Abstract
In the present study, the immune effect of turtle shell extract on normal and cyclophosphamide (CP)-treated mice was determined. Mice treated with the turtle shell extract and CP had significantly (p<0.05) elevated levels of serum IgG, ATL, ATS and CREA compared with CP-treated mice. The turtle shell extract-treated mice had significantly elevated serum IgG (p<0.001), reduced serum CREA level (p<0.05), but the serum ATL, ATS and BUN did not change significantly compared with normal mice. Treatment with the turtle shell extract significantly improved the CD3+, CD4+CD8−, CD4−CD8+ and CD4+CD8+ cells percentages (p<0.05) in normal mice, and also significantly improved CD3+ and CD4+CD8− cells percentages in CP-treated mice (p<0.05). There was significant difference (p<0.001) in the proliferation rate of PHA-stimulated spleen lymphocyte in vitro between the turtle shell extract + CP-treated mice and CP-treated mice, and also between the turtle shell extract-treated mice and normal mice (p<0.05). These results demonstrate that the turtle shell extract can up-regulate the immune states of normal and CP-treated mice, and shows some protective effect on liver and kidney damage in CP-induced mice.
Introduction
Of all the body's systems, the immune system may be the most challenging to co-ordinate, as it is composed of a disparate, far-flung collection of individual immune cells, immune cell aggregates, immune tissues, and immune organs (Roitt et al. Citation1998). The balance of the immune system is important to organisms, which are involved in the aetiology as well as the pathophysiological mechanisms of many diseases. For many years, researchers have been interested in modulation of the immune response to alleviate diseases. Th1 lymphocytes promote cell-mediated immunity, and Th2 lymphocytes promote humoral antibody-mediated immune response (Carter et al. Citation1996; Stephens et al. Citation2002).
For many years in China, Chinese traditional medicines have been widely used to modulate body conditions and treat diseases, such as autoimmune diseases and cancers (Li Citation1991). A large number of plants and their isolated constituents have been shown to potentiate immunity, such as Astragali Radix extract (Kang et al. Citation2004) and Mangifera indica L. extract (García et al. Citation2003). Compared with plant-derived immunomodulating active material, less information is available documenting immunomodulating active material derived from animals.
Trachemys scripta elegans (Red-eared Turtle) has been widely raised and consumed in China as an important tonic material, and turtle shell is a traditional Chinese medicine. Turtle cell-free tissue extracts (Turdyev et al. Citation1985), splenic extract (Prus et al. Citation1994) and blood cell extract (Turdyev et al. Citation1998), have been shown to increase the survival rate of irradiated rats and mice, and have an immunostimulating effect. Oral administration of soft-shelled turtle powder can reduce fatigue, accelerate recovery from stress-induced deficient sexual behavior in mice (Feng et al. Citation1996a), and has anti-tumor effects (Feng et al. Citation1996b). Tortoise (Testudo graeca) shell extraction can kill a large amount of liver and colon carcinomas and the myosarcoma cells in G2, M and G0 phases (Bayazit Citation2004). But, to date, there is little understanding as to the mechanisms underlying the immunomodulating effect of turtle shell powder or its extract.
Cyclophosphamide (CP) mainly acts as a non-specific cytotoxic agent, and it is effective against a wide variety of cancers, such as breast cancer (Tada et al. Citation2006) and scleroderma lung disease (Martinez et al. Citation2006). However, its adverse side effects cause great damage to the body in CP-based chemotherapy, such as immunotoxicity, mutagenicity, and teratogenicity (Colvin et al. Citation1981). Therefore, many attempts have been made to control or minimize the CP toxic effects. For example, the combined application of Miltefosine with Epirubicine and CP decreased the destructive cytotoxic effects of CP on mouse spermatogenic and haematopoietic cells (Martinova et al. Citation2006). Captopril can reduce the genotoxicity of CP in bone marrow cells (Hosseinimehr et al. Citation2005). Lipoic acid can protect CP-induced oxidative injury of rat liver and testis (Selvakumar et al. Citation2004, Citation2005). Shi-Quan-Da-Bu-Tang, a traditional Chinese preparation, has been reported to be effective against toxicitities, such as anorexia, nausea, vomiting, haematotoxicity, immunosuppression, leucopoenia, etc., of CP in cancer patients (Zee-Chang Citation1992). But, it is not known whether turtle shell extract has protective effects on the function of the liver and kidney in CP-treated mice.
The objectives of the present study were to evaluate the immunomodulating effect of turtle shell extract in normal and CP-treated mice, and to examine its protective effect on the functions of the liver and kidney in CP-treated mice.
Materials and methods
Materials
Fluorescein isothiocyanate (FITC)-labeled CD3 anti-mouse monoclonal antibody, phycoerytherin (PE)-labeled CD4 anti-mouse monoclonal antibody, and allophycocyanin (APC)-labeled anti-mouse CD8 antibodies were purchased from eBioscience (USA). HRP-labeled goat anti-mouse IgG antibody and rabbit anti-mouse IgG antibody were from Bethyl (USA). Mouse IgG was purchased from Sigma (USA). CP was from Sigma-Aldrich (USA). MTT and PHA were purchased from Amersco (USA). Foetal bovine serum, l-glutamine, penicillin, streptomycin and RPMI 1640 were from Sangon Co. (Shanghai). Aspartate transaminase test kit, alanine transaminase test kit, blood urea nitrogen test kit and creatinine test kit were from the Shanghai Institute of Biological Products (China). All other chemicals and reagents used were of analytical reagent grade.
Animals
BALB/c mice (20–22 g), supplied by Medical Laboratory Animal Centre of Guangdong province (China), were used in this study. All mice were kept at the animal facilities under specific pathogen-free conditions until use. The mice were housed six per cage. Sterile food and water were supplied ad libitum. Animal welfare and experimental procedures were carried out strictly in accordance with the guidelines for the care and use of laboratory animals and the related ethical regulations in China.
Preparation of turtle shell extracts
Trachemys scripta elegans was obtained from a market and identified by a specialist. The turtle was sacrificed and, immediately, the shell was separated from the turtle tissues and dried at 80°C for 12 h. The turtle shell was used to make aqueous extract as described (Dias et al. Citation2001) with slight modifications. Briefly, the dried turtle shell (100 g) was extracted with cell culture grade water (800 ml) at 100°C for 5 h each time. After three times, the supernatants from each extraction were mixed and centrifuged at 2000×g, then the precipitation was discarded and the supernatant condensed to 100 ml with 7.6% of yields (W/W) and kept at −20°C. The turtle shell water extract solution was diluted to 440 mg/ml with sterile saline.
Dosage
To determine the immunomodulating activities of turtle shell extract in vivo, the mice were pre-treated by force-feeding through a stomach tube. CP was suspended in normal saline. CP and turtle shell extract were administrated orally via a stomach tube. Mice were divided into four groups, each group comprising a minimum of six animals. Normal mice (Group I) were administered normal saline (0.5 ml per day p.o.) for 30 days. CP-treated animals (Group II) were administered with normal saline (0.5 ml per day) for 29 days, and CP (0.5 ml, 50 mg/kg, per day) was given in a single dose on the 30th day. Turtle shell extract + CP-treated mice (Group III) were given turtle shell extract (0.5 ml, 220 mg/kg, per day) for 29 days, and a single injection of CP (0.5 ml, 50 mg/kg, per day) on the 30th day. Turtle shell extract-treated animals (Group IV) was administered turtle shell extract (0.5 ml, 220 mg/kg, per day) for 29 days, and normal saline (0.5 ml per day) was given in a single dose on the 30th day. The animals were sacrificed by cervical dislocation 24 h after the last dose.
Separation of spleen cell
The mouse spleen was removed aseptically and teased into a single cell suspension in RPMI 1640 supplemented with 10% foetal bovine serum, 2 mM l-glutamine, penicillin (100 U/ml), and streptomycin (100 µg/ml). Red blood cells were removed by lysis with 0.14 M Tris-buffered NH4Cl. The remaining cells were washed twice with culture medium. Finally, the cells were re-suspended in 5 ml RPMI 1640 medium and subjected to cell count.
Proliferation of mouse spleen lymphocytes
Spleen cells were cultured in a 96-well flat-bottomed culture plate at a density of 5×105 cells/well in RPMI 1640 medium (0.2 ml), and stimulated with a final concentration of 2.5 µg/ml PHA for 72 h at 37°C in 5% CO2/air. Then, cell growth was evaluated with modified 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sargent et al. Citation1989). Briefly, MTT (10 µl/well) in RPMI 1640 medium was added for a further incubation period of 4 h. Then, 150 µl supernatant was removed from each well and 150 µl dimethyl sulphoxide (DMSO) was added to dissolve the crystals. The absorbance was read on an ELISA reader (Sunrise Remote/Touch Screen, TECAN, Austria) at 550 nm, and the spleen lymphocyte proliferation rate was calculated using the following formula:
Flow cytometric analysis
The percentages of T lymphocyte subsets in the spleen were measured using flow cytometric analysis (Guo et al. 2000). Spleen single-cell suspensions were triple labeled with FITC-conjugated anti-mouse CD3, phycoerythrin (PE)-conjugated anti-mouse CD4 and APC-conjugated anti-mouse CD8 antibodies for 30 min at 4°C. Following the initial staining with antibody and washing with staining buffer, enumeration was performed on a Becton Dickinson FACScan Flow Cytometer.
Measurement of IgG by sandwich ELISA
The microtitre plate for ELISA was coated overnight at 4°C with 1 µg/ml of purified rabbit anti-mouse IgG in 50 mM PBS pH 7.2. After washing four times (8 min each time) with PBS containing 0.05% Tween-20, the plate was blocked with 0.5% non-fat dry milk at room temperature for 1.2 h, and then washed four times (8 min each time) with PBS containing 0.05% Tween-20. The diluted serum was added to the first antibody-coated wells in aliquots (100 µl), and the plate was incubated at room temperature for 1.2 h. After the plate was washed four times with PBS-Tween, 100 µl of 1×4000 dilution of HRP labeled goat anti-mouse IgG was added and incubated at room temperature for 1.2 h. After washing the wells with PBS-Tween four times (8 min each time), each well was incubated with 100 µl o-phenylenediamine dihydrochloride (OPD) solution, and after 10 min, the absorbance at 490 nm was measured using a microplate reader.
Liver and kidney function tests
Activities of serum aspartate transaminase (AST, EC: 2.6.1.1), alanine transaminase (ALT, EC: 2.6.1.2), blood urea nitrogen (BUN) and creatinine (CREA) were estimated using the appropriate kits.
Statistical analysis
Results were expressed as mean±SE. Each experiment included triplicate sets in vitro, and each group included six animals in vivo. Statistical analyses were performed using Student's t-test when only two value sets were compared, and one-way ANOVA followed by Dunnett's test when the data involved three or more groups. Significant differences were set at p<0.05 and p<0.01.
Results
Effect of turtle shell extract on spleen lymphocyte proliferation
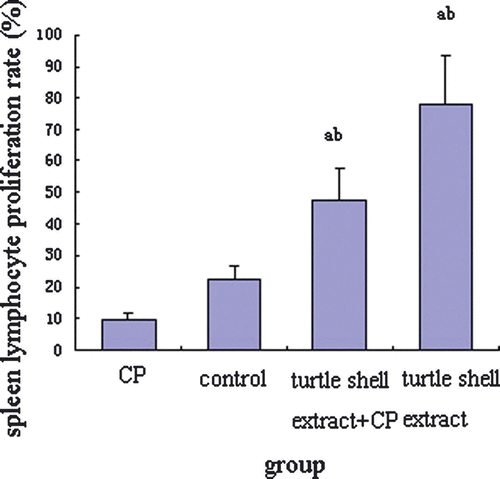
The effect of turtle shell extract on spleen lymphocyte proliferation is presented in . Turtle shell extract significantly recovered CP-treated mice (p<0.001) and enhanced normal mice (p<0.05) in PHA-stimulated spleen lymphocyte proliferation. CP decreased the PHA-stimulated spleen lymphocyte proliferation in normal mice, but there was no significant difference between CP-treated mice and normal mice.
Figure 1. Effect of pre-treatment with turtle shell extract (220 mg/kg, p.o. for 29 days) on PHA-induced spleen lymphocyte proliferation in normal and CP-treated mice. Values are means±SE (n=6). (a) Indicates significant difference in data between control and other groups (p<0.05), and (b) between CP and other groups (p<0.001).
Effect of turtle shell extract on serum IgG level
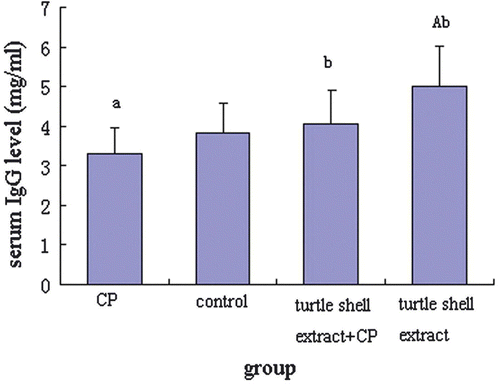
The serum IgG of four groups was determined by sandwich ELISA (). Turtle shell extract significantly recovered the serum IgG level of CP-treated mice (p<0.001) to about normal condition (p>0.05, normal mice compared with extract + CP treated mice), and enhanced normal mice serum IgG level (p<0.001), whereas treatment with CP significantly decreased the serum IgG level of normal mice (p<0.05).
Figure 2. Effect of pre-treatment with turtle shell extract (220 mg/kg BW, p.o. for 29 days) on serum IgG level in normal and CP-treated mice. Values are means±SE (n=6). (a) and (A) indicate that, when compared with control group, the data of CP and extract groups were significantly different (p<0.05 and p<0.001, respectively). (b) Indicates significant difference in data when CP group was compared with other groups (p<0.001).
Effect of turtle shell extract on spleen T lymphocyte subsets
The effect of turtle shell extract on spleen T lymphocyte subsets was examined using flow cytometric analysis, and the results are shown in . Turtle shell extract administered orally, significantly increased percentages of CD3+, CD4+CD8−, CD4−CD8+ and CD4+CD8+ in normal mice (p<0.05), and also significantly increased the percentages of CD3+ and CD4+CD8− cells in CP-treated mice (p<0.05). CP increased the percentages of CD3+, CD4+CD8− and CD4−CD8+ in normal mice, but the differences were not significant (p>0.05). There was no significant difference in CD4/CD8 data among all groups.
Table I. Effect of pre-treatment with turtle extract (220 mg/kg BW, p.o. for 29 days) on spleen T cells in normal and CP-treated male BALB/c mice.
Liver and kidney function tests
In order to investigate the effect of pre-treatment with turtle shell extract (220 mg/kg p.o. for 29 days) on the functions of the liver and kidney in normal and CP-treated mice, the serum ALT, AST, CREA and BUN were measured by commercial kits, and the results are shown in Figures . CP significantly (p<0.05) decreased serum ALT, AST, CREA and BUN levels in normal mice. Turtle shell extract significantly (p<0.05) recovered the serum ALT and AST and CREA levels in CP-treated mice to about normal condition (p>0.05) (normal mice compared with extract + CP treated mice) and only deceased normal serum CREA level (p<0.05).
Figure 3. Effect of pre-treatment with turtle shell extract (220 mg/kg BW, p.o. for 29 days) on serum ATL level in normal and CP-treated mice. Values are means±SE (n=6). (a) Indicates that, when compared with CP-treated mice, the serum ATL level in other groups increased significantly (p<0.05).
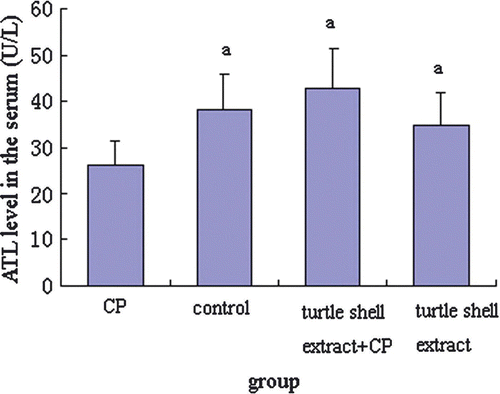
Figure 4. Effect of pre-treatment with turtle shell extract (220 mg/kg BW, p.o. for 29 days) on serum AST level in normal and CP-treated mice. Values are means±SE (n=6). (a) Indicates that, when compared with CP treated mice, the serum AST level in both control and extract + CP-treated mice increased significantly (both p<0.05).
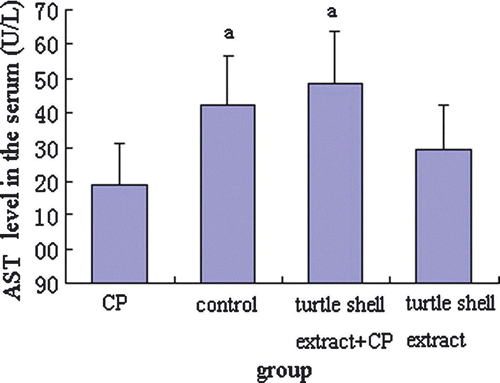
Figure 5. Effect of pre-treatment with turtle shell extract (220 mg/kg BW, p.o. for 29 days) on the serum CREA level in normal and CP-treated mice. Values are means±SE (n=6). (a) and (A) indicate that, when compared with CP-treated mice, the serum CREA level in control and extract + CP-treated mice were both significantly increased (p<0.05 and p<0.001, respectively). (b) Indicates that, when compared with control group, the serum CREA level of other groups were all significantly different (all p<0.05).
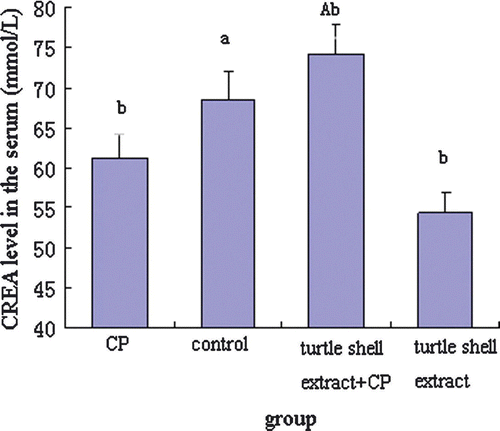
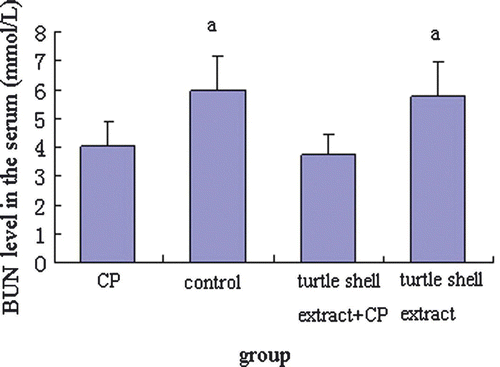
Figure 6. Effect of pre-treatment with turtle shell extract (220 mg/kg BW, p.o. for 29 days) on the serum BUN level in normal and CP-treated mice. Values are means±SE (n=6). (a) Indicates that, when compared with control mice, the serum BUN level of CP and extract + CP-treated mice were all significantly decreased (both p<0.05).
Discussion
After antigen stimulating, T lymphocytes proliferate rapidly and produce various cytokines, which activate other immune cells to proliferate and differentiate into effector cells. Therefore, T lymphocytes play an important role in regulating the immune responses. Recent studies indicate that the ratio of these two Th cell types, Th1 and Th2, is closely correlated with the outcome of many diseases, and controlling the Th1/Th2 ratio has been demonstrated as a therapeutic strategy for various diseases (Singh et al. Citation1999; Boothby et al. Citation2001; Spellberg et al. Citation2001). The present study demonstrated that turtle shell extract via oral administration (Groups III and IV), improved not only CD3+, CD4+CD8−, CD4−CD8+ and CD4+CD8+ percentages in normal mice (p<0.05), but also CD3+ and CD4+CD8− percentages in CP-treated mice (p<0.05). CP increased CD3+, CD4+CD8− and CD4−CD8+ percentages in normal mice (Group I), but the difference was not significant. This may be because when normal mice were given low-dose CP (100 mg/kg, 2 days), the percentage of both CD4+CD8− and CD4−CD8+ subsets were increased, and the CD4+/CD8+ ratio remained unchanged (Tzai et al. Citation1996).
Lymphocyte proliferation is considered indispensable in the activation of the effector T lymphocytes. In the present study, treatment with turtle shell extract significantly (p<0.05) recovered the CP-treated mice and improved normal mice, as shown by PHA stimulating spleen lymphocyte proliferation rates (). The improvement of spleen lymphocyte proliferation by turtle shell extract may be due to stimulating T lymphocytes and antagonizing the suppressive effect of CP on immunity. Hence, turtle shell extract not only up-regulates Th1 and/or Th2 number in normal mice and CP-treated mice, but also, to some extent, enhances the functions of T lymphocyte.
Immune response of the body is mainly composed of specific and non-specific immunity. The specific immune response includes humoral immunity and cellular immunity. The humoral immunity involves the interaction of B cells with the antigen and their subsequent proliferation and differentiation into antibody-secreting plasma cells. The antibody functions as the effector of the humoral response by binding to antigen and neutralizing it or facilitating its elimination. Therefore, in the present study, to evaluate the effect of turtle shell extract on humoral response, its influence was examined by measuring serum IgG level (). The results showed that antibody production was inhibited significantly by CP, whereas, turtle shell extract could significantly enhance the production of circulating antibody (p<0.05). At normal states, oral administration of turtle shell extract can also enhance serum IgG level, suggesting the enhancement of Th2 cellular responses and B cell function.
CP metabolites cause toxicities. Therefore, intervention of CP metabolism to decreases its toxicity has been studied by some investigators, and many materials have been reported effective in reducing CP toxicity, such as Cassia occidentalis (Sharma et al. Citation2000a), E. Officinalis (Sharma et al. Citation2000b) and Cinnamomum cassia (Sharma et al. Citation2001). Figures show that treatment with CP (Group I) significantly (p<0.05) reduced the serum ATL, ATS, CREA and BUN levels in normal mice. Oral administration of turtle shell extract (Group III) significantly increased the serum ATL, ATS and CREA levels in CP-treated mice to about normal levels, indicating that turtle shell extract has some protective functions on liver and kidney damage caused by CP.
In conclusion, the immunomodulatory effects of turtle shell extract in normal and CP-treated mice, and its protective effect on the liver and kidney in CP-treated mice make it a promising candidate for combination therapy with CP.
Acknowledgements
This work was supported in part by grants from the National Natural Science Foundation of China to AXL, and the China National Science Funds for Distinguished Young Scientists (no. 30225033) to XQZ. The authors are grateful to Professor Yang De-Po for providing equipment for the preparation of turtle shell extract and technical assistance.
References
- Bayazit , V . 2004 . Cytotoxic effects of some animal and vegetable extracts and some chemicals on liver and colon carcinoma and myosarcoma . Saudi Med J , 25 : 156 – 163 .
- Boothby , M , Mora , AL , Aronica , MA , Youn , J , Sheller , JR and Goenka , S . 2001 . IL-4 signalling, gene transcription regulation, and the control of effector T cells . Immunol Res , 23 : 179 – 191 .
- Carter , LL and Dutton , RW . 1996 . Type 1 and type 2: A fundamental dichotomy for all T-cell subsets . Curr Opin Immunol , 8 : 336 – 342 .
- Colvin , M and Hilton , J . 1981 . Pharmacology of cyclophosphamide and metabolites . Cancer Treat Rep , 65 : 89 – 95 .
- Dias , PC , Foglio , MA , Possenti , A , Nogueira , DC and de Carvalho , JE . 2001 . Antiulcerogenic activity of crude ethanol extract and some fractions obtained from aerial parts of Artemisia annua L . Phytother Res , 15 : 670 – 675 .
- Feng , H , Matsuki , N and Saito , H . 1996a . Improvement of fatigue and acceleration of recovery from stress-induced deficient sexual behavior in mice following oral administration of soft-shelled turtle powder . Biol Pharm Bull , 19 : 1447 – 1450 .
- Feng , H , Yamazaki , M , Matsuki , N and Saito , H . 1996b . Anti-tumor effects of orally administered soft-shelled turtle powder in mice . Biol Pharm Bull , 19 : 367 – 368 .
- García , D , Leiro , J , Delgado , R , Sanmartín , ML and Ubeira , FM . 2003 . Mangifera indica L. Extract (Vimang) and Mangiferin modulate mouse humoral immune responses . Phytother Res , 17 : 1182 – 1187 .
- Hosseinimehr , SJ and Karami , M . 2005 . Chemoprotective effects of captopril against cyclophosphamide-induced genotoxicity in mouse bone marrow cells . Arch Toxicol , 79 : 482 – 486 .
- Kang , H , Ahn , KS , Cho , CW and Bae , HS . 2004 . Immunomodulatory effect of Astragali Radix extract on murine Th1/Th2 cell lineage development . Biol Pharm Bull , 27 : 1946 – 1950 .
- Li , XY . 1991 . Immunomodulating Chinese herbal medicines . Mem Inst Oswaldo Cruz , 86 : 159 – 164 .
- Martinez , FJ and McCune , WJ . 2006 . Cyclophosphamide for scleroderma lung disease . N Engl J Med , 354 : 2707 – 2709 .
- Martinova , Y , Topashka-Ancheva , M , Konstantinov , S , Petkova , S , Karaivannva , M and Berqer , M . 2006 . Miltefosine decreases the cytotoxic effect of epirubicine and cyclophosphamide on mouse spermatogenic, thymic and bone marrow cells . Arch Toxicol , 80 : 27 – 33 .
- Prus , EK , Turdyev , AA , Trifonov , IuA , Voloshin , SV and Usmanov , RB . 1994 . The radiotherapeutic effect of the CM-5 fraction of turtle spleen extract . Radiat Biol Radioecol , 34 : 138 – 142 .
- Roitt , I , Brostoff , J and Male , D . 1998 . Immunology , 5th ed , Philadelphia : Mosby .
- Sargent , JM and Taylor , CG . 1989 . Appraisal of the MTT assay as a rapid test of chemosensitivity in acute myeloid leukaemia . Br J Cancer , 60 : 206 – 210 .
- Selvakumar , E , Prahalathan , C , Mythili , Y and Varalakshmi , P . 2004 . Protective effect of dl-α-lipoic acid in cyclophosphamide induced oxidative injury in rat testis . Reprod Toxicol , 19 : 163 – 167 .
- Selvakumar , E , Prahalathan , C , Mythili , Y and Varalakshmi , P . 2005 . Mitigation of oxidative stress in cyclophosphamide-challenged hepatic tissue by dl-α-lipoic acid . Mol Cell Biochem , 272 : 179 – 185 .
- Sharma , N , Trikha , P , Athar , M and Raisuddin , S . 2000a . In vitro inhibition of carcinogen-induced mutagenicity by Cassia occidentalis and Emblica officinalis . Drug Chem Toxicol , 23 : 477 – 484 .
- Sharma , N , Trikha , P , Athar , M and Raisuddin , S . 2000b . Inhibitory effect of Emblica officinalis on the in vivo clastogenicity of benzo[a]pyrene and cyclophosphamide in mice . Hum Exp Toxicol , 19 : 377 – 384 .
- Sharma , N , Trikha , P , Athar , M and Raisuddin , S . 2001 . Inhibition of benzo[a]pyrene-andcyclophosph-amide-induced mutagenicity by Cinnamomum cassia . Mutat Res , 480–481 : 179 – 188 .
- Singh , VK , Mehrotra , S and Agarwal , SS . 1999 . The paradigm of Th1 and Th2 cytokines: Its relevance to autoimmunity and allergy . Immunol Res , 20 : 147 – 161 .
- Spellberg , B and Edwards , JE Jr . 2001 . Type 1/Type 2 immunity in infectious diseases . Clin Infect Dis , 32 : 76 – 102 .
- Stephens , R , Eisenbarth , SC and Chaplin , DD . 2002 . T helper type 1 cells in asthma: Friend or foe? . Curr Opin Allergy Clin Immunol , 2 : 31 – 37 .
- Tada , K , Ito , Y , Takahashi , S , Iijima , K , Miyagi , Y and Nishimura , S . 2006 . Tolerability and safety of classic cyclophosphamide, methotrexate, and fluorouracil treatment in Japanese patients with early breast cancer . Breast Cancer , 13 : 279 – 283 .
- Turdyev , AA , Usmanov , RB , Madzhidova , DKh and Nigmatov , Z . 1985 . Postradiation recovery of hematopoiesis in mice administered cell-free tissue extracts from the central Asiatic turtle . Radiobiologiia , 25 : 665 – 669 .
- Turdyev , AA , Aleksandrov , VV , Usmanov , RB , Batyrbekov , AA , Ivanov , VI , Filiutovich , OS and Tagaialieva , NA . 1998 . Hemo- and immunostimulating effect of an extract from blood cells of the Central Asian tortoise . Radiat Biol Radioecol , 38 : 207 – 214 .
- Tzai , TS , Lin , JS and Chow , NH . 1996 . Modulation of antitumor immunity of tumor-bearing mice with low-dose cyclophosphamide . J Surg Res , 65 : 139 – 144 .
- Zee-Chang , RK . 1992 . Shi-Quan-Da-Bu-Tang (ten significant tonic decoction), SQT. A potent Chinese biological response modifier in cancer immunotherapy, potentiation and detoxification of anticancer drugs . Cancer Res , 14 : 725 – 736 .