 mg kg−1) with WSDP 7, 3, 1 days before, simultaneously or 1, 2 days after immunization with 4×108 SRBC in 0.5 ml of medium RPMI 1640. Animals were killed 4 days after immunization with SRBC. Values are mean (±SE) from 12–15 samples (5 mice in triplicate). Significantly (p ♦*○<0.05) different from untreated controls. Significantly (p ♦<0.05) different from treatment with WSDP 1 day before, simultaneously, 1 or 2 days after immunization with 4×108 SRBC. Significantly (p ○<0.05) different from treatment with WSDP simultaneously, 1 or 2 days after immunization with 4×108 SRBC.
Discussion
Activated macrophages were shown to be a major component of host defence against neoplastic growth in experimental tumour systems (Bašic et al. Citation1998, Oršolić and Bašic Citation2003). The purpose of this study was to examine the effects of WSDP and polyphenolic compounds of propolis on macrophage activation and their effect on other immunological effectors responsible for the destruction of tumour cells. The analysis of the total number of cells present in the peritoneal cavity revealed that all the experimental groups inoculated with tumour cells in the presence of WSDP or polyphenolic compounds of propolis exhibited significantly lower number of cells in peritoneal cavity than that in tumour control group. Moreover, the survival rates of EAT-bearing mice were increased after treatment with test components (see ). It was shown that animals treated with the immunostimulants resist, in various degrees, subsequent inoculation of tumour cells as evidenced by the reduced “tumour take”, slowed growth of the tumours, and prolonged survival of recipients (Scheller et al. Citation1989, Matsuno Citation1995, Kimoto et al. Citation1998, Hayashi et al. Citation2000). This paper presents data showing that macrophages activation by WSDP and related polyphenolic compounds of propolis is likely to be the most important effector mechanism of antitumour activity of test compounds in vivo. These results suggest that test components might interfere with the growth of EAT cells by activation of macrophages.
Considering the other possible mode of antitumour effect of WSDP we already discussed that antitumour activity, at least in part, was due to stimulation of the immune system (Bašić et al. Citation1998). In addition, these results indicate that treatment of tumour-bearing mice with WSDP caused a reduction in the ratio between CD4+ and CD8+ lymphocytes; 1.4±0.5 in control compared to 0.8±0.3 in both groups of the WSDP treated mice, respectively. The elevation of both CD4+ and CD8+ T-cell subsets in tumour-bearing mice after treatment with WSDP showed a dose dependent stimulation by WSDP that lead to progressive reduction of the immuno-index (CD4+/CD8+ ratio) in favour of CD8+ cells. These results are consistent with the observations by Kimoto et al. (Citation1998) who reported that the artepilin C from Brazilian propolis suppressed the tumour growth after intratumour injection of 500 mg; however, results showed that increasing the ratio of CD4/CD8 was favourable to CD4 cells.
Our results suggest that the antitumour activities of WSDP were, at least in part, the consequence of immunomodulation of the host responses and interaction of immunomodulated effectors with tumour cells (Oršolic et al. Citation2003 Citation2004). It is, therefore, possible that increased lymphocyte proliferation leads to enhanced macrophage activation and thus to an amplification of the general immunological responses. The interaction of T cells with macrophages results in production of several cytokines including IL-1, interferon-γ (IFN-γ) and tumour necrosis factor α (TNF-α) that have been implicated in host resistance to tumour cells and are known to play a role in macrophage activation. Furthermore, cytokines might lead to induction of NO synthase in macrophages which may contribute to the direct and indirect cytotoxic effect to tumour cells. It is known that cytotoxicity of NO is mainly mediated by intracellular iron loss, inhibition of mitochondrial respiration, inhibition of aconitase and of ribonucleotide reductase, inhibition of DNA synthesis which induces cytostasis in tumour cells (Stuehr and Nathan Citation1989, MacMicking et al. Citation1997).
The number of IgM PFC of spleen was significantly increased in WSDP-treated mice when SRBC were injected into mice at the time of injection of WSDP and 24 or 48 h before WSDP respectively; the most pronounced effect was achieved in the group of mice receiving SRBC 24 h before WSDP treatment. This data suggests an adjuvant effect of the WSDP. Although PFC is an endpoint to evaluate the humoral immune response, the response to SRBC requires the cooperation of a number of cell populations, including B cells, T helper cells, and macrophages. Findings from these experiments confirm that WSDP can strongly activate the processes included in production of antibodies. These findings, however, confirm that a dose of WSDP is an important factor for the activation of the mechanisms involved in antibody production as well as the time intervals between antigen introduction and treatment with WSDP. WSDP increased, in a time-dependent manner, the levels of γ-globulins in treated mice when mice were given WSDP before immunization with antigen; γ-globulin levels were higher when time between WSDP and antigen injection were longer. Treatment with WSDP at the time of antigen injection resulted in the lowest level of γ-globulins.
Results on macrophage spreading revealed that the treatment with test components affects the functional state of macrophages. In spite of the non-occurrence of an increase in the number of MN cells in the peritoneal cavity of treated mice (data not shown), a significant increase was observed in the activity of peritoneal macrophages in test component-treated groups. The increase of macrophage activity might have been responsible for the slower growth of tumour cells. It is well known that MN cells, mainly macrophages, are the major cells involved in tumour destruction (Oršolic and Bašic Citation2003, Kimoto et al. 1988). Since the strongest antitumour effect was achieved by WSDP it is likely that the antitumour activity of WSDP is the result of synergistic activities of polyphenolic compounds present in WSDP. Stimulation of macrophages might induce production and release of several cytokines such as IL-1, IL-6, IL-8, TNF-α (Dimov et al. Citation1991 Citation1992, Oršolic and Bašic Citation2003) and NO. Some of these cytokines may express direct cytotoxic effect on tumour cells while others act on NK cells and cytotoxic T lymphocytes. In addition, these cytokines might stimulate production of antibody, C-reactive protein and complement factor C3 that could act as opsonins to tumour cells (Bellelli and Sezzi Citation1976) and as such activate antibody-dependent cellular toxicity. The combination of these effects might impede tumour growth and lead to elimination of tumour cells. The other possible mechanisms of antitumour influence of test components, as we described previously, include immunomodulatory activity of these products (Oršolic and Bašic Citation2003, Oršolić et al. Citation2003) their cytotoxic activity to tumour cells (Oršolic et al. Citation2004) their capability of inducing changes in the cellular level of glutathione (Oršolic et al. Citation2004) and their capability to induce apoptosis and/or necrosis (Oršolic et al. Citation2003 Citation2004). It is likely that WSDP and its polyphenolic compounds can trigger various host defence mechanisms. Destruction of tumour cells observed with test components may be the result of one or more mechanisms described above.
References
- Akao , Y , Maruyama , H , Matsumoto , K , Ohguchi , K , Nishizawa , K , Sakamoto , T , Araki , Y , Mishima , S and Nozawa , Y . 2003 . Cell growth inhibitory effect of cinnamic acid derivatives from propolis on human tumour cell lines . Biologic Pharmaceut Bull , 26 : 1057 – 1059 .
- Bankova , V , Christov , R and Popov , S . 1994 . Volatile constituents of propolis . Zeitschrift für Naturforschung , 49 : 6 – 10 .
- Bašic , I and Varga , E . 1979 . Immunogenicity of mammary carcinoma and a fibrosarcoma of CBA mice . Periodicum Biologorum , 81 : 335 – 337 .
- Bašic , I , Oršolić , N , Tadic , Z , Macedo Fereire Alcici , N , Brbot Saranovic , A , Bendelja , K , Krsnik , B and Rabatic , S . 1998 . Antimetastatic effect of propolis, caffeic acid phenethyl ester and caffeic acid on mammary carcinoma of CBA mouse. 17th International Cancer Congress, Rio de Janeiro . Brazil. , 1 : 63 – 75 .
- Bellelli , L and Sezzi , ML . 1976 . C3 participation in the rejection of some experimental tumours . Oncology , 33 : 215 – 218 .
- Bhimani , RS , Troll , W , Grunberger , D and Frenkel , K . 1993 . Inhibition of oxidative stress in HeLa cells by chemopreventive agents . Cancer Res , 53 : 4528 – 4533 .
- Dimov , V , Ivanovska , N , Bankova , V and Popov , S . 1992 . Immunomodulatory action of propolis. IV. Prophylactic activity against gram-negative infections and adjuvant effect of the water-soluble derivate . Vaccine , 10 : 817 – 823 .
- Dimov , V , Ivanovska , N , Manolova , N , Bankova , V , Nikolov , N and Popov , S . 1991 . Immunodulatory action of propolis. Influence on anti-infectious protection and macrophage function . Apidologie , 22 : 155 – 162 .
- Femia , AP , Caderni , G , Buzzigoli , C , Cocca , E , Salvadori , M and Dolara , P . 2001 . Effect of simple phenolic compounds on azoxymethane-induced aberrant crypt foci in rat colon . Nutrit Cancer , 41 : 107 – 110 .
- Frenkel , K , Wei , H , Bhimani , R , Ye , J , Zadunaisky , JA , Huang , MT , Ferraro , T , Conney , AH and Grunberger , D . 1993 . Inhibition of tumour promoter-mediated processes in mouse skin and bovine lens by caffeic acid phenethyl ester . Cancer Res , 53 : 1255 – 1261 .
- Galati , G , Teng , S , Moridani , MY , Chan , TS and O'Brien , PJ . 2000 . Cancer chemoprevention and apoptosis mechanisms induced by dietary polyphenolics . Drug Metabol Drug Interact , 17 : 311 – 349 .
- Ghisalberti , E . 1979 . Propolis: A review . Bee World , 60 : 59 – 84 .
- Grange , JM and Davey , RW . 1990 . Antibacterial properties of propolis (bee glue) . J Royal Soc Med , 83 : 159 – 160 .
- Greenaway , W , May , J , Scaysbrook , T and Whatley , FR . 1991 . Identification by gas chromatography-mass spectrometry of 150 compounds in propolis . Zeitschrift für Naturforschung , 46 : 111 – 121 .
- Grunberger , D , Banerjee , R , Eisinger , K , Oltz , EH , Efros , L , Caldwell , M , Estevez , V and Nakanishi , K . 1988 . Preferential cytotoxicity on tumour cells of caffeic acid phenethyl ester isolated from propolis . Experientia , 44 : 230 – 232 .
- Hayashi , A , Gillen , AC and Lott , JR . 2000 . Effects of daily oral administration of quercetin chalcone and modified citrus pectin on implanted colon-25 tumour growth in Balb-c mice . Alt Med Rev , 5 : 546 – 552 .
- Heo , MY , Sohn , SJ and Au , WW . 2001 . Anti-genotoxicity of galangin as a cancer chemopreventive agent candidate . Mutat Res , 488 : 135 – 150 .
- Jerne , KN and Nordin , AA . 1963 . Plaque formation of single antibody-production cells . Science , 140 : 405 – 408 .
- Kawabe , M , Lin , C , Kimoto , N , Sano , M , Hirose , M and Shirai , T . 2000 . Modifying effects of propolis on MeIQx promotion of rat hepatocarcinogenesis and in a female rat two-stage carcinogenesis model after multiple carcinogen initiation . Nutrit Cancer , 37 : 179 – 186 .
- Kenneth , VH and Chavin , W . 1975 . An improved automated Biuret method for the determination of microgram protein concentration . Analyt Biochem , 68 : 230 – 235 .
- Kimoto , T , Arai , S , Kohguchi , M , Aga , M , Nomura , Y , Micallef , MJ , Kurimoto , M and Mito , K . 1998 . Apoptosis and suppression of tumour growth by artepillin C extracted from Brazilian propolis . Cancer Detect Prevent , 22 : 506 – 515 .
- Kimoto , T , Koya-Miyata , S , Hino , K , Micallef , MJ , Hanaya , T , Arai , S , Ikeda , M and Kurimoto , M . 2001 . Pulmonary carcinogenesis induced by ferric nitrilotriacetate in mice and protection from it by Brazilian propolis and artepillin C . Virchows Archiv , 438 : 259 – 270 .
- Kobayashi , N , Unten , S , Kakuta , H , Komatsu , N , Fujimaki , M , Satoh , K , Aratsu , C , Nakashima , H , Kikuchi , H , Ochiai , K and Sakagami , H . 2001 . Diverse biological activities of healthy foods . In Vivo , 15 : 17 – 23 .
- Kohn J . 1968 . In : Smith I , editor . Chromatographic and electrophoretic technique . Vol. II . London : William Heinemann Ltd . pp 84 – 146 .
- Kuo , SM . 1996 . Antiproliferative potency of structurally distinct dietary flavonoids on human colon cancer cells . Cancer Lett , 110 : 41 – 48 .
- Luo , J , Soh , JW , Xing , WQ , Mao , Y , Matsuno , T and Weinstein , IB . 2001 . PM-3, a benzo-gamma-pyran derivative isolated from propolis, inhibits growth of MCF-7 human breast cancer cells . Anticancer Res , 21 : 1665 – 1671 .
- MacMicking , J , Xie , Q-W and Nathan , C . 1997 . Nitric oxide and macrophage function . Ann Rev Immunol , 15 : 323 – 350 .
- Marcucci , MC . 1995 . Propolis: Chemical composition, biological properties and therapeutic activity . Apidologie , 26 : 83 – 99 .
- Matsuno , T . 1995 . A new clerodane diterpenoid isolated from propolis . Zeitschrift für Naturforschung Section , C50 : 93 – 97 .
- Nikolov , N , Marekov , N , Bankova , V , Popov , S , Ignatova , R and Vladimirova , I . 1987 . Method for the preparation of water-soluble derivative of propolis . Bulg J Patol Appl , 79903/28 : 05
- Orsi , RO , Funari , SRC , Soares , AMVC , Calvi , SA , Oliveira , SL , Sforcin , JM and Bankova , V . 2000 . Immunomodulatory action of propolis on macrophage activation . J Venom Animals Toxins , 6 : 1 – 11 .
- Oršolic , N , Bendelja , K , Brbot-Šaranovic , A and Bašic , I . 2004 . Effects of caffeic acid phenethyl ester and caffeic acid, antioxidants from propolis, on inducing apoptosis in HeLa human cervical carcinoma and Chinese hamster lung V79 fibroblast cells . Period Biolog , 106 : 367 – 372 .
- Oršolic , N , Macedo Fereire Alcici , N , Bendelja , K , Lojkic , M and Bašic , I . 2001 . Immunostimmulatory and antitumour activity of water-soluble derivative of propolis (WSDP) . Period Biolog , 103 : 255 – 261 .
- Oršolic , N , Šver , L , Terzic , S , Tadic , Z and Bašic , I . 2003 . Inhibitory effect of water-soluble derivative of propolis (WSDP) and its polyphenolic compounds on tumour growth and metastasing ability: A possible mode of antitumour action . Nutrit Cancer , 47 : 156 – 163 .
- Oršolic , N and Bašic , I . 2003 . Immunomodulation by water-soluble derivative of propolis (WSDP) a factor of antitumour reactivity . J Ethnopharmacol , 84 : 265 – 273 .
- Piantelli , M , Maggiano , N , Ricci , R , Larocca , LM , Cappelli , A , Scambia , G , Isola , G and Natali , P . 1995 . Type-II oestrogen-binding sites in human melanoma and growth-inhibitory effect of tamoxifen and quercetin . J Investig Dermatol , 105 : 248 – 253 .
- Rabinovitch , M , Manejias , RE , Russo , M and Abbey , EE . 1977 . Increased spreading of macrophages from mice treated with interferon inducers . Cell Immunol , 29 : 86 – 95 .
- Rao , CV , Desai , D , Kaul , B , Amin , S and Reddy , BS . 1992 . Effect of caffeic acid esters on carcinogen-induced mutagenicity and human colon adenocarcinoma cell growth . Chemico-Biolog Interact , 84 : 277 – 290 .
- Rao , CV , Desai , D , Rivenson , A , Simi , B , Amin , S and Reddy , SB . 1995 . Chemoprevention of colon carcinogenesis by phenylethyl-3-methylcaffeate . Cancer Res , 55 : 2310 – 2315 .
- Saha , S and Mondal , S . 2000 . Suppression of Ehrlich ascites tumour growth by immunization with ganglioside GT1b of its origin, its IgM antibody or anti-idiotype of the anti-GT1b IgM . Ind J Exp Biol , 38 : 1207 – 1216 .
- Sato , MN , Yamashiro-Kanashiro , EH , Tanji , MM , Kaneno , R , Higuchi , ML and Duarte , AJS . 1992 . CD8+ cells and natural cytotoxic activity among spleen, blood and heart lymphocytes during the acute phase of Trypanosoma cruzi infection in rats . Infection Immunity , 60 : 1024 – 1030 .
- Scheller , S , Krol , W , Swiacik , J , Owczarek , S , Gabrys , J and Shani , J . 1989 . Antitumour property of ethanolic extract of propolis in mice-bearing Ehrlich carcinoma, as compared to bleomycin . Zeitschrift für Naturforschung , 44C : 1063 – 1065 .
- Scheller , S , Wilczok , T , Imielski , S , Krol , W , Gabrys , J and Shani , J . 1990 . Free radical scavenging by ethanol extract of propolis . Int J Radiation Biol , 57 : 461 – 465 .
- Siegel , S and Castellan , NJ Jr . 1988 . Nonparametric statistics for the behavioural sciences , 2nd ed , New York : McGraw-Hill .
- Stato , K and Kasai , H . 1965 . Electrophoresis of serum proteins on gelatinized cellulose acetate . Int Med Biol , 70 : 195 – 198 .
- Stuehr , DJ and Nathan , C . 1989 . A macrophage product responsible for cytostasis and respiratory inhibition in tumour target cells . J Exp Med , 169 : 1543 – 1555 .
- Su , ZZ , Lin , J , Prewett , M , Goldstein , NI and Fisher , PB . 1995 . Apoptosis mediates the selective toxicity of caffeic acid phenethyl ester (CAPE) toward oncogene-transformed rat embryo fibroblast cells . Anticancer Res , 15 : 1841 – 1848 .
- SudLina , GF , Mirzoeva , OK , Pushkareva , MA , Korshunova , GA , Sumbatyan , NV and Varfolomeev , SD . 1993 . Caffeic acid phenethyl ester as a lipoxygenase inhibitor with antioxidant properties . FEBS Letts , 329 : 21 – 24 .
- Walker , P and Crane , E . 1987 . Constituents of propolis . Apidologie , 18 : 327 – 334 .
- Wang , L , Mineshita , S , Ga , I , Shigematsu , T and Matsuno , T . 1993 . Anti-inflammatory effect of propolis . Jap J Pharmacol Therap , 24 : 223 – 224 .