Abstract
Intestinal epithelial cells are confronted with many noxious stimuli which play an important role in the mucosal immune response. In the present study, Caco-2 cells treated with hydrogen peroxide were used as a model system for studying inflammatory responses and induction of cell death. Live lactobacilli and their concentrated spent culture supernatant (SCS) were applied for studying possible short- and long-term protective effects against hydrogen peroxide-mediated oxidative stress in Caco-2 cells. The secretion of pro-inflammatory cytokine interleukin-8 (IL-8) was investigated in non-filter grown Caco-2 cells, while transepithelial electrical resistance and cell death was studied in filter grown Caco-2 cells. Pre-incubation of Caco-2 cells with Lactobacillus plantarum 2142 did not decrease IL-8 levels induced by 1 mM hydrogen peroxide, nor did lactobacilli suppress IL-8 levels induced by hydrogen peroxide when Caco-2 cells were treated simultaneously with 1 mM hydrogen peroxide and L. plantarum 2142. Thus, lactobacilli did not exert a long-term protective effect against hydrogen peroxide in non-filter grown Caco-2 cells. However, the concentrated SCS of lactobacilli was able to reduce IL-8 levels by more than 6-fold, as determined 24 h after treatment. A short-term effect of lactobacilli was observed in filter grown Caco-2 cells, as they inhibited cell death induced by hydrogen peroxide in the concentration range 10-40 mM. Pre-incubation of epithelial cells with L. plantarum 2142 or simultaneous exposure to hydrogen peroxide and lactobacilli protected Caco-2 cells against cell death. In spite of the presence of lactobacilli, the permeability of membrane increased (transepithelial electrical resistance decreased), and exhibited a similar characteristic pattern as under treatment with hydrogen peroxide alone.
Introduction
Intestinal epithelium serves as the first line of defence against invading bacteria, toxins, oxidative stress and various chemical agents. In intestinal epithelial cells, such noxious stimuli cause secretion of pro-inflammatory cytokines, such as interleukin-8 (IL-8), which play an important role in the mucosal immune response (Yamamoto et al. Citation2003). IL-8 attracts and directs neutrophil granulocytes to the site of inflammation (Baggiolini et al. Citation1995). Its production is regulated by nuclear factor-kappaB (NF-κB) in human epithelial cells (Li & Verma Citation2002).
Increasing secretion of IL-8 has been observed in intestinal epithelial cells in response to pro-inflammatory cytokines, such as TNF-α, or to oxidative stress induced by hydrogen peroxide (Lang et al. Citation2004). Although production of IL-8 is part of the immune response, persistent secretion of this cytokine often causes chronic inflammation which usually leads to cell and tissue damage. High levels of IL-8 were detected in inflammatory bowel disease (IBD), e.g. Crohn's disease or ulcerative colitis (Gijsbers et al. Citation2006). Hence, treatment of these diseases aims at reducing the high levels of IL-8 or inhibition of its signalling pathways.
In addition, hydrogen peroxide is a reactive oxygen species (ROS) which can cause necrotic cell death. It was observed that during necrosis, cell volume and membrane permeability increase (Fink & Cookson Citation2005), so that water soluble components can pass through the membrane. Membrane permeability can be investigated either by water soluble protein-binding dyes (Trypan blue) or fluorescence dyes which show high affinity to DNA (DAPI, 4′,6-diamino-2 phenylindole).
Culturing of Caco-2 cells on permeable filter supports enables the investigation of transcellular transport processes. Under this experimental set-up, due to the formation of tight junctions, transepithelial electrical resistance (TER) will increase and reach a maximum level when the cells are completely differentiated (Anderson et al. Citation1995).
Lactic acid bacteria attached to the intestinal epithelium play an important role in strengthening of the gut barrier function. They are normal inhabitants of the healthy gut microflora, and are present in several fermented foods, such as cheese and milk. It has been shown that some lactobacilli strains exert positive effects following oral administration. They can improve the intestinal microbial balance, confer protection against potential enteropathogenic bacteria, and prevent or cure intestinal diseases (Fuller Citation1989; Brassart & Schiffrin Citation1997; Campieri et al. Citation2000; Gionchetti et al. Citation2000). These effects are mediated via the production of antimicrobial metabolites, such as various organic acids (acetate, propionate, butyrate), hydrogen peroxide or bacteriocins, and competition for nutrients or adhesion receptors (Hudault et al. Citation1997; Cocconier et al. Citation2000; Malago et al. Citation2005). Moreover, lactic acid bacteria can stimulate the immune system and regulate cytokine production. They suppress the synthesis of transforming growth factor (TGF-β) and TNF-α by the intestinal epithelial cells (Reid et al. Citation2001; Wallace et al. Citation2003). Although some lactobacilli inhibited IL-8 production by intestinal epithelial cells, and some were implicated in prevention and treatment of IBD (Campieri et al. Citation2000; Gionchetti et al. Citation2000), a direct link between down-regulation of IL-8 and alleviation of these diseases as exerted by lactobacilli remains to be established.
Against this background, we hypothesised that the presence of lactobacilli or their antimicrobial products in the spent culture supernatant (SCS) have protective effects against oxidative stress, and tested the hypothesis by treatment of the enterocyte-like Caco-2 cells with hydrogen peroxide.
We first assessed whether the non-starter lactobacilli Lactobacillus plantarum 2142 and their microbial products present in SCSs have any long-term protective effect on hydrogen peroxide-induced IL-8 production. For this, we used enterocyte-like Caco-2 cells as an in vitro model of the human intestinal epithelium. Caco-2 cells differentiate in culture and acquire characteristics both structurally and functionally of villus cells (14-day-old Caco-2 cells) of the small intestine (Koninkx Citation1995; Ovelgönne et al. Citation2000; Malago et al. Citation2003). These types of Caco-2 cell cultures were grown on normal tissue culture plates, and are referred to as ‘‘non-filter grown Caco-2 cells’’.
Furthermore, we investigated the possible short-term protective effects of L. plantarum 2142 against hydrogen peroxide-induced necrosis and transepithelial membrane permeability. We tested whether lactobacilli strengthen the measurable barrier function of Caco-2 cells by measuring TER after exposure to various concentrations of hydrogen peroxide. In these types of experiments, 21-day-old fully differentiated Caco-2 cells grown on permeable supports were used which mimic the in vivo conditions in the colon. These types of Caco-2 cells are referred to as ‘‘filter grown Caco-2 cells’’.
Materials and methods
Cell culture
The human colon adenocarcinoma cell line (Caco-2), obtained from Dr. G. Mózsik (Department of Internal Medicine, Medical University of Pécs, Hungary), was cultured in Dulbecco modified Eagle medium (DMEM) (D5671, Sigma) at 37°C in a humidified atmosphere with 5% (v/v) CO2 in air. DMEM was supplemented with 1% (v/v) MEM non-essential amino acid solution 100×, 50 mg/l gentamicin sulphate, 100 mg/l kanamycin sulphate, 4 mM glutamine, 25 mM hepes buffer, 1 mM sodiun pyruvate, and 10% (v/v) heat inactivated foetal bovine serum (FBS) (Gibco). Supplemented DMEM devoid of antibiotics and FBS is referred to as ‘‘plain DMEM’’. All treatments were carried out in plain DMEM. Caco-2 cells were seeded at 4×104 cells/cm2 in 24-well tissue culture plates (2 cm2/well, Costar) or on Transwell, polycarbonate filter inserts in 6-well tissue culture plates (4.2 cm2/well, 0.4-µm pore size, Nunc).
Non-filter grown Caco-2 cells were cultured for 14 days to obtain differentiated villus-like cells, and filter grown Caco-2 cells for 21 days to achieve fully differentiated completely polarised cell population. In normal tissue culture plates, after 14 days, 4×105 cells/well were present, while on filter inserts after 21 days, 1.5×106 cells/well were present. The cell culture medium was replaced three times a week.
Bacterial strains and SCS
Lactobacillus plantarum 2142 (L. plantarum 2142) has been isolated from Italian cheese and obtained from the Culture Collection of the Institute of Dairy Microbiology, Agricultural Faculty of Perugia. For the propagation of lactobacilli, DeMan, Rogosa, Sharpe (MRS) broth (Oxoid Ltd., Basingstoke, Hampshire, UK) was applied.
MRS broth was inoculated with a stationary culture of L. plantarum 2142 (1% inoculum) and the bacteria were grown for 24 h at 30°C. They were subcultured at least twice before the experiments.
The lactobacillus culture was centrifuged for 15 min at 6000×g (25°C) to obtain the cell pellet and the SCS. SCS was concentrated by centrifugation for 1 h at 4000×g (25°C) using Centricon Plus-20 (50 kDa, Millipore, Bedford, MA), according to the manufacturer's guidelines.
The collected lactobacilli (2×109 cfu/ml) were subsequently suspended in plain DMEM, diluted five-fold and added to Caco-2 cells.
Long-term effect of L. plantarum 2142 or its SCS on hydrogen-peroxide induced IL-8 secretion in non-filter grown Caco-2 cells
To characterise the possible long-term inhibitory effect of lactobacilli and their SCS respectively, on hydrogen peroxide-induced IL-8 secretion, we treated non-filter grown Caco-2 cells with 1 mM hydrogen peroxide alone (as control) or in combination with lactobacilli. In the first set of experiments, non-filter grown Caco-2 cells were pre-incubated with 4×108 cfu/ml bacteria for 1 h. After incubation, the bacteria were removed and the cells were washed twice with plain DMEM. The washing step was followed by exposure of Caco-2 cells to 1 mM hydrogen peroxide for 1 h. When hydrogen peroxide was removed and the Caco-2 cells were regenerated by washing the cells twice with plain DMEM, they were allowed to recover for 0, 2, 4, 5, 6 and 24 h, and the supernatants were collected for IL-8 determination. In the second set of experiments, Caco-2 cells were treated for 1 h with 1 mM hydrogen peroxide and 4×108 cfu/ml bacteria simultaneously. After exposure, Caco-2 cells were washed twice with plain DMEM and the cells were recovered for up to 24 h. Supernatants were collected for IL-8 assay after 0, 2, 4, 5, 6 and 24 h of recovery.
In the third set of experiments, either 150 µl MRS or concentrated SCS was added per well to 850 µl hydrogen peroxide for testing the effects of MRS broth or SCS on IL-8 secretion in Caco-2 cells; 1 mM hydrogen peroxide was used as control. After treatment, cells were washed and allowed to recover for 0, 2, 4, 5, 6 and 24 h.
Determination of IL-8 secretion by sandwich ELISA
IL-8 concentrations were determined using the IL-8 Cytosets Antibody Pair Kit containing matched, pre-titered and fully optimised capture and detection antibodies, recombinant standard and streptavidin-horseradish peroxidase (Biosource). The assay was carried out according to the manufacturer's specifications.
Short-term effect of L. plantarum 2142 on transepithelial electrical resistance in filter grown Caco-2 cells
Mucosal integrity of polarised, filter grown Caco-2 cells seeded (4×104 cells/cm2) on polycarbonate, 0.4-µm pore size tissue culture inserts in 6-well plates (insert growth area) was verified by measuring the TER with a Millicell-ERS Volt/Ohm Meter. This device contains a pair of chopstick electrodes, which facilitated the measurements. Filter grown monolayers (apical volume: 2 ml; basolateral volume: 2 ml) were equilibrated with plain DMEM under cell culture conditions. Subsequently, 2 ml of apical DMEM was removed and replaced by the same volume of DMEM containing hydrogen peroxide (as control) or the combination of lactobacilli and hydrogen peroxide. In these types of experiments, Caco-2 cells were pre-incubated with 4×108 cfu/ml bacteria for 1 h. The bacteria were removed by washing the Caco-2 cell twice with plain DMEM. Following washing, 10, 20, 30 or 40 mM hydrogen peroxide was added to Caco-2 cells for 1 h. The initial and final TER was measured. Alternatively, Caco-2 cells were exposed to hydrogen peroxide (10, 20, 30 and 40 mM) and lactobacilli simultaneously, and after 1 h of incubation, TER was measured.
Short-term effects of L. plantarum 2142 and hydrogen peroxide on cell death in filter grown Caco-2 cells
After treatment with hydrogen peroxide and lactobacilli-as described above – necrosis was determined by DAPI (4′,6-diamino-2 phenylindole; Sigma)-staining of filter grown Caco-2 cells. As Trypan blue dye cannot be applied in situ, DAPI was used in the experiments. DAPI can bind to DNA only after the cell membrane has become permeable. At first, Caco-2 cells were washed with plain DMEM (2 ml) per well at both the apical and basolateral side. Then the monolayers were stained on the apical side with 1 µg/ml DAPI in PBS (pH 7.4) for 15 min. DAPI was removed by washing with PBS (2 ml) six times. Cells were fixed with 4% (v/v) formaldehyde/PBS solution. The polycarbonate membrane rings covered with the fixed Caco-2 cells were cut off and placed on glass slides. The membranes were covered with glycerol/PBS (3/1) and stored at 5°C until analysis. Samples were analysed using a fluorescence microscope.
Statistical analysis
Data values are expressed as the mean ± SD. Data were analysed by Student's t-test for multiple variable comparison. A p-value of <0.05 was considered statistically significant. For comparison of slopes, a linear R program was used (R Development Team Citation2006).
Results and discussion
Long-term effect of L. plantarum 2142 and their SCS on hydrogen peroxide-induced IL-8 synthesis in non-filter grown Caco-2 cells
Exposure of Caco-2 cells to 1 mM hydrogen peroxide resulted in a significant increase of IL-8 levels during recovery, and reached the highest value at 24 h (399.3±15.6 pg/ml). To investigate the possible long-term protective effect of lactobacilli against hydrogen peroxide, Caco-2 cells were pre-incubated with L. plantarum 2142 or simultaneously exposed to lactobacilli and hydrogen peroxide. Pre-incubation of Caco-2 cells with lactobacilli did not prevent or diminish induction of IL-8 by hydrogen peroxide in Caco-2 cells (), as no significant difference was detected between the IL-8 levels induced by hydrogen peroxide alone or after pre-treatment with lactobacilli.
Figure 1. Influence of pre-incubation with L. plantarum 2142 on hydrogen peroxide-induced IL-8 synthesis of Caco-2 cells. Caco-2 cells were pre-treated with L. plantarum 2142 for 1 h. After removing lactobacilli by washing with plain DMEM, Caco-2 cells were exposed to 1 mM hydrogen peroxide for 1 h. Caco-2 cells exposed to 1 mM hydrogen peroxide for 1 h without pre-treatment served as positive control. After hydrogen peroxide was removed by washing, cells were allowed to recover in plain DMEM medium for up to 24 h. In the supernatants IL-8 was determined. Mean and SD of triplicates are given. There were no significant differences (p<0.05) between the IL-8 levels in the lactobacilli pre-treated cells and non-pre-treated cells.
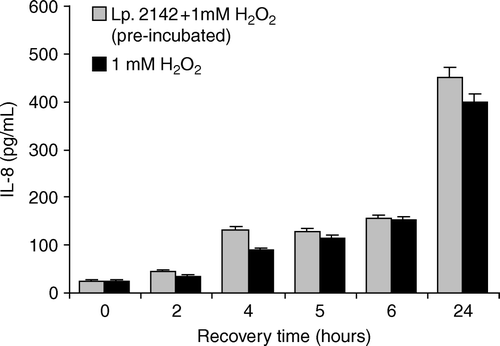
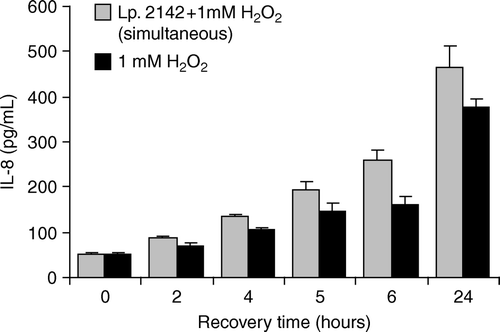
When Caco-2 cells were treated simultaneously with 1 mM hydrogen peroxide and L. plantarum 2142 for 1 h, the IL-8 level also did not decrease, compared to that induced by 1 mM hydrogen peroxide alone. After treatment of Caco-2 cells with 1 mM hydrogen peroxide, at the end of the recovery period (24 h), IL-8 concentration was 377.5±18.62 pg/ml, and after simultaneous exposure to 1 mM hydrogen peroxide and L. plantarum, it was 2142 465.7±46.9 pg/ml (). Thus, L. plantarum 2142 was not effective in the suppression of IL-8 caused by 1 mM hydrogen peroxide.
Figure 2. Effect of simultaneous application of Lactobacillus plantarum 2142 and 1 mM H2O2 on IL-8 production of Caco-2 cells. Caco-2 cells were treated for 1 h either with the combination of L. plantarum 2142 and 1 mM hydrogen peroxide or with 1 mM hydrogen peroxide alone. After removing lactobacilli and hydrogen peroxide by washing with plain DMEM, Caco-2 cells were allowed to recover in plain DMEM medium for up to 24 h. In the supernatants IL-8 was determined. Mean and SD of triplicates are given. There were no significant differences (p<0.05) between the IL-8 levels in supernatants of Caco-2 cells exposed either to lactobacilli and hydrogen peroxide concomitantly or to hydrogen peroxide alone.
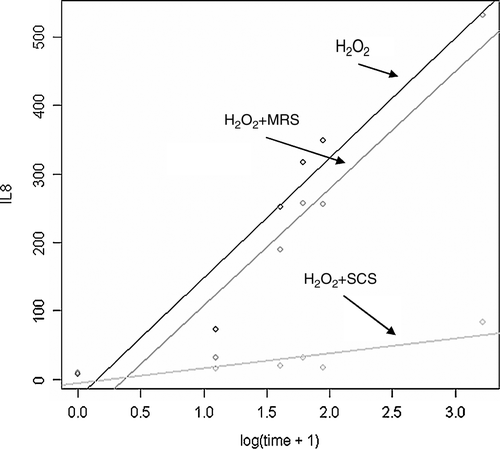
Fresh MRS medium together with hydrogen peroxide caused a slight decrease in IL-8 levels, especially at the beginning of the recovery period, which was statistically not significant, compared to 1 mM hydrogen peroxide alone. However, SCS of L. plantarum 2142 significantly inhibited the synthesis of IL-8 induced by 1 mM hydrogen peroxide in Caco-2 cells, and this could be observed from the beginning of the recovery period. At the end of the recovery period (24 h), IL-8 production was more than 30-fold lower in the presence of SCS of lactobacilli compared to that induced by 1 mM hydrogen peroxide alone ().
Figure 3. Effect of SCS of L. plantarum 2142 on hydrogen peroxide-induced IL-8 production by Caco-2 cells. Non-filter grown Caco-2 cells were treated for 1 h with 1 mM hydrogen peroxide and the combinations of 150 µl MRS broth or SCS with 850 µl of hydrogen peroxide. After the treatment period, cells were washed with plain DMEM, and allowed to recover for 24 h. In the supernatants, IL-8 was determined. Linear equations were calculated by R program. H2O2: −27.178 + 175.293*log(time + 1); H2O2+MRS: −62.682 + 171.092*log(time + 1); H2O2+SCS:−5.454 + 21.791*log(time + 1). There was no significant difference between the linear equations of treatment with H2O2 and H2O2+MRS. H2O2+SCS was significantly different from H2O2 or H2O2+MRS.
Short-term effects of L. plantarum 2142 and hydrogen peroxide on the TER and necrosis in filter grown Caco-2 cells
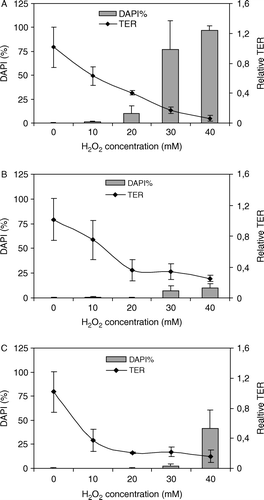
Treatment of filter grown Caco-2 cells with 10, 20, 30 and 40 mM of hydrogen peroxide respectively, resulted in a dose-dependent decrease of the relative TER. After exposure of Caco-2 cells to 40 mM hydrogen peroxide, the relative TER was 0.061±0.038 at the end of the treatment period (A). Thus, the presence of lactobacilli did not result in strengthening of the barrier function of the epithelium, as neither pre-incubation nor simultaneous exposure with lactobacilli resulted in inhibition of hydrogen peroxide-induced TER (B,C).
Figure 4. Effect of hydrogen peroxide and L. plantarum 2142 on TER and necrosis in filter grown Caco-2 cells. Caco-2 cells were exposed to various concentrations of hydrogen peroxide (A) or simultaneously to hydrogen peroxide and L. plantarum 2142 (B) for 1 h. Furthermore, Caco-2 cells were pre-treated with L. plantarum 2142 for 1 h, and after removing the bacteria by washing with plain DMEM, the cells were exposed to the hydrogen peroxide concentrations for 1 h (C). At the end of the treatment periods, TER was measured. After washing, cells were stained with DAPI. TER is expressed as relative TER, necrosis as percentage of DAPI-stained nuclei. Means and SD of triplicates are given.
After the TER measurements were completed, Caco-2 cells were stained with DAPI to determine the percentage of necrotic cells.
The percentage of DAPI-stained nuclei (i.e. percentage of necrotic cells) increased in a hydrogen peroxide dose-dependent manner (A). Exposure of Caco-2 cells to 10 and 20 mM hydrogen peroxide resulted in necrosis in 1.37±0.52 and 10±8.31% of cells, respectively. The highest doses of hydrogen peroxide (40 mM) caused virtually complete necrosis (97.25±4.56%).
Although the integrity of the epithelium was affected by the presence of L. plantarum 2142, as manifested by a decline of TER, there were significantly less necrotic cells, compared to that induced by hydrogen peroxide alone.
Treatment of cells with 30 mM hydrogen peroxide caused necrosis in 76.87±28.81% of cells (A), pre-incubation with lactobacilli reduced necrosis to 2.18±2.06% (C), while simultaneous incubation yielded 6.875±5.312% necrotic cells (B). When Caco-2 cells were pre-treated with lactobacilli and then exposed to 40 mM hydrogen peroxide, 41.56±19% of cells was found to be necrotic at the end of the treatment period (C), while simultaneous exposure of Caco-2 cells to 40 mM hydrogen peroxide and lactobacilli resulted in 10.31±3.93% necrosis (B). That means the lactobacilli pre-treatment suppressed necrosis by more than two-fold, and simultaneous exposure by more than nine-fold.
Figure 5. Protective effect of L. plantarum 2142 against 30 mM hydrogen peroxide-induced necrosis in filter grown Caco-2 cells. Caco-2 cells were exposed to 30 mM hydrogen peroxide (A) or a combination of 30 mM hydrogen peroxide with L. plantarum 2142 (B) for 1 h. Furthermore, Caco-2 cells were pre-treated with L. plantarum 2142 for 1 h followed by incubation with 30 mM hydrogen peroxide for 1 h (C). After the treatments, cells were stained with DAPI.
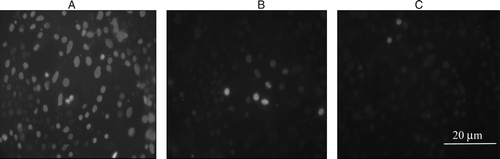
Thus, although L. plantarum 2142 did not exhibit the long-term effect of reducing IL-8 levels, it exerted a short-term effect of protecting filter grown Caco-2 against hydrogen peroxide-induced necrosis.
Yan and Palk (Citation2002) reported that the probiotic L. rhamnosus GG (LGG) prevented cytokine-induced apoptosis in mouse and human intestinal epithelial cells. Culture of LGG with colon cells activated the anti-apoptotic Akt/protein kinase B and inhibited the activation of pro-apoptotic p38/mitogen-activated protein kinase by tumour necrosis factor (TNF), interleukin-1 and α- or γ-interferon. Thus, LGG protected the survival of intestinal epithelial cells through the regulation of both anti-and pro-apoptotic signal transduction pathways. Bacterially produced factors were found to be present in L. rhamnosus GG culture broth, which could regulate signal transduction or cell survival pathways (Yan & Palk Citation2002). In another study, it was reported that L. reuteri inhibited the constitutive synthesis of IL-8 induced by TNF-α in T84 and HT-29 colon cancer cells. According to the report of Ma et al. (Citation2004), to evoke the inhibitory effect, the epithelial cells must be pre-incubated with adherent and live lactobacilli. Zhang et al. (Citation2005) found that live, antibiotic treated and heat-killed L. rhamnosus GG reduced TNF-α-induced IL-8 production; however, by itself, it caused only a relatively small increase in IL-8 production. Son et al. (Citation2005) reported that carnosine, a histidine-containing dipeptide, inhibited hydrogen-peroxide induced IL-8 secretion in Caco-2 cells, and histidine had an anti-inflammatory effect. Yamamoto et al. (Citation2003) studied the inhibitory effect of an antioxidant on the hydrogen peroxide-induced enhancement of IL-8 expression. N-acetylcysteine was found to inhibit the oxidative stress induced enhancement of IL-8 mRNA expression and secretion in both Caco-2 and ACBR1519 cells.
The effects of lactic acid bacteria or their SCS on hydrogen peroxide-induced IL-8 synthesis, TER and necrosis in enterocyte-like Caco-2 cells were studied. Although the presence of L. plantarum 2142 itself did not inhibit hydrogen peroxide-induced IL-8 production in non-filter grown Caco-2 cells, antimicrobial products present in their SCS were able to do so. A similar inhibitory effect of this lactic acid bacteria strain and its SCS against Salmonella enteritidis 857 was reported in an earlier study of ours (Nemeth et al. Citation2006). The adhesion activity of live L. plantarum 2142 to Caco-2 cells was very low (0.19±0.14%) after 1 h of incubation (data not shown), which could be the reason why the bacteria themselves did not decrease hydrogen peroxide-induced IL-8 levels.
TER decreased in a hydrogen peroxide dose-dependent manner in spite of the presence of L. plantarum 2142. Necrosis was inhibited by pre-incubation with lactobacilli, as well as by simultaneous exposure with them, at any of the applied hydrogen peroxide concentrations. Attached lactobacilli might partly neutralise the oxidative effect of hydrogen peroxide, but this needs further investigations. As the antimicrobial components present in SCS of lactobacilli proved effective in the reduction of hydrogen peroxide-induced IL-8 synthesis in epithelial cells, this appears to be a new perspective as a food ingredient in the treatment of IBD, with high IL-8 levels in intestinal epithelial cells.
In the future, we will focus on the analysis of the antimicrobial components of SCS for identification of anti-inflammatory components and effect of cell death.
References
- Anderson , JM and Van Itallie , CM . 1995 . Tight junctions and the molecular basis for regulation of paracellular permeability . Am J Physiol , 269G : 467 – 475 .
- Baggiolini , M , Loetscher , P and Moser , B . 1995 . Interleukin-8 and the chemokine family . Int J Immunopharmacol , 17 : 103 – 108 .
- Brassart , D and Schiffrin , EJ . 1997 . The use of probiotics to reinforce mucosal defense mechanisms . Trends Food Sci Technol , 9 : 321 – 326 .
- Campieri , M , Rizzello , F , Venturi , A , Poggioli , G , Ugolini , F , Helwig , U , Amadini , C , Romboli , E and Gionchetti , P . 2000 . Combination of antibiotic and probiotic treatment is efficacious in prophylaxis of post-operative recurrence of Crohn's disease: A randomised controlled study vs mesalamine . Gastroenterology , 118 : 4179
- Cocconier , MH , Liévin , V , Lorrot , M and Servin , AL . 2000 . Antagonistic activity of Lactobacillus acidophilus LB against intracellular Salmonella enterica Serovar typhimurium infecting human enterocyte-like Caco-2/TC-7 cells . Appl Environ Microbiol , 66 : 1152 – 1157 .
- Fink , SL and Cookson , BT . 2005 . Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells . Infect Immunol , 73 : 1907 – 1916 .
- Fuller , R . 1989 . Probiotics in man and animals . J Appl Bacteriol , 66 : 365 – 378 .
- Gijsbers , K , Geboes , K and Van Damme , J . 2006 . Chemokines in gastrointestinal disorders . Curr Drug Targets , 7 : 47 – 64 .
- Gionchetti , P , Rizzello , F , Venturi , A , Brigidi , P , Matteuzzi , D , Bazzocchi , G , Poggioli , G , Miglioli , M and Campieri , M . 2000 . Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: A double-blind, placebo-controlled trial . Gastroenterology , 119 : 305 – 309 .
- Hudault , S , Liévin , V , Bernet-Camard , MF and Servin , AL . 1997 . Antagonistic activity exerted in vitro and in vivo by Lactobacillus casei (strain GG) against Salmonella typhimurium C5 infection . Appl Environ Microbiol , 63 : 513 – 518 .
- Koninkx , JFJG . 1995 . “ Enterocyte-like Caco-2 cells as a tool to study lectin interaction ” . In Lectins: Biomedical perspectives , Edited by: Pusztai , A and Bardocz , S . 81 – 101 . London : Taylor and Francis .
- Lang , A , Lahav , M , Sakhnini , E , Barshack , I , Fidder , HH , Avidan , B , Bardan , E , Hershkoviz , R , Bar-Meir , S and Chowers , Y . 2004 . Allicin inhibits spontaneous and TNF-alpha induced secretion of proinflammatory cytokines and chemokines from intestinal epithelial cells . Clin Nutr , 5 : 1199 – 1208 .
- Li , Q and Verma , IM . 2002 . NF-kappaB regulation in the immune system . Nat Rev Immunol , 2 : 725 – 734 .
- Ma , D , Forsythe , P and Bienenstock , J . 2004 . Live Lactobacillus reuteri is essential for the inhibitory effect on tumor necrosis factor alpha-induced interleukin-8 expression . Infect Immunol , 72 : 5308 – 5314 .
- Malago , JJ , Koninkx , JFJG , Ovelgönne , HH , Van Asten , FJAM , Swennenhuis , JF and Van Dijk , JE . 2003 . Expression levels of heat shock proteins in enterocyte-like Caco-2 cells after exposure to Salmonella enteritidis . Cell Stress Chaperones , 8 : 94 – 203 .
- Malago , J , Koninkx , J , Tooten , P , Van Liere , EA and Van Dijk , J . 2005 . Anti-inflammatory properties of heat shock protein 70 and butyrate on Salmonella-induced interleukin-8 secretions in enterocyte-like Caco-2 cells . Clin Exp Immunol , 141 : 62 – 71 .
- Nemeth , E , Fajdiga , S , Malago , J , Koninkx , J , Tooten , P and Van Dijk , J . 2006 . Inhibition of Salmonella-induced IL-8 synthesis and expression of Hsp 70 in enterocyte-like Caco-2 cells after exposure to non-starter lactobacilli . Int J Food Microbiol , 112 : 266 – 274 .
- Ovelgönne , HH , Koninkx , JFJG , Pusztai , A , Bardocz , S , Kok , W , Ewen , SW , Hendriks , HGCJM and Van Dijk , JE . 2000 . Decreased levels of heat shock proteins in gut epithelial cells after exposure to plant lectins . Gut , 46 : 679 – 687 .
- R Development Team . 2006 . A language and environment for statistical computing Available : http://www.R-project.org .
- Reid , G , Howard , J and Gan , BS . 2001 . Can bacterial interference prevent infection? . Trends Microbiol , 9 : 424 – 428 .
- Son , DO , Satsu , H and Shimizu , M . 2005 . Histidine inhibits oxidative stress- and TNF-α-induced interleukin-8 secretion in intestinal epithelial cells . FEBS Letts , 579 : 4671 – 4677 .
- Wallace , TD , Bradley , S , Buckley , ND and Green-Johnson , JM . 2003 . Interactions of lactic acid bacteria with human intestinal epithelial cells: Effects on cytokine production . J Food Protect , 66 : 466 – 472 .
- Yamamoto , K , Kushima , R , Kisaki , O , Fujiyama , Y and Okabe , H . 2003 . Combined effect of hydrogen peroxide-induced oxidative stress and IL-1α on IL-8 production in Caco-2 cells (a human colon carcinoma cell line) and normal intestinal epithelial cells . Inflammation , 27 : 123 – 128 .
- Yan , F and Polk , B . 2002 . Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells . J Biol Chem , 277 : 50959 – 50965 .
- Zhang , L , Li , N , Caicedo , R and Neu , J . 2005 . Alive and dead Lactobacillus rhamnosus GG decrease tumor necrosis factor-α-induced interleukin-8 production in Caco-2 cells . J Nutr , 135 : 1752 – 1756 .