Abstract
In this study, the anti-tumour activity of selenium-protein polysaccharide (SPP), a water extract of the rich selenium Agaricus blazei, was tested both in vivo and in vitro. The results of in vivo experiments show that SPP at doses of 50 and 100 mg/kg inhibits proliferation of implanted Sarcoma 180 by 22 and 37.69%, respectively, and promotes lymphocyte transformation and natural killer (NK) cells activity in tumour bearing mice. During the in vitro experiment, we treated the tumour and non-tumour bearing mice with SPP, and prepared serum treated with SPP (SerumSPP). The results show that SerumSPP, whether from tumour or non-tumour bearing mice, significantly inhibits K562 cells proliferation and induces their apoptosis, and also significantly increases caspase-3 activity of K562 cells. However, the difference in anti-tumour activity of SerumSPP between tumour and non-tumour bearing mice is significantly different (p<0.01). The results, according to the studies both in vivo and in vitro, imply that SPP extracted from rich selenium A. blazei can inhibit growth of implanted Sarcoma 180 and promote lymphocyte transformation and NK cells activity in vivo. Additionally, SerumSPP can inhibit proliferation and cause apoptotic morphological changes and the fragmentation of internucleosomal DNA, and increase caspase-3 activity of K562 cells in vitro, which indicates that apoptosis of K562 cells induced by SerumSPP may be related to up-regulation of caspase-3.
Introduction
Agaricus blazei Murill, a native species of Brazil, has been widely planted in Japan and Indonesia since 1988, and its main components, including steroid, polysaccharide, glycoprotein and nucleic acid, have been separated and purified. Among them, polysaccharide executes the activities of anti-tumour (Ito et al. Citation1997; Bina & Fujimiya Citation1998; Mizuno et al. Citation1999; Ohno et al. Citation2001; Takaku et al. Citation2001), immunity improving (Mizuno et al. Citation1998; Sorimachi et al. Citation2001a), anti-mutagenic (Delmanto et al. Citation2001; Luiz et al. Citation2003), and bacteria killing (Osaki et al. Citation1994; Sorimachi et al. Citation2001b). In 2000, this fungus was successfully cultivated in Ziyang Country of Shan'xi Province, which is the second richest selenium area in China. However, the anti-tumour effect of the rich selenium A. blazei cultivated in China is still unclear. In this study, we have isolated selenium-protein polysaccharide (SPP) from the rich selenium A. blazei and explored its anti-tumour activity in vivo and in vitro. For the in vitro experiment, we applied the serum pharmacology method to treating the tumour and non-tumour bearing mice with SPP, and prepared its serum to investigate the anti-proliferation and apoptosis-inducing effects on K562 cells, and subsequently to detect the variation of caspase-3 activity.
The serum pharmacology method, which was initiated by Iwama and Ogihara (Citation1987), has been used in some researches on anti-tumour agents, such as Chinese herbal medicinal mixture (Zhang et al. Citation2000). The serum treated with herbs contains pharmacological components, and may also have some immune molecules activated after the herb is metabolised in vivo. Although, in many researches, serum treated with herbs prepared from normal or pathological animals seems to have good anti-tumour effects, the difference in anti-tumour activity between them is still unknown.
Therefore, the purposes of this study are two folds: (1) to evaluate the anti-tumour effects of SPP extracted from rich selenium A. blazei in vivo and in vitro; and (2) to explore the difference in anti-tumour activity of serum treated with SPP (SerumSPP) between normal and pathological mice.
Materials and methods
Reagents
Roswell Park Memorial Institute (RPMI) 1640 medium, 3-(4,5-dimethylthiazo 1-2-yl)-2, 5-diphenylte trazolium bromide (MTT), sodium dodecyl sulphate (SDS), acridine orange, 100 bp DNA Ladder Marker, and ethidium bromide (EB) were purchased from Sigma. Fetal bovine serum (FBS) was from Gibco-BRL. Caspase-3 cellular assay kit was from Biomol.
Animals and cell lines
Male BALB/c mice, weighing 18–22 g, were raised in a sterile room at a controlled temperature (22±1°C) and humidity (55%). Animals had access to tap water ad libitum. Chronic Myeloid Leukaemia K562 cell line and Sarcoma 180 cell line were purchased from Type Culture Collection of Chinese Academy of Sciences, Shanghai, China.
Preparation of SPP
The rich-selenium A. blazei Murill was supplied by Ziyang Kangyuan Bioengineering Co., Ltd. The dried fruit bodies were pulverised, and then extracted with hot water (60°C) twice under reflux. Both extracts were combined, filtered and concentrated under reduced pressure. The concentrate was precipitated with 80% ethanol. The precipitate was dissolved in distilled water and shaken. After the undissolved matter was removed by centrifugation, the supernatant was subjected to D101 macro reticular resin column using distilled water. The SPP was obtained from the fractions eluted from this column, and was again precipitated with 80% ethanol and spray-dried. Using Lowry-hydroxybenzene, phenol-vitriol and 2, 3-two amido naphthalene fluorescence colorimetric method respectively, the content of protein, selenium and polysaccharide of SPP were 16.32, 0.70 and 81.20%, respectively.
Preparation of SerumSPP
Some 60 BALB/c mice were randomly divided into non-tumour and tumour bearing groups, 30 mice in each group. The non-tumour bearing group were then randomised into three subgroups, 10 mice in each subgroup. Mice in the three non-tumour bearing subgroups were administered oral high-dose (100 mg/kg), low-dose (50 mg/kg) of SPP and 0.9% NaCl-solution (NS) respectively, once a day from the first to the tenth day. Tumour bearing groups were also randomised into three subgroups, 10 mice in each subgroup. (Sarcoma 180 cells were allowed to replicate for two generations in the mice abdomen. Cell solution during the logarithmic growth phase had a density of 1×107 cells/ml. All mice were then implanted subcutaneously in the right groin with 0.1 ml of Sarcoma 180 cells.) The latter treatment is the same as that of the non-tumour bearing group. On the tenth day, the SerumSPP were prepared from the blood of all experimental mice, treated at 56°C for 30 min and stored at –20°C for subsequent studies.
Effect of SPP on growth of Sarcoma 180 in tumour bearing mice
Tumour bearing mice, as described above, were established, and then randomised into four groups, 10 mice in each group. Mice in the four experimental groups were administered respectively, oral high-dose (100 mg/kg), low-dose (50 mg/kg) of SPP, cytoxan (20 mg/kg) and 0.9% NS, once a day, from the first to the 10 th day. On the tenth day, all mice were sacrificed and tumours were cut and weighed.
Lymphocyte transformation assay
After anaesthetising the experimental mice, an operation was performed aseptically. The spleen was extracted and thinly sliced with scissors, and then filtered through a fine nylon mesh. The spleen cell suspension was washed three times in Hanks’ balanced salt solution. Cells were finally suspended in RPMI 1640 medium supplemented with 10% FBS, benzyl penicillin 100 kU/l, and streptomycin 100 mg/l. Micro plate wells received spleen cell suspension (2×106/well) with Concanavalin A (2.5 mg/l) added, and incubated at 37°C, 5% CO2 for 72 h. Cell proliferation was estimated based on the method of MTT.
Assay of NK killing activity
For effector cells, mice spleens were made into single cell suspension by the method mentioned above, and the cell density was adjusted to 1×107/ml in RPMI 1640 containing 10% FBS. The rate of viable effector cells submitted for measurement of NK cell activity was usually >95%, as assessed by Trypan blue dye exclusion test. For target cells, exponentially growing YAC-1 cells were adjusted to 2×105/ml cell suspension in RPMI 1640 medium containing 10% FBS. A 50-µl effector cell suspension and a 50-µl target cell suspension were added into each well in a 40-well plate as the experimental group, three wells in parallel. On the other hand, 50 µl/well effector cells and 50 µl/well RPMI 1640 were used as the effector group, and 50 µl RPMI 1640 containing 10% FBS and 50 µl target cells were used as the target group. After 24 h of culture in 5% CO2 at 37°C, 10 µl of MTT solution (5 mg/ml) was added to each well, and the cells were incubated for another 4 h. Then, 100 µl of 10% SDS was added, and another 12 h culture was performed. Cells were lysed with vibration. Measurement was carried out at 570 nm with an ELISA reader (Bio-RAD, Ultramark™ Micro plate system, USA).
Cell culture
Chronic myeloid Leukaemia K562 cells were cultured in RPMI 1640 medium supplemented with 100 U/ml penicillin, 100 mg/ml streptomycin, and 10% (v/v) FBS. The cells were incubated at 37°C, 5% CO2 in fully humidified air and sub-cultured twice weekly. Exponentially growing cells were used throughout the study.
Measurement of cell viability
The sensitivity of K562 cells to SerumSPP was determined using the MTT assay as described previously (Mosman Citation1983). Briefly, 1×104 K562 cells were cultured with 10% SerumSPP from non-tumour and tumour bearing groups in a 96-well micro plate. After 24, 48 and 72 h of culture respectively, 10 µl of MTT solution (5 mg/ml) was added to each well and the cells were incubated for another 4 h. Then, 100 µl of 10% SDS was added, and another 12 h culture was performed. The absorbance at 570 nm was measured using a spectrophotometer (Bio-RAD, Ultramark™ Micro plate system), and the data were expressed as optical density (OD570).
Chromatin condensation
Acridine orange staining was performed according to Hare and Bahler (Citation1986). The cells were incubated with 1.2 µg/ml acridine orange for 30 min. An aliquot of the cells was transferred to a microscope slide and fitted with a cover slip. DNA was visualised with a Fluorescence Olympus Microscope (IX-71, Japan) and photographed. The cells that exhibited condensed chromatin and fragmented nuclei were scored as apoptotic. At least 200 cells were scored from each group, and data were expressed as percentage of apoptotic cells in a total of 200 cells.
Agarose gel electrophoresis
The 5×106 K562 cells cultured with 10% SerumSPP for 72 h were washed twice in phosphate-buffered saline (PBS; containing (in mM): 136 NaCl, 2.7 KCl, 8.2 Na2HPO4 12H2O, and 15 KH2PO4). The cell pellets were then lysed with a 500 L lysis buffer (1% Triton X-100, 50 mM Tris–HCl, pH 7.4, and 20 mM EDTA). The lysates were centrifuged at 1000×g for 10 min. The supernatants were incubated for 3 h at 37°C with 100 l, 1% SDS 10 l, TE/RNase (RNase 10 mg/ml, 10 mM Tris–HCl, pH 7.5, 15 mM NaCl), and 50 l proteinase K (1.0 mg/ml). The DNA was extracted by the standard phenol-chloroform-isoamyl alcohol extraction procedure as described previously (Yoshida et al. Citation1999), precipitated with ethanol, and re-suspended in a 30 l TE buffer (10 mM Tris–HCl, pH 8.0, 1.0 mM EDTA). Each DNA sample was electrophoresed through 1.8% agarose gel containing ethidium bromide. The DNA bands were visualised by MultiImage™ light cabinet (AlphaImager™ 2200, USA) and photographed.
Analysis of intracellular caspase-3 activity
Intracellular caspase-3 activity was measured using a caspase-3 cellular assay kit. Briefly, cells were cultured in the presence of 10% SerumSPP for 12, 24, 48 h respectively, and collected by centrifugation, washed twice with PBS, and re-suspended in cell lysis buffer (50 mM HEPES, pH 7.4, 0.1% CHAPS, 1 mM dithiothreitol (DTT) and 0.1 mM EDTA). The cells were then centrifuged again at 10,000×g at 4°C for 10 min, and the supernatant was used for the following assay. Supernatant (10 L) was mixed with 80 L assay buffer (50 mM HEPES, pH 7.4, 100 mM NaCl, 0.1% CHAPS, 10 mM DTT, 1 mM EDTA and 10% glycerol) in each well of a micro plate and incubated at 37°C for 10 min. Finally, 10 l Ac-DEVD-pNA substrate was added, and after 12, 24, 48 h respectively, the absorbance at 405 nm was measured using micro plate reader (Bio-RAD, Ultramark™ Micro plate system). The results were expressed as optical density (OD405).
Statistical analysis
Data were expressed as means±standard deviation (SD), and the statistical comparison was carried out by ANOVA followed by the Student's t-test.
Results
Effect of SPP on growth of Sarcoma 180 in tumour bearing mice
SPP at doses of 50 and 100 mg/kg inhibits proliferation of implanted Sarcoma 180 by 22 and 37.69% respectively ().
Table I. The effect of SPP on growth of implanted Sarcoma 180.
Effects of SPP on lymphocyte transformation and NK killing activity of tumour bearing mice
As illustrated in , the results revealed that SPP could significantly promote lymphocyte transformation and NK killing activity of tumour bearing mice.
Table II. Effects of SPP on lymphocyte transformation and NK killing activity.
Effects of SerumSPP on the proliferation of K562 cells
To assess the effects of SerumSPP on the proliferation of K562 cells, cells were treated with various concentrations of SerumSPP, and the viability was determined by MTT assay. As shown in , SerumSPP could inhibit the proliferation of K562 cells in dose-dependent and time-dependent manners. However, compared with the non-tumour bearing group, SerumSPP of the tumour bearing group had much more potent effect on the inhibition of cell proliferation.
Table III. Effect of SerumSPP on the growth of K562 cells.
Effect of SerumSPP on apoptosis of K562 cells
After treatment with SerumSPP, vital cells appeared as regular rounded nuclei, intensely stained with acridine orange, while apoptotic cells showed cell shrinkage, membrane blebbing, reduction in cell volume, nuclear fragmentation and apoptotic bodies, which are the typical morphological changes of apoptosis under fluorescent microscope (). As described in Material and methods, a total of 200 K562 cells were counted and apoptosis rates were evaluated as a percent of apoptotic cells in the total 200 cells. Changes in apoptosis were dependent on the time of exposure and the dose of SPP ().
Figure 1. Acridine orange staining of K562 cells. (A) Control serum in non-tumour bearing group; (B) serum treated with 100 mg/kg of SPP in non-tumour bearing group, 72 h exposure; (C) control serum in tumour bearing group; (D) serum treated with 100 mg/kg of SPP in tumour bearing group, 72 h exposure. Nuclear morphology typical for apoptosis is seen in (B) and (D) (×100).
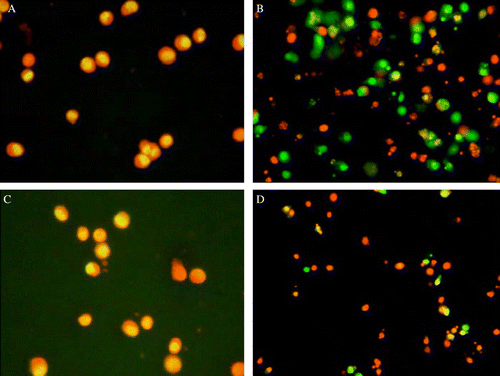
Table IV. Effect of SerumSPP on apoptosis of K562 cells.
DNA fragmentation
In addition to the morphological change, apoptosis induced by SerumSPP was ascertained by using an assay developed to measure DNA fragmentation, a biochemical hallmark of apoptosis. As illustrated in , agarose gel electrophoresis of DNA extracted from K562 cells cultured with SerumSPP for 72 h revealed a ‘ladder’ pattern.
Figure 2. The effect of SerumSPP on the appearance of the DNA apoptotic ladder in K562 cells. (A) Control serum in non-tumour bearing group; (B) serum treated with 50 mg/kg of SPP in non-tumour bearing group, 72 h exposure; (C) serum treated with 100 mg/kg of SPP in non-tumour bearing group, 72 h exposure; (A1) control serum in tumour bearing group; (B1) serum treated with 50 mg/kg of SPP in tumour bearing group, 72 h exposure; (C1) serum treated with 100 mg/kg of SPP in tumour bearing group, 72 h exposure. (M) 100 bp DNA ladder as molecular size markers.

Effect of SerumSPP on activating caspase-3 of K562 cells
As shown in , activity of caspase-3 in K562 cells was significantly increased when cells were exposed to SerumSPP for 12–48 h. Compared with the same dose on the non-tumour bearing group, the SerumSPP of the tumour bearing group had much more potent effect on activating caspase-3 in K562 cells.
Table V. Effect of SerumSPP on activations of caspase-3 in K562 cells.
Discussion
Agaricus blazei extract was previously reported to show the effects of inhibiting growth on Meth A implanted in mice (Itoh et al. Citation1994), and increasing secretion of TNF-a, IL-8 and NO by macrophages activated in vitro (Sorimachi et al. Citation2001a). A selective anti-tumour effect of soluble glycoprotein extracted from A. blazei, mediated via NK-cell activation and by the induction of apoptosis, was demonstrated by Fujimiya et al. (Citation1999). Delmanto et al. (Citation2001) used mice to show that the medicinal properties of A. blazei might be influenced by different strains or growth conditions. Our results showed that SPP, the extraction of rich selenium A. blazei cultivated in China, markedly inhibited proliferation of implanted Sarcoma 180 (), and promoted lymphocyte transformation and NK killing activity of tumour bearing mice (), which indicated the anti-tumour effect of SPP involved in enforcement of host immunity. Sorimachi et al. (Citation2001a) reported that A. blazei fractions did not show direct anti-tumour activity in vitro. However, our results demonstrated that SerumSPP significantly inhibited proliferation of K562 cells in vitro, and caused morphological typical changes of apoptosis, such as the formation of nuclear fragmentation and apoptotic bodies by acridine orange staining (). Apoptosis induced by SerumSPP was also confirmed by the formation of internucleosomal DNA fragmentation () which is a biochemical indicator of apoptosis (Wyllie Citation1980; Cohen & Duke Citation1984; Arends et al. Citation1990; Cohen et al. Citation1992).
To explore the possible mechanism of apoptosis of K562 cells induced by SerumSPP, we observed caspase-3 activity that is closely related with apoptosis of the tumour cells. Caspase belongs to a Plasmosinase family with aminothiopropionic acid activity, and has a high homologisation with ced-3 gene of eelworm cells with 14 species. Caspase activation is a central event in the execution phase of apoptosis (McIlroy et al. Citation1999; Zhuang & Simon Citation2000; Kim et al. Citation2001, Citation2002; Zhang et al. Citation2003). Among caspase family members, caspase-3 plays a crucial role in mediating apoptotic DNA fragmentation during apoptosis (Virag et al. Citation1998; Wolf et al. Citation1999; Jiang et al. Citation2001; Anuradha et al. Citation2000; Turner et al. Citation2003). Our studies showed that SerumSPP could significantly increase caspase-3 activity of K562 cells in vitro, which was strongly suggested that apoptosis of K562 cells induced by the SerumSPP might be related to upregulation of caspase-3.
Although culture in vitro has many merits, such as simplicity, easy control of experimental condition and detecting result etc., but the object cultured could not be controlled and regulated by organism. The serum pharmacology method, which was initiated by Iwama and Ogihara (Citation1987), is partially regarded as the combination of experiments in vivo and in vitro. In the present study, we applied the serum pharmacology method to evaluating the effects of SPP extracted from rich selenium A. blazei on anti-tumour activity. This study, to our knowledge, is the first report on the difference of anti-tumour activity of serum treated with herbs between normal and pathological status animals. Our results showed that both SerumSPP from tumour and non-tumour bearing mice can inhibit growth, induce apoptosis and increase caspase-3 activity of K562 cells in vitro. However, the difference of anti-tumour activity between them is significant (p<0.01).
In conclusion, the SPP extracted from rich selenium A. blazei can inhibit growth of implanted Sarcoma 180 and enhance lymphocyte transformation and NK cells activity in vivo. SerumSPP can also restrain proliferation and cause apoptotic morphological changes and the fragmentation of internucleosomal DNA, and increase caspase-3 activity of K562 cells in vitro, suggesting that caspase-3 involves in the apoptosis induced by SerumSPP.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 30470320) and the Ministry of Education Science Foundation of Gansu Province, China (No. J 02-4).
References
- Anuradha , CD , Kanno , S and Hirano , S . 2000 . RGD peptide-induced apoptosis in human leukemia HL-60 cells requires caspase-3 activation . Cell Biol Toxicol , 16 : 275 – 283 .
- Arends , MT , Morris , RG and Wyllie , AH . 1990 . Apoptosis: The role of the endonuclease . Am J Pathol , 136 : 593 – 608 .
- Bina , T and Fujimiya , Y . 1998 . Anti-tumour effect of a peptide-glucan preparation extracted from Agaricus blazei in a double-grafted tumour system in mice . Biotherapy , 11 : 259 – 265 .
- Cohen , JJ and Duke , RC . 1984 . Glucocorticoid activation of a calcium-dependent endonuclease in thymocyte nuclei leads to programmed cell death . J Immunol , 132 : 38 – 42 .
- Cohen , JJ , Duke , RC , Fadok , VA and Sellins , KS . 1992 . Apoptosis and programmed cell death in immunity . Annu Rev Immunol , 10 : 267 – 293 .
- Delmanto , RD , deLima , PL , Sugui , MM , daEira , AF , Salvadori , DM , Speit , G and Ribeiro , LR . 2001 . Antimutagenic effect of Agaricus blazei Murrill mushroom on the genotoxicity induced by cyclophosphamide . Mutat Res , 496 : 15 – 21 .
- Fujimiya , Y , Susuki , Y , Katakura , R and Ebina , T . 1999 . Tumour-specific cytocidal and immunopotentiating effects of relatively low molecular weight products derived from the basidiomycete, Agaricus blazei Murill . Anticancer Res , 19 : 113 – 118 .
- Hare , JD and Bahler , DW . 1986 . Analysis of Plasmodium falciparum growth in culture using acridine orange and flow cytometry . J Histochem Cytochem , 34 : 215 – 220 .
- Ito , H , Shimura , K , Itoh , H and Kawade , M . 1997 . Anti-tumour effects of a new polysaccharide protein complex (ATOM) prepared from Agaricus blazei (Iwade strain 101) ‘Himematsutake’ and its mechanisms in tumour-bearing mice . Anticancer Res , 17 : 277 – 284 .
- Itoh , H , Ito , H , Amano , H and Noda , H . 1994 . Inhibitory action of a (1→6)-beta-d-glucan-protein complex (F III-2-b) isolated from Agaricus blazei Murill (‘Himematsutake’) on Meth A fibrosarcoma-bearing mice and its anti-tumour mechanism . Jpn J Pharmacol , 66 : 265 – 271 .
- Iwama Amagaya , S and Ogihara , Y . 1987 . Effect of Shosaikoto, a Japanese and Chinese herbal medicinal mixture, on the mitogenic activity of lipopolysaccharide: a new pharmacological testing method . Ethnopharmacology , 21 : 45 – 53 .
- Jiang , D , Jha , N , Boonplueang , R and Andersen , JK . 2001 . Caspase-3 inhibition attenuates hydrogen peroxide-induced DNA fragmentation but not cell death in neuronal PC12 cells . J Neurochem , 76 : 1745 – 1755 .
- Kim , T , Jung , U , Cho , DY and Chung , AS . 2001 . Se-methylselsnocysteine induces apoptosis through caspase activation in HL-60 cells . Carcinogenesis , 22 : 559 – 565 .
- Kim , HS , Rhim , H , Jeong , SW , Kim , JW and Kim , IK . 2002 . Induction of apoptosis dependent on caspase activities and growth arrest in HL-60 cells by PGA2 . Prostaglandins Other Lipid Mediat , 70 : 169 – 183 .
- Luiz , RC , Jordao , BQ , daEira , AF , Ribeiro , LR and Mantovani , MS . 2003 . Mechanism of anticlastogenicity of Agaricus blazei Murill mushroom organic extracts in wild type CHO (K(1)) and repair deficient (xrs5) cells by chromosome aberration and sister chromatid exchange assays . Mutat Res , 528 : 75 – 79 .
- McIlroy , D , Sakahira , H , Talanian , RV and Nagata , S . 1999 . Involvement of caspase-3 activated DNase in internucleosomal DNA cleavage induced by diverse apoptotic stimuli . Oncogene , 18 : 4401 – 4408 .
- Mizuno , M , Morimoto , M , Minato , K and Tsuchida , H . 1998 . Polysaccharides from Agaricus blazei stimulate lymphocyte T-cell subsets in mice . Biosci Biotechnol Biochem , 62 : 434 – 437 .
- Mizuno , M , Minato , K , Ito , H , Kawade , M , Terai , H and Tsuchida , H . 1999 . Anti-tumour polysaccharide from the mycelium of liquid-cultured Agaricus blazei mill . Biochem Mol Biol Int , 47 : 707 – 714 .
- Mosman , T . 1983 . Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays . J Immunol Method , 65 : 55 – 63 .
- Ohno , N , Furukawa , M , Miura , NN , Adachi , Y , Motoi , M and Yadomae , T . 2001 . Anti-tumour beta glucan 9 from the cultured fruit body of Agaricus blazei . Biol Pharm Bull , 24 : 820 – 828 .
- Osaki , Y , Kato , T , Yamamoto , K , Okubo , J and Miyazaki , T . 1994 . Antimutagenic and bactericidal substances in the fruit body of a Basidiomycete Agaricus blazei . Yakugaku Zasshi , 114 : 342 – 350 .
- Sorimachi , K , Akimoto , K , Ikehara , Y , Inafuku , K , Okubo , A and Yamazaki , S . 2001a . Secretion of TNF-alpha, IL-8 and nitric oxide by macrophages activated with Agaricus blazei Murill fractions in vitro . Cell Struct Funct , 26 : 103 – 108 .
- Sorimachi , K , Ikehara , Y , Maezato , G , Okubo , A , Yamazaki , S , Akimoto , K and Niwa , A . 2001b . Inhibition by Agaricus blazei Murill fractions of cytopathic effect induced by western equine encephalitis (WEE) virus on VERO cells in vitro . Biosci Biotechnol Biochem , 65 : 1645 – 1647 .
- Takaku , T , Kimura , Y and Okuda , H . 2001 . Isolation of an anti-tumour compound from Agaricus blazei Murill and its mechanism of action . J Nutr , 131 : 1409 – 1413 .
- Turner , C , Devitt , A , Parker , K , MacFarlane , M , Giuliano , M , Cohen , GM and Gregory , CD . 2003 . Macrophage-mediated clearance of cells undergoing caspase-3-independent death . Cell Death Differ , 10 : 302 – 312 .
- Virag , L , Marmer , DJ and Szabo , C . 1998 . Crucial role of apopain in the peroxynitrite-induced apoptotic DNA fragmentation . Free Radic Biol Med , 25 : 1075 – 1082 .
- Wolf , BB , Schuler , M , Echeverri , F and Green , DR . 1999 . Caspase-3 is the primary activator of apoptotic DNA fragmentation via DNA fragmentation factor-45/inhibitor of caspase-activated DNase inactivation . J Biol Chem , 274 : 30651 – 30656 .
- Wyllie , AH . 1980 . Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation . Nature , 284 : 555 – 556 .
- Yoshida , A , Shao , RG and Pommier , Y . 1999 . “ Assessment DNA damage during apoptosis ” . In Apoptosis , Edited by: Studzinski , GP . 41 – 55 . London : Oxford University Press .
- Zhang , QH , Yu , DH and Lin , ZB . 2000 . Study on the anti-tumour mechanism of ganodermalucidum extract (GLE) by serologic pharmacological method . J Beijing Med Univ , 32 : 210 – 213 .
- Zhang , WB , Wang , CY , Ho , Kj , Lu , FJ , Chang , TC and Lee , WS . 2003 . Magnolol induces apoptosis in human leukemia cells via cytochrome C release and caspase activation . Anticancer Drugs , 14 : 211 – 217 .
- Zhuang , S and Simon , G . 2000 . Peroxynitrite-induced apoptosis involves activation of multiple caspases in HL-60 cells . Am J Physiol Cell Physiol , 279 : C341 – C351 .