Abstract
Screening and confirmatory methods for the determination of Salbutamol (SAL) and Clenbuterol (CL) in swine urine were established. The polyclonal antibody against SAL was prepared, which was used to develop the indirect competitive enzyme-linked immunosorbent assay (ELISA) with the limit of detection of 0.5 ng/ml, and to prepare the immunoaffinity chromatography column. The immunoaffinity column could extract and purify SAL and CL simultaneously, with a binding capacity of 400 ng for SAL and 416 ng for CL. After purification, the swine urine samples were screened by ELISA for the presence of SAL and/or CL, and the positive samples were further confirmed and quantified by gas chromatography-mass spectrometry (GC-MS). The results showed that 95% of the positive samples were confirmed by GC-MS with various levels of SAL (1.1–4.6 ng/ml) and/or CL (1.9–229.1 ng/ml) residues in the incurred samples, and all the negative samples were confirmed with no SAL and/or CL residues.
Introduction
Salbutamol (SAL) and Clenbuterol (CL) are β2-agonists, originally used in the treatment of bronchial diseases. However, SAL and CL have been shown to increase the lean-to-fat ratio in cattle (Ricks et al. Citation1984), sheep (Baker et al. Citation1984), and swine (Jones et al. Citation1985), so called “repartition agents”. Consumption of edible tissues containing CL residues has resulted in some accidental poisonings in humans (Martinez-Navaaro Citation1990), therefore, β2-agonists are banned for use as growth promoters in China and the European Union. Nevertheless, the illicit use of CL and SAL still exists in China, and the illegal use are few in the breeding of other livestock.
In order to supervise the illegal use of CL and SAL, enzyme-linked immunosorbent assay (ELISA) (for screening) and gas chromatography-mass spectrometry (GC-MS) (for confirmation) were established as the official detection methods for monitoring the abuse of SAL and CL in China. Many methods for detection of SAL and CL in animal tissues have been reported in the past, such as enzyme immunoassay (Degand & Duyckaerts Citation1993; Elliott et al. Citation1993; Courthyn et al. Citation1994), liquid chromatography (Lawrence & Menard Citation1997; Koole et al. Citation1999; Rashid et al. Citation1999), GC-MS (Montrade et al. Citation1993; Wilson et al. Citation1994; Bocca et al. Citation2003), and others (Traynor et al. Citation2003). In these methods, the conventional extraction procedures (solid phase extraction or liquid–liquid extraction) were used, which requires a large amount of organic solvents and usually involves a few steps. The immunoaffinity chromatography (IAC) technique, based on the specific interaction of antigen–antibody, is a good alternative method, which can provide a simple and selective means of purifying extracts and reduce the use of organic solvents. Some researchers have reported the use of the IAC technique for purification of SAL and/or CL residues in animal tissues (Pickett & Sauer Citation1993; Kunakar et al. Citation1994; Lawrence & Menard Citation1997; Koole et al. Citation1999; Rashid et al. Citation1999). The general IAC method could purify only one kind of analyte or use mixed antibodies to extract several analytes (Van Ginkel et al. Citation1992; Cooper & Shepherd Citation1996).
The excretion of CL and SAL was primarilyythrough urine (Smith Citation1998), which enabled the sampling of livestock, in contrast to muscle or liver, which was only available after slaughter. Therefore, the aim of the present study was to develop an IAC column, immobilised only anti-SAL antibody cross-reactive for CL, for extraction and purification of SAL and CL in swine urine. After clean-up by IAC, the urine samples were detected by ELISA to give a P/N (positive/negative) result, and further confirmed by GC-MS for multi-analysis of SAL and CL. At the same time, the correlation of the two methods was evaluated.
Experimental
Reagents and chemicals
SAL and CL were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Bovine serum albumin (BSA) and ovalbumin (OA) were from the Hua Mei Biological Technique Co. (Beijing, China). Methanol and CNBr activate Sepharose 4B was obtained from Sigma (St. Louis, MO, USA). N,O(trimethylsilyl) trifluoracetamine (BSTFA) with 1% TMS was from Sigma-Aldrich (Bellefonte, PA, USA). All other chemical reagents were of analytical grade (Beijing Chemical Co., Beijing, China). Acetate buffer (0.1 M, pH 4.0) was prepared by dissolving 2.45 g sodium acetate and 4.7 ml acetic acid in 1000 ml demineralised water. PBS (pH7.2) was prepared by dissolving 0.2 g KH2PO4, 0.2 g KCl, 1.15 g Na2HPO4, and 8.0 g NaCl in 1000 ml demineralised water. IAC elution buffer (5 ml) consisted of 2 ml of ethanol/water/acetate buffer (80/15/5, v/v/v) and 3 ml of methanol.
Equipment
ELISA plate reader was model SUNRISE (Tecan, Durham, USA). Milli-Q Plus water purification system was from Millipore (Bedford, MA, USA). GC-MS was performed with an Agilent (Palo Alto, CA, USA) model 6890 gas chromatograph, and an Agilent model 5973 mass selective detector. The GC column was a 0.25 mm×30 m×0.25 µm HP-5 ms capillary column. Data acquisition and integration were achieved by use of MSD ChemiStation software.
GC-MS conditions
A splitless injection model of 1 µl was performed at an injection port temperature of 250°C. Helium (purity >99%) was used as the carrier gas with a flow rate of 1.0 ml/min. The column temperature programme was 70°C held for 2 min, increased to 200°C at 18°C/min, then increased to 250°C at 5°C/min, finally increased to 300°C at 25°C/min and held 5 min. The mass spectrometer was operated in electron impact (EI) ionisation mode with electron energy of 70 eV, source temperature of 230°C, and detector temperature of 280°C. Acquisition in selected-ion-monitoring (SIM) mode was performed with a dwell time of 100 ms.
Antibody preparation
The anti-SAL antibody was produced as described by others (Degand & Duyckaerts Citation1993). Briefly, the hapten was synthesised by coupling SAL with succinate anhydride, and then the hapten was coupled to bovine serum albumin (SAL-BSA) as immunogen, and to ovalbumin (SAL-OA) as coating antigen using the mixed anhydride method. Six rabbits were initially immunised subcutaneously on the dorsal region with SAL-BSA in Freund's complete adjuvant. Then, the rabbits were boosted with SAL-BSA in Freund's incomplete adjuvant subcutaneously eight times at intervals of 2 weeks, and the antibody titer and cross-reactivity with CL was monitored throughout the eight boosters. After the sera was collected, the IgG was isolated using the saturated [NH4]2SO4 precipitation method. The anti-SAL IgG was used to develop the indirect competitive ELISA and the IAC column.
IAC column preparation
The IAC column preparation procedure was as follows: approximately 1 g of CNBr activated Sepharose 4B powder was swollen in 200 ml 1 mM HCl (3.5–4 ml) and equilibrated with NaHCO3 solution (0.1 M, pH 8.4). The gel was then transferred into the IgG solution (30 mg IgG dissolving in 10 ml of NaHCO3, 0.1 M, pH 8.4), and the mixture was gently stirred at 4°C for 24 h to prepare the immunosorbent. After being washed with 100 ml PBS, the immunosorbent was resuspended in 20 ml Tris-HCl buffer (0.1 M, pH 8.0), and stirred gently at 4°C for 2 h to cap the uncoupled groups. After the gel was washed alternately with acetate buffer (0.1 M, pH 4.0) and Tris-HCl buffer (0.1 M, pH 8.0) for three cycles, the gel of 1 ml bed volume (containing about 8 mg IgG) was transferred to a glass column (8×100 mm, i.d.), and stored in PBS at 4°C when not in use. The column capacity of each newly prepared IAC column was determined by application of successive SAL or CL solution onto the column until the concentration of eluate reached the loading concentration. Then, the column was eluted with 5 ml IAC elution buffer, and the amounts of analytes retained were measured. The recovery (loaded 50% of the column capacity) and the recycle performance of the IAC column were evaluated on three different columns.
Urine clean-up by IAC
Before use, the IAC column was preconditioned with 20 ml PBS. The fortified or unknown urine sample (2 ml) was diluted 10-fold with PBS, and the solution was loaded onto the column and allowed to pass through the column under gravity. Then, the column was washed with 30 ml deionised water to remove the impurities, and the analytes were eluted with 5 ml IAC elution buffer. Regeneration of the IAC column was by sequent washing with 5 ml of IAC elution buffer, 10 ml of deionised water, and 20 ml of PBS. The eluate was evaporated to dryness under a stream of nitrogen in a 45°C water bath. Finally, the dry residue was dissolved in PBS for screening by ELISA or derivatised using BSTFA (with 1% of TMS) for confirmation by GC-MS.
ELISA screening
An indirect competitive ELISA was performed as described below. The anti-SAL IgG dilution and urine extracts with same volume (50 l) were added into the wells of a microplate already coated with SAL-OA to allow the competitive reaction at 37°C for 1 h. After washing with PBS, horseradish peroxidase labelled goat-anti-rabbit IgG solution (100 l) was added to the wells and incubated for 1 h. Then, the wells were washed and the TMB (3,3′,5,5′-tetramethylbenzidine) substrate system (100 l) was added. Finally, 2 M H2SO4 (50 l) was added to stop the reaction, and the plate was read on an ELISA plate reader at 450 nm.
GC-MS confirmation
The dry residue of IAC 50 l of BSTFA (with 1% of TMS), and the solution was incubated at 80°C for 1 h. After cooling to room temperature, the solution was transferred to the injection vial and GC-MS analysis was performed.
Unknow samples
The unknown swine urine samples (n=500) were collected from several farms in China. All the samples, after purification by IAC column, were screened by ELISA, and the samples with positive result were further confirmed and quantitated by GC-MS.
Results and discussion
Antibody and IAC column preparation
The polyclonal antibody prepared here showed good specificity toward SAL with IC50 value of 0.5 ng/ml, and exhibited a cross-reactivity of 106% toward CL. Therefore, the anti-SAL antibody could recognise simultaneously, and the limit of detection (LOD) of the ELISA was determined as 0.5 ng/ml, i.e. IC50, for both SAL and CL.
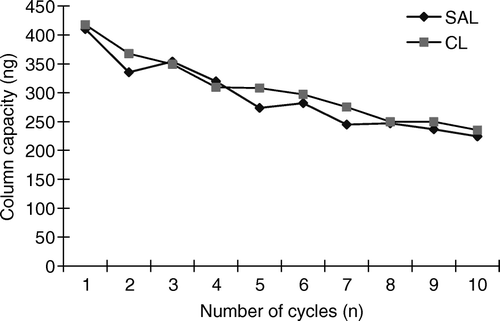
A number of researchers have reported the use of the IAC technique for purification of tissue extracts containing CL and/or SAL. The IAC column used, immobilised with only anti-SAL antibody, could extract and clean-up SAL and CL simultaneously, with a column capacity of 400 ng for SAL and 416 ng for CL. These column capacity values were higher than or similar to other researchers’ reports (45 ng of CL (Lawrence & Menard Citation1997), 200 ng of CL (Rashid et al. Citation1999), 300 ng of CL (Koole et al. Citation1999), 440 ng of CL and 270 ng of SAL (Cooper & Shepherd Citation1996), 200 ng of CL and SAL (Van Ginkel et al. Citation1992). The reason that only anti-SAL antibody was used to prepare the ELISA and the IAC column was obvious-SAL and CL have similar molecule structures, both have an N-tert-butyl group in their molecular structure, so the SAL antibody could detect and bind SAL and CL simultaneously. Therefore, the IAC column prepared in this study could extract SAL and CL with a high column capacity, which was advantageous compared to other researchers’ columns. After fortification with SAL and CL at different levels, the fortified urine samples were purified by the column, and the recovery of target analytes was determined by GC-MS (). It can be seen from the results that the IAC column yielded high recoveries, 81.2 − 112.9% for SAL and 70.9 − 114.6% for CL. The performance of IAC clean-up is shown in . In contrast to the conventional extraction procedure, IAC procedure has the advantages of time saving, material saving and simplicity, but the specific antibody had to be prepared in advance. In this study, all the analyte retained on the column was eluted by 5 ml of IAC elution buffer, with no carry over (). After 10 cycles’ application, the capacity of the IAC column decreased to 220 ng for SAL and CL respectively, which was still sufficient for the purification of the residues of the two illegal drugs ().
Figure 1. Total ion chromatograms of SAL and CL standard (20 ng/ml) (A), and the fortified level of 10 ng/ml in blank urine (B).
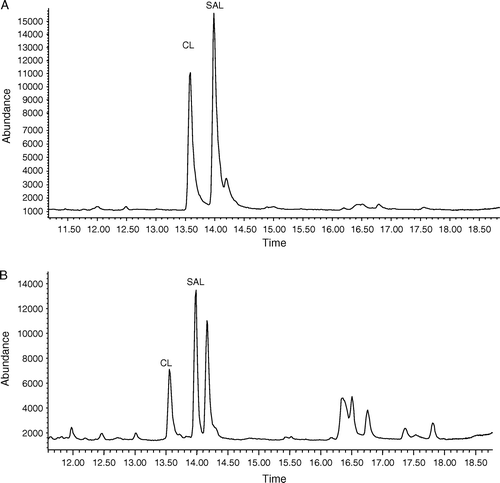
Figure 2. Recovery of eluted SAL and CL with 5 ml of elution buffer. The loaded sample was SAL or CL standard solution diluted with PBS.
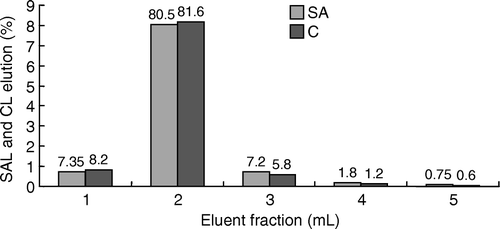
Table I. Recovery of SAL and CL from fortified blank urine samples by IAC-GC-MS (n=6).
Screening and conformation
In order to supervise the illegal use of β2-agonists in animal husbandry, there is an urgent need for a rapid and simple method, i.e. ELISA, for multi-screening of these drugs. Degand and Duyckaerts (Citation1993) developed an ELISA to detect SAL in cattle urine (IC50 of 0.32 ng/ml), in which anti-SAL antibody with cross-reactivity of 115% for CL was used, but they did not put the detection of CL into practical use. The present ELISA with anti-SAL antibody was developed for screening SAL and CL residues simultaneously in unknown swine urine samples (). The LOD of the ELISA was 0.5 ng/ml for both SAL and CL, which was a little higher than that of the previous reports. In this study, ELISA was just a screening method with a result of P/N format that suggested the non-specificity of the antibody, i.e. recognising SAL and CL simultaneously, would not influence the detection results. As the identification of every analyte was based on the four ions monitored during GC-MS analysis, the positive samples screened by ELISA (concentration ≥0.5 ng/ml) were confirmed and quantified by GC-MS.
Table II. Determination results of unknown urine samples with positive result by ELISA screening.
The GC-MS analysis was a confirmatory method for supervising SAL and/or CL in China, and other researchers developed GC-MS for determination of the two analytes (Montrade et al. Citation1993; Pickett & Sauer Citation1993). According to EC criteria for reference methods, identification of a compound must be based on four diagnostic ions in select-ion-monitor (SIM) acquisition mode; the analytes eluted from the GC column must have a response significantly higher than the average noise, and the response ratios must be in agreement with the ratios of corresponding standard compound. Therefore, only the four diagnostic ions for SAL and CL were monitored in this study: m/z 86 (79%), 350 (3%), 369 (100%), and 440 (1%) for SAL; m/z 86 (100%), 243 (5%), 262 (13%), and 277 (2%) for CL. The most abundant ion was determined as the quantitative ions, i.e. m/z 369 and 86 for SAL and CL, respectively. The LOD, defined as the signal/noise ≥3, was in the range of 0.5–1 ng/ml toward the two analytes, and the limit of quantification (LOQ) was 2.0 ng/ml. The determination of LOD and LOQ was based on the four diagnostic ions and the response ratios of the SAL and CL standard. The retention time was 13.51 min for CL and 13.94 min for SAL on the total ion chromatogram. Van Ginkel et al. (1993) developed a GC-MS procedure for determination of SAL and CL in swine urine with an LOD of 0.05–0.2 ng/ml, and the LOQ, based on four diagnostic ions, was 1–2 ng/ml. Montrade et al. (Citation1993) reported a GC-MS procedure for multi-analysis of β2-agonists in cattle urine with the LOD at the low ng/ml level. The LOD of their study and of the present study could all meet the requirement of residue detection for SAL and CL.
Unknown samples
After purification by IAC, all the unknown urine samples were screened by ELISA, and the positive samples were further confirmed by GC-MS. There were 20 positive samples out of the 500 unknown urine samples screened by ELISA, and 19 out of the 20 positive samples were confirmed by GC-MS containing SAL and/or CL (see ). The one false positive sample screened by ELISA maybe due to the non-specific binding of the anti-SAL IgG to an impurity or the concentration was too low to be quantified by GC-MS. It was calculated from the 19/20 positive sample result that the two methods showed a good identity with a correlation of 95%. It was evident from the results that there were higher levels of CL residue (up to 229.1 ng/ml) than that of SAL residue (<4.6 ng/ml) in the unknown swine urine samples, and that the illicit use of β2-agonists, in particular CL, still exists in pig husbandry in China. About 20% of the ELISA negative samples selected randomly were confirmed by GC-MS, and the results showed that these samples contained no SAL or CL residues. It can be assumed from the above results that all the negative urine samples screened by ELISA were negative samples with no SAL or CL residues.
Conclusion
In the present study, an IAC column was prepared based on anti-SAL antibody, which could multi-extract and clean-up SAL and CL in swine urine samples, and screening and confirmatory methods for SAL and CL determination were developed. Experiments with fortified samples showed good accuracy and precision results, and the IAC column was shown to be a simple and satisfactory extraction tool for urine samples. Results from analysis of a high number of real swine urine samples by ELISA was in good agreement with that obtained by GC-MS. The two procedures might, therefore, have the potential to detect SAL and CL residues in animal tissues.
Acknowledgements
We acknowledge with thanks the assistance of all faculty members of the Department of Veterinary Pharmacology and Toxicology, College of Veterinary Medicine, China Agricultural University.
References
- Baker , PK , Dalrymple , RH , Ingle , DL and Ricks , CA . 1984 . Use of a β-adrenergic agonist to alter muscle and fat deposition in lambs . J Anim Sci , 59 : 1256 – 1265 .
- Bocca , B , Fiori , M , Cartoni , C and Brambilla , G . 2003 . Simultaneous determination of Zilpaterol and other beta agonists in calf eye by gas chromatography/tandem mass spectrometry . J AOAC Int , 86 ( 1 ) : 8 – 14 .
- Cooper , AD and Shepherd , MJ . 1996 . Evaluation of a novel immunoaffinity phase for the purification of cattle liver extracts prior to high-performance liquid chromatographic determination of β-agonists . Food Agric Immunol , 8 : 205 – 213 .
- Courthyn , D , Baakeroot , V and de Volder , F . 1994 . Multi-residue enzyme immunoassays for screening for beta-agonists in faeces and feeds . Food Agric Immunol , 6 : 131 – 139 .
- Degand , G and Duyckaerts , AB . 1993 . Determination of β-agonists in urine by an enzyme immunoassay bases on the use of an anti-salbutamol antiserum . Anal Chim Acta , 275 : 241 – 247 .
- Elliott , CT , Mcccaughey , WJ and Shortt , HD . 1993 . Residues of the Beta-agonist clenbuterol in tissues of medicated farm animals . Food Addit Contam , 10 ( 2 ) : 231 – 244 .
- Jones , RW , Easter , RA and McKelth , FK . 1985 . Effect of the beta-adrenergic agonist cimaterol (CL 263,780) on the growth and carcass characteristics of finishing swine . J Anim Sci , 61 : 905 – 913 .
- Koole , A , Bosman , J , Franke , JP and de Zeeuw , RA . 1999 . Multiresidue analysis of β-agonists in human and calf urine using multimodal solid-phase extraction and high-permance liquid chromatography with electrochemical detection . J Chromatogr B , 726 : 149 – 156 .
- Kunakar , P , Ong , H and Adam , A . 1994 . Combined immunoextraction approach coupled to a chemiluminescence enzyme immunoassay for the determination of trace levels of salbutamol and clenbuterol in tissue sample . Analyst , 119 : 2659 – 2662 .
- Lawrence , JF and Menard , C . 1997 . Determination of clenbuterol in beef liver and muscle tissue using immunoaffinity chromatography cleanup and liquid chromatography with ultraviolet absorbance detection . J Chromatogr B , 696 : 291 – 297 .
- Martinez-Navaaro , JF . 1990 . Food poisoning related to consumption of illicit β-agonist in liver . The Lancet , 336 : 1311 – 1315 .
- Montrade , MP , Le Bizec , B , Monteau , F , Siliart , B and Andre , F . 1993 . Multi-residue analysis for β-agonist drugs in urine of meat-producing animals by gas chromatography-mass spectrometry . Anal Chim Acta , 275 : 253 – 268 .
- Pickett , RJH and Sauer , MJ . 1993 . Determination of clenbuterol in bovine urine by enzyme immunoassay following concentration and clean-up by immunoaffinity chromatography . Anal Chim Acta , 275 : 269 – 273 .
- Rashid , BA , Kwasowski , P and Stevenson , D . 1999 . Solid phase extraction of clenbuterol from plasma using immunoaffinity followed by HPLC . J Pharmaceut Biomed , 21 : 635 – 639 .
- Ricks , CA , Dalrymple , RH , Baker , PK and Ingle , DL . 1984 . Use of clenbuterol to alter muscle and fat accretion in swine . Fed Proc , 59 : 1247 – 1255 .
- Smith , DJ . 1998 . The pharmacokinetics, metabolism, and tissue residues of β-adrenergic agonists in livestock . J Anim Sci , 76 : 173 – 194 .
- Traynor , IM , Crooks , SRH and Elliott , CT . 2003 . Detection of multi-β2-agonist residues in liver matrix by use of a surface plasma resonance biosensor . Anal Chim Acta , 483 : 187 – 191 .
- Van Ginkel , LA , Stephany , RW and Van Rossum , HJ . 1992 . Development and validation of a multiresidue method for beta-agonists in biological samples and animal feed . J AOAC Int , 75 : 554 – 560 .
- Wilson , RT , Groneck , JM , Holland , KP and Henry , AC . 1994 . Determination of clenbuterol in cattle, sheep, and swine tissues by electron ionization gas chromatography-mass spectrometry . J AOAC Int , 77 : 917 – 924 .