Abstract
The competitive enzyme immunoassays for detection of Campylobacter jejuni, C. coli and C. fetus subsp. fetus have been developed. Rabbit and hen immunoglobulins were prepared for these purposes. The working conditions of ELISAs, such as the concentrations of immunoreactants, incubation temperatures and time, and the composition of the substrate have been established. The detection limits were in the range 5.0 104–3.2 106 cfu/ml. The application of chemiluminescent substrates did not result in any significant improvement of the assay's detectability and sensitivity. Prepared antibodies showed rather high specificity and cross-reactivity profiles, and both rabbit and hen immunoglobulins were similar. Only IgY to C. jejuni cross-reacted with seven strains of C. jejuni and two other Campylobacter spp.
A limited number of naturally and artificially contaminated food samples were tested. The results obtained by means of an enzyme immunoassay were compared with those obtained from PCR or commercially available Singlepath® Campylobacter GLISA-Rapid Test. Poultry products were naturally contaminated with Campylobacters. The wild species were identified as C. jejuni and C. coli.
Introduction
Campylobacter spp. is zoonotic bacterium frequently associated with human diarrhea in both industrialized and developing countries. Campylobacter infections lead to economic losses in terms of decreased productivity and medical costs. The incidence of campylobacteriosis is increasing, and the number of illnesses caused by Campylobacter spp. is similar or exceeds cases of salmonellosis in many developed countries (Jones et al. Citation1991; Lacey Citation1993; Phillipps Citation1995). In the Czech Republic, an increase in campylobacteriosis has been recognized since 1995. According to the National Reference Centre of Analysis of Epidemiological Data, 22 713 cases of Campylobacter enteritidis were reported and the morbidity associated with Campylobacter reached 221.6 cases per 100 000 inhabitants in 2006 (www.szu.cz/cema/epidat/epidat.htm). The genus Campylobacter comprises multiple species of which Campylobacter jejuni, C. coli, C. lari are most important (Tauxe et al. Citation1985; Allos & Blaser Citation1995). Published reports, however, indicate that less common Campylobacters, such as Campylobacter fetus subsp. fetus, C. hyointestinalis, C. sputorum subsp. sputorum and C. upsaliensis are increasingly implicated in human disease (Harvey & Greenwood Citation1983; Fennel et al. Citation1986; Edmonds et al. Citation1987; Lindblom et al. Citation1995).
The acute enterocolitis caused by entheropathogenic Campylobacter exhibits symptoms such as malaise, fever, severe abdominal pain and watery to bloody diarrhea. The incubation period varies from 1 to 11 days, typically 1–3 days. Rare extraintestinal infections may appear (Skirrow & Blaser Citation2000). Post-infection complications are also rare, and include reactive arthritis (Schaad Citation1982), Reiter's syndrome (Peterson Citation1994) and autoimmune-mediated disorders of the peripheral nervous system, named Guillain-Barré syndrome (GBS) and its variant Miller-Fisher syndrome (Molnar et al. Citation1982; Othsuka et al. Citation1988; Mishu & Blaser Citation1993).
Campylobacters are common commensals in the intestinal tract of many wild and domestic animals and avian species (Kapperud & Rosef Citation1983; Pacha et al. Citation1988; Maruyama et al. Citation1990; Atabay & Corry Citation1998; Ridsdale et al. Citation1998; Stanley et al. Citation1998; Steinhauserová et al. Citation2000; Aydin et al. Citation2001). From this reason, these animals are considered potential reservoirs for the human infections induced by Campylobacters (Skirrow Citation1994). Transmission of the organism to humans is usually associated with the consumption of contaminated food, water (Jones & Roworth Citation1996), and raw or inadequately pasteurized milk (Fahey et al. Citation1995). Campylobacter spp. has been reported to occur on chicken and red meat and meat products (Madden et al. Citation1998; Atanassova & Ring Citation1999; Whyte et al. Citation2004), oysters and mussels (Endtz et al. Citation1997; Whyte et al. Citation2004), mushrooms (Whyte et al. Citation2004), and vegetables (Kumar et al. Citation2001). The foods of animal origin are usually contaminated during the slaughter process, scalding, mechanical eviscerating, washing, water chilling, and cutting (Berndtson et al. Citation1996; Ono & Yamamoto Citation1999; Logue et al. Citation2003; Nesbakken et al. Citation2003). Campylobacters are heat sensitive, but are able to survive refrigeration temperatures for 112 days of storage (Moorhead & Dykes Citation2002). Therefore, infection is most likely from handling or eating undercooked products or cross-contaminated food not subsequently heated (De Boer & Hahné Citation1990).
The conventional methods of Campylobacter detection in foods takes approximately 5 days and involves selective cultural enrichment followed by isolation from a selective agar. Obtained bacteria are finally characterized by few biochemical tests. These standard microbiological techniques are very sensitive, but also very laborious and time-consuming. These limitations emphasize the necessity of rapid, reliable and sensitive methods for detection of Campylobacter species in foods. Immunochemical assays appear to accommodate such requirements. These techniques are alternative and complementary methods for the detection of microorganisms in foods, environmental and clinical samples because of their sensitivity, reliability, simplicity and cost-effectiveness. In comparison with DNA-based methods, they do not require expensive and highly sophisticated instrumentation, and it is possible to adapt them for field measurement. The major component of all immunoassays is the antibody that is specific to antigens located on the surface of the Campylobacter cells. The specific antibodies are used not only for the detection or serotyping of Campylobacters (Penner & Hennessy Citation1980; Lior et al. Citation1982; Frost et al. Citation1998; Hoorfar et al. Citation1999; Borck et al. Citation2002), but also for the isolation of these bacteria from different sample matrices (Docherty et al. Citation1996).
This study focused on the development of the indirect competitive ELISA for the detection of C. jejuni subsp. jejuni, C. coli, and C. fetus subsp. fetus in foods and foodstuffs. The working conditions of the assays were established. Hen and rabbit polyclonal antibodies were prepared for this purposes and cross-reactivity was determined.
Materials and methods
Materials
Bacteria were purchased from the Czech Collection of Microorganisms (CCM) in Brno (Czech Republic), the Czech National Collection of Type Cultures (CNCTC) in Prague (Czech Republic) or they were obtained from the collection of microorganisms at the Institute of Chemical Technology, Department of Biochemistry and Microbiology (DBM). Iva Steinhaserová from the Veterinary University in Brno proved the strain of Campylobacter upsaliensis (ATCC 43954). The C. jejuni serotypes characterized by means of two different serotyping schemes were obtained from CCM and CNCTC. Bacteria typed according to the Penner serotyping scheme (Penner & Hennessy Citation1980) is described by characters O: followed by the seroserotype number. For example, C. jejuni the Penner serotype 23 is described as C. jejuni O:23. If Kahlich's serotyping scheme is used (Kahlich et al. Citation1985), the serotypes are highlighted by the word serotype followed by the relevant number in the text. For example, C. jejuni Kahlich's serotype 10 is described as C. jejuni serotype 10.
Swine anti-rabbit IgG immunoglobulin-horseradish peroxidase conjugate (7.62 mg IgG/ml, molar ratio peroxidase/IgG = 1.63) was purchased from Sevapharma (Prague, Czech Republic) and rabbit anti-hen IgY immunoglobulin-horseradish peroxidase complex (8.0 mg IgG/ml, molar ratio peroxidase/IgG = 0.91) was purchased from Sigma-Aldrich (Prague, Czech Republic).
Immunogen and standard preparation
Campylobacter jejuni subsp. jejuni O:23 (CCM 6214), C. coli (CCM 6211), and C. fetus subsp. fetus (CCM 6213) were chosen as immunogens for the preparation of both rabbit and hen antibodies. The microorganisms were cultivated in an anaerobic jar (Oxoid, Basingstoke, USA) in Park and Sanders broth (HiMedia, Mumbai, India) supplemented with 1% (v/v) of haemin solution HEM-VIT, and 1% (v/v) of VIT A solution containing mixture of vitamins, amino acids, and other essential compounds (both Dulab, Dubné, Czech Republic) at 37°C for 72–96 h. Microaerophilic conditions were ensured by application of the commercial gas generating kit CampyGen™ (Oxoid). The cells enumeration was performed by means of a plate count procedure on CCDA agar (Oxoid) without any additives. After cultivation, the cells were separated from the cultivating broth by centrifugation at 8000×g at 4°C for 15 min, and then washed twice with 0.01 M PBS pH 7.4. Then, formaldehyde killed bacterial cells were prepared according to Rice et al. (Citation1997) as an immunogen. In the case of standard preparation, the bacterial cells were resuspended in 10 ml of PBS pH 7.4 without formaldehyde treatment and stored at 0°C until use.
Immunization of animals and antibody preparation
Adult White Leghorn hens and adult New Zealand White rabbits were chosen for generation of the specific antibodies. Each animal obtained 6 doses of 5 109 cfu at 2- or 3-week intervals, respectively. The first dose was complete Freund's adjuvant and incomplete Freund's adjuvants and Al Span-Oil one were applied alternately in the remaining five doses. In the course of immunization, ELISA continuously evaluated the increase in antibody titre. Eggs were collected daily and stored at 4°C until antibody preparation. Blood was collected by intracardial puncture 2 weeks after the final dose.
Yolk globulins were prepurified by the method of Akita and Nakai (Citation1992) and crude precipitate was purified according to Jacob et al. (Citation1994) by means of a thiophilic adsorption chromatography on Fractogel EMD TA column (Merck, Darmstadt, Germany). The method was slightly modified by using only 0.8 M ammonium sulphate in neutral pH.
Rabbit gamma globulins were purified from hyperimmune sera by affinity chromatography on Prosep A High Capacity column (Bioprocessing Ltd., Medomsley, UK). The feedstock should be diluted 1:1 v/v with PBS pH 7.4 and after filtration through Whatman 3 paper was adsorbed in binding PBS buffer. IgG fraction was eluted by 0.1 M citrate buffer pH 3.0. Specific activity was evaluated by sandwich ELISA and positive fraction was dialyzed against ammonium hydrogencarbonate and freeze-dried.
Indirect competitive ELISA
The suspension of antigen (the whole cells of C. jejuni subsp. jejuni O:23 (Penner serotype); C. coli or C. fetus subsp. fetus) was diluted by 0.01 M PBS pH 7.4 to a working concentration. Some 0.1 ml of the diluted antigen was added to the wells of the polystyrene microplate, type U (Costar Corning, Cambridge, USA). The content of the wells was incubated at 37°C for 1 h followed by an additional incubation at 4°C overnight. Then, 50 µl of 0.5% (v/v) glutaraldehyde was added, and after 15 min incubation at room temperature, the reaction mixture was removed and the microplate wells were washed three times with 0.2 ml of 0.01 M PBS pH 7.4 containing 0.05% (v/v) Tween-20 (Fluka Chemica, Buchs, Germany) (PBS-Tween-20). As a blocking agent, 0.1 ml of 2% (w/v) bovine serum albumin (Imuna, Šarišské Michalany, Slovakia) in PBS pH 7.4 was added for 1 h at room temperature. The microplate was washed with 0.2 ml of PBS-Tween 20 pH 7.4 three times. For the next step, 50 µl of the diluted standard and sample was added to the wells. Then, 50 µl of hen IgY or rabbit IgG raised to particular Campylobacter species diluted with PBS pH 7.4 to working concentration was added and kept to react for 1.5 h at laboratory temperature. The reaction mixture was removed and the wells were washed three times with 0.2 ml of 0.1% (w/v) BSA in PBS-Tween-20 pH 7.4. Then, 0.1 ml of the rabbit anti IgY immunoglobulin-horseradish peroxidase conjugate or swine anti-rabbit IgG immunoglobulin-horseradish peroxidase conjugate diluted with 0.1% (w/v) BSA in PBS-Tween-20 pH 7.4 to optimal concentration was pippetted into the microplate wells. The conjugate was kept for 1 h at room temperature. Finally, the wells were washed four times with 0.2 ml of PBS-Tween-20 pH 7.4, once with 0.2 ml of deionized water, and then 0.1 ml of 2.7 mM solution of o-phenylenediamine dihydrochloride (Sigma-Aldrich, Prague, Czech Republic), and 0.03% (v/v) H2O2 in 0.05 M citrate-phosphate buffer pH 5.0 was added. The enzyme reaction was terminated by the addition of 50 µl 2 M H2SO4 after 20 min. The absorbance was measured directly in the wells at wavelength 492 nm using the microplate reader SLT RainBow (Tecan, Hombrechtikon, Switzerland).
Chemiluminescent ELISA
Chemiluminescent ELISA was performed according to the protocol described above. The chemiluminescent substrates Renaissance® Enhanced Luminol Nucleic Acid Chemiluminescence Reagent (Nen™ Life Science Products, Boston, MA, USA) and SuperSignal West Pico Chemiluminescent Substrate (Pierce, Oud-Beijerland, The Netherlands) were prepared according to the suppliers’ instructions. On injecting the substrate (100 µl) into individual wells on the plate, after a 2-s delay the light emission was measured for 8 s (time of integration) by means of a luminometer Labsystem LUMINOSCAN RT (Labsystem OY, Helsinki, Finland).
Detection of Campylobacter by means of gold-labeled immunosorbent assay
The detection of campylobacteria in food samples by means of Singlepath® Campylobacter GLISA-Rapid Test (Merck, KgaA) was performed according to the manufacturer's instructions. It is an immunochromatographic method based on gold-labeled antibodies. The sample applied to the chromatography paper via the sample port is absorbed to the reaction zone containing colodial, gold-labeled antibodies specific to Campylobacter spp. Any Campylobacter antigen is captured with the antibody and the created complex migrates until it encounters a binding zone in the test area. The binding zone contains another anti-Campylobacter-antibody, which immobilizes migrating antigen-antibody complex. Due to gold labeling, a distinct red line is formed.
Polymerase chain reaction
For PCR analysis, bacterial DNA from samples was isolated by standard phenol-chloroform extraction (Sambrook et al. Citation1989). A species-specific PCR assay was used for the detection of C. fetus and C. hyointestinalis (Bastyns et al. Citation1994). It is possible to perform PCR for the detection of both strains in a single tube, using the primers HYO1, FET1 and 69ar (KRD, Czech Republic). The DNA amplification was performed in 25 µl of reaction mixture containing 100 ng DNA template, 1×PCR buffer (10 mM Tris–HCl, pH 8.8, 50 mM KCl), 1.5 mM MgCl2, 200 µM each nucleotide, 2 U of Taq DNA polymerase (MBI Fermentas, Lithuania) and 10 pmol each primer. After 3 min of initial denaturation at 95°C, the samples were subjected to 27 amplification cycles of 1 min at 94°C, 1 min at 54°C, and 1 min at 72°C. PCR was carried out in MiniCycler PTC-150 (MJ Research, USA). Discrimination between C. fetus and C. hyointestinalis is possible by size differentiation of the resulting amplification products, after separation on 1% agarose gel. The PCR product size for C. fetus and C. hyointestinalis was 835 and 656 bp, respectively.
Preparation and analysis of intentionally contaminated and non-contaminated food samples
All samples were purchased in a local supermarket. Exactly 25 g of sample (or 25 ml of liquid sample) was added to 225 ml of Park and Sanders broth supplemented with 1% (v/v) of haemin solution HEM-VIT, 1% (v/v) of VIT A, and 0.2% (v/v) of Park and Sanders Selective Supplement I (HiMedia) and homogenized by means of a peristaltic homogenizator for 2 min. The spiked samples were prepared with the addition of 1% (v/v) of freshly prepared inoculum of Campylobacter spp. Both spiked and non-spiked samples were handled and characterized as described in the direction CSN ISO 10272 ‘Microbiology of food and animal stuffs – horizontal method for detection of thermotolerant Campylobacter’. A resuscitation step involving the incubation at 37°C for 4 h without the presence of antibiotics in broth with subsequent incubation at 37°C for 2 h in the presence of antibiotics was introduced for the recovery of the cold-stressed Campylobacter cells. The final incubation took place under microaerophilic conditions at 42°C (thermophilic Campylobacters) or at 37°C (C. fetus subsp. fetus). The obtained isolates were characterized applying the following tests: Gram-staining, oxidase and catalase test, utilization of glucose, lactose and sucrose from TSI agar, growth at 25°C, hydrolysis of hippurate and sensitivity to nalidixic acid and cephalotin. The hippurate hydrolysis was tested with the commercially available HIPPURATEtest (Pliva-Lachema, Brno, Czech Republic). The oxidase test was performed with OXItest (Pliva-Lachema). The susceptibility of the tested strains to nalidixic acid and cephalotin was examined with 30-µg discs (HiMedia) placed on the surface of the inoculated Muller-Hinton agar (HiMedia) plates.
Results evaluation
The calibration curve has been calculated according to the four-parameter equation (Karpinski Citation1990):
The detection limit was calculated as the average value of absorbance at zero standard concentration minus 3 standard deviations.
Results
Optimization of enzyme immunoassay
The optimization procedures were performed as described previously (Hochel et al. Citation2001). The assay in which rabbit anti-Campylobacter spp IgG as a primary antibody was used is highlighted as ELISA 1 in further text. Similarly, the assay using hen IgY as a primary antibody is described as ELISA 2. The optimal concentrations of all immunoreactants and achieved detection limits are given in and . A linear part of the calibration curves covered antigen concentrations in approximately 8 106–2.0 108, 2.0 107–3 108, and 1 106–1 108 cfu/ml for ELISA 1 of C. jejuni, C. coli, and C. fetus subsp. fetus, respectively.
Table I. Determination of optimal concentration of immobilized antigen, rabbit IgG, and swine anti-IgG antibody-peroxidase conjugate in ELISA of Campylobacter sp.
Table II. Determination of optimal concentration of immobilized antigen, hen IgY, and rabbit anti-IgY antibody-peroxidase conjugate in ELISA of Campylobacter sp.
The activation of the microplates was performed by passive adsorption of the whole bacterial cells with subsequent fixation by glutaraldehyde. After saturation of the microplate surface with the inert protein (BSA) and after drying at room temperature, it was possible to store the activated microplates at 4°C for more than 2 months without affecting the assay detectability or sensitivity (data not shown).
The shortest incubation time of a standard (free antigen) and primary antibody having no effect on the assay parameters was 1.5 h. The minimal time period necessary for the interaction of the labeled antibody in all enzyme immunoassays was 1 h.
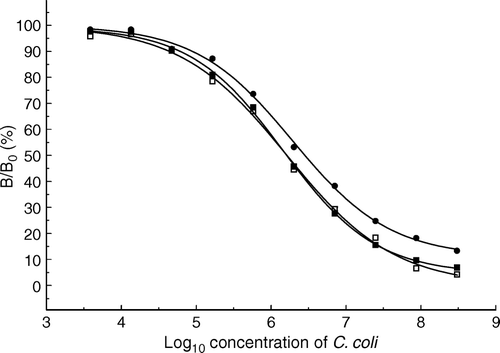
Another important factor affecting the assay sensitivity is the composition of the enzyme substrate. As described previously (Hochel et al. Citation2004), the best sensitivity of enzyme immunoassay was achieved with 0.03% H2O2 in 0.05 M citrate-phosphate buffer pH 5.0 containing 2.7 mM of o-phenylenediamine as a chromogen or with 0.003% solution of H2O2 in 0.1 M acetate buffer pH 6.0 containing 0.4 mM 3,3′,5,5′-tetramethylbenzidine. The ELISA 2 of C. coli has been adapted for the chemiluminescent detection of the complex antigen-antibody. In this case, the assay was carried out in EIA/RIA black plates Costar 3601 with a clear bottom, which are designed for fluorescent or chemiluminescent analysis. The optimization of immunoreactant concentrations was performed again for this type of microplates. In this case, the optimal concentration of immobilized cells of C. coli was 3.1 106 cfu/ml. The optimal concentrations of polyclonal hen IgY and rabbit anti-IgY immunoglobulin labeled by horseradish peroxidase were 3.1 and 8.0 µg/ml, respectively. Under these conditions, the detection limit of ELISA of C. coli with chromogenic end-point reached 1.4 105 cfu/ml. Using Renaissance® Enhanced Luminol Nucleic Acid Chemiluminescence Reagent or SuperSignal West Pico Chemiluminescent Substrate, the detection limits would be 1.7 105 and 1.1 105 cfu/ml, respectively. The detectability and sensitivity of chemiluminescent ELISA are comparable with a chromogenic one ().
Figure 1. Comparison of competitive enzyme immunoassay of Campylobacter coli with chemiluminescent and chromogenic end-point. Substrates: •, o-phenylendiamine; ▪, Renaissance Enhanced Luminol Nucleic Acid Reagent; □, SuperSignal West Pico Chemiluminescent Substrate. The concentration of C. coli is expressed as cfu/ml.
Cross-reactivity
The cross-reactions of 19 Campylobacter species and different species of aerobic and facultative anaerobic bacteria which colonize the gastrointestinal tract of animals and man or grow on the surface and/or cause tainting of meat, fish and poultry product were studied. For the calculation of percentage cross-reactions, the cell concentrations of the studied microorganism (proving 50% inhibition of antibody) were compared with that of the standard that showed the antibody inhibition in the same range. As follows from and , hen IgY against the C. jejuni O:23 interacted with seven C. jejuni serotypes from nine strains tested. On the other hand, rabbit IgG against the same immunogen did not bind any of the other tested heterologeneous serotype of C. jejuni. The types of recognized epitopes give the different specificity of both antibodies. Hen IgY against C. jejuni O:23 is bound to the cell surface proteins, whereas rabbit IgG is predominantly bound to heat-stable surface antigens (lipopolysaccharides). A detailed study using immunoblot, indicated that this antibody is bound to the cell proteins of C. jejuni O:23, O:3, O:9, and C. jejuni serotype 4, 10 and 22. The recognized proteins had apparent molecular weight around 64, 44 and 22–42 kDa (data not shown). Other antibodies showed rather high specificity. For example, IgG against C. fetus subsp. fetus bound only the cells of C. hyointestinalis (13%), C. fetus subsp. veneralis (CCM 3051) and C. fetus subsp. veneralis (CCM 5681). No cross-reactivity, however, was observed with C. fetus subsp. veneralis (CCM 5992). All antibodies showed the interactions with the same tested species among Campylobacteraceae. Except for the antibodies raised against C. jejuni, both rabbit and hen immunoglobulins had similar cross-reactivity profiles ( and ). In addition, all antibodies had not bound the cells of the aerobic or facultative anaerobic bacteria. In several cases, one cannot quantify the interactions of immunoglobulins with the heterologous antigens due to shared reactivity (see below) of the studied heterogeneous antibodies (Berzofsky & Schechter Citation1981).
Table III. Cross-reactivity of the rabbit antibodies against Campylobacter sp.
Table IV. Cross-reactivity of the hen antibodies against Campylobacter sp.
Food samples
A limited number of the naturally and intentionally contaminated food samples were tested. All samples were purchased in a local supermarket and prepared as described in Material and methods. In both competitive ELISA 1 and ELISA 2, the levels of Campylobacters presented in the food samples were quantified. For each measurement, the detection limits were calculated. The results obtained from developed ELISAs were compared with those obtained by means of PCR and commercially available Singlepath® Campylobacter GLISA-Rapid Test (Tables ). All spiked samples were Campylobacter spp. positive. As follows from Tables , the ELISA 1 and ELISA 2 provided results comparable with those obtained from other methods. Only ELISA 1 for detection of C. jejuni showed false negative results for naturally contaminated chicken headcheese and turkey, i.e. the presence of C. jejuni cells in the sample was not recognized. On the other hand, this phenomenon was not observed in ELISA 2 (). The false-positive results were observed if ELISA 2 of C. jejuni and ELISA 1 and ELISA 2 of C. coli in non-spiked headcheese were performed. Moreover, the cultivation method failed when the non-spiked chicken headcheese was tested. The PCR, ELISA 1 and ELISA 2, however, established the presence of the Campylobacter cells in this sample. Finally, a PCR method failed to detect C. fetus subsp. fetus in spiked chicken liver.
Table V. Detection of Campylobacter jejuni in food samples.
Table VI. Detection of Campylobacter coli in food samples.
Table VII. Detection of Campylobacter fetus subsp. fetus in food samples.
The wild strains of thermophilic Campylobacter spp. were found in all poultry products. C. jejuni was found in chicken wings, chicken headcheese, and turkey. C. coli was recognized in samples of the chicken legs.
Discussion
The concentration of reactants, the capacity of the solid phase, the concentration of marker, incubation time and the choice of detection system can influence the assay sensitivity achieved. One of the major problems with whole bacteria is to achieve persistent binding of the antigen to the solid phase. The adsorptive properties of the solid phase for antigens are limited, thereby restricting the measurable analyte range. For that reason, many different ways to enhance the attachment of whole cells or other particulate antigens have been reported (Challacombe Citation1988). In our case, the adsorption of the bacterial cells suspended in 0.01 M PBS pH 7.4 at 4°C overnight followed by glutaraldehyde fixation provided satisfactory results.
A detectability of ELISA is also limited by amount of enzyme label product that should accumulate before measurements can be made. The chemiluminescent substrates may significantly lower the detection limit. For example, ELISA with chemiluminescent end-point provided about 100 times lower detection limit in comparison with the chromogenic enzyme immunoassay (Pronovost et al. Citation1981). In our case, however, the detectability and sensitivity of chemiluminescent ELISA of C. coli was comparable with those obtained from chromogenic end-point method (). There are the limiting factors, such as the avidity of specific antibodies, the avidity of polyclonal anti-globular conjugates, the presence of heterofilic antibodies in a conjugate, the presence of contaminating oxidative/reductive agents, and antigen-conjugate interference which decrease the sensitivity of chemiluminescent ELISA. These factors take effect especially at low concentrations of analyte. Due to the increased background of natural samples, the amplification mode does not enhance the detectability.
The important factor that affects the quality of any immunochemical technique is the specificity of the antibodies. As follows from the and , except for hen IgY, against C. jejuni all antibodies are rather specific, interacting with only a few heterologeneous antigens. The hen IgY against C. jejuni was unable to recognize the homogeneous strain between C. jejuni O:23 and C. jejuni O:3. Moreover, this antibody interacted with C. jejuni O:9, C. jejuni serotype 4, 10, 18, 22, and with non-serotyped strain C. jejuni (CCM 6189). Unfortunately, there are no cross-references between Penner and Kahlich's serotyping schemes. The differences are given not only in serotyping methods, but also in the origin of the detected antigen and in the method of the antisera preparation (Penner & Hennessy Citation1980; Kahlich et al. Citation1985, Citation1989).
We also carried out some preliminary experiments to determine which specific antigens are responsible for the reaction involved. The absorbance was rather reduced after saturation of rabbit anti-C. jejuni IgG with the heat-treated microorganism. This phenomenon indicates that this antibody is bound to both heat-labile and heat stable determinants. On the contrary, the absorbance remained unchanged when saturated hen anti-C. jejuni IgY was applied, and this antibody, therefore, recognized only heat-labile surface antigens in all probability. The detailed characterization of this antibody showed that cell proteins at molecular weight 22–42, 44 and 64 kDa are recognized. All our antibodies also showed interactions among the members of Campylobacter genus, indicating the presence of similar determinants on the cell surface of the different Campylobacter species. Lu et al. (Citation1997) prepared monoclonal antibodies against C. jejuni interacted with C. coli, and C. sputorum subsp. bubulus. Several of these antibodies cross-reacted also with C. fetus subsp. fetus, C. fetus subsp. veneralis, C. upsaliensis and C. hyointestinalis. The main antigens responsible for the binding of monoclonal antibodies have been recognized as flagellin (67 kDa), flagellar hook protein (92 kDa) or 31 kDa protein. Similarly, some antibodies against heat-stable antigen of C. jejuni used in Frost serotyping scheme cross-reacted with some strains of C. coli (Frost et al. Citation1998).
‘True cross-reactivity’ between two antigens and the same antibody describes the case where both antigens are bound to the same binding site of the antibody, but with different affinities. The partial cross-reactivity, shared reactivity or determinant sharing (Berzofsky & Schechter Citation1981) is based on heterogeneity with respect to determinants recognized on the antigen. For example, if an antigen 1 has determinants A and B and an antigen 2 has either determinant A or determinants A and C (where C does not cross-react with B) and heterogeneous antibody is raised to the antigen 1, then antigen 2 will react with some immunoglobulins (those specific for A) but not with these specific for B, which it lacks. In this case, a competition curve would appear to reach a plateau at <100% inhibition, and quantification of the cross-reactivity of the antigen 2 becomes impossible until the antibody is fractioned.
Factors affecting yields of microorganisms are incubation temperature and time. Most protocols are focused on isolation of thermophilic Campylobacters and incubation usually takes place at 42 or 43°C. Under such conditions, these protocols are ineffective at isolating other species. The prolonged incubation time at 37°C enables higher recoveries of non-thermophilic Campylobacter spp. (Corry & Atabay Citation2001) and slowly growing strains. According to our experience, the incubation time of 72–92 h is sufficient to give rise to the visible and distinct colonies of the slowly growing campylobacteria.
Results obtained from a limited number of naturally and intentionally contaminated food samples tested by developed enzyme immunoassays corresponded with those obtained from PCR, commercially available Singlepath® Campylobacter GLISA-Rapid Test and cultivation method. Only two false-negative results were obtained with ELISA 1, when turkey and chicken headcheese was analyzed (). On the other hand, the less specific hen antibody against C. jejuni weakly interacted with these wild strains. Although, enzyme immunoassays usually show a high detection threshold in the range 104–106 cfu/ml, this phenomenon cannot significantly attribute to false-negative results as reported Hoorfar et al. (Citation1999) and Borck et al. (Citation2002). According to our concept, the false negative results are mainly caused by the inability of the antibody to capture some Campylobacter serotypes. A solution to this problem consists in increasing the spectrum of detectable serotypes by an application of cocktails of the specific antibodies in an assay. Jacobs-Reitsma et al. (Citation1995) used a similar procedure by grouping the different specific antisera into 12 pools in order to simplify the serotyping of 62 Campylobacter serotypes. The false-positive responses may be due to cross-reactivity of the used antibody or due to matrix effects.
All poultry products were naturally contaminated by Campylobacter spp. Campylobacter jejuni was identified in chicken wings, chicken headcheese, and turkey, and C. coli was found in chicken legs. No Campylobacter spp. was found in the remaining food samples. A number of studies reported the high contamination of poultry and poultry product by Campylobacters and the predominance of C. jejuni in these samples (Atanassova & Ring Citation1999; Denis et al. Citation2001; Domínguez et al. Citation2002). Whyte et al. (Citation2004) isolated Campylobacter spp. from raw chicken, turkey and duck samples. Lower isolation rates were observed for raw beef, pork and lamb. A low prevalence of the organism was isolated from oysters, fresh mushrooms and raw milk. Ono and Yamamoto (Citation1999) found no Campylobacters in retail beef and pork samples. Similarly, no Campylobacters were found on lamb and pork carcasses and in retail packs of beef and pork. However, 38% of retail packs of chicken pieces (n=120) were Campylobacter spp. positive. The C. jejuni and C. coli were found in approximately equal numbers (Madden et al. Citation1998).
This paper describes the development of an immunoassay to detect Campylobacter spp. Used polyclonal antibodies interacted with only a few strains among the genus Campylobacter which circumscribed their application in the routine assay. The ELISA could be used as a model for the development of methods for the detection of not only a broader spectrum of Campylobacter serotypes, but also for the detection of other important food contaminating microorganis ms.
Acknowledgements
This study was supported by the Grant Agency of Czech Republic, the project GAČR 525/02/287 ‘Development of screening ELISA and PCR methods for detection of Campylobacters in foods’.
References
- Akita , EM and Nakai , S . 1992 . Immunoglobulins from egg yolk: Isolation and purification . J Food Sci , 57 : 626 – 634 .
- Allos , BM and Blaser , MJ . 1995 . Campylobacter jejuni and the expanding spectrum of related infection . Clin Infect Dis , 20 : 1092 – 1101 .
- Atabay , HI and Corry , JEL . 1998 . The isolation and prevalence of Campylobacters from dairy cattle using variety methods . J Appl Microbiol , 84 : 733 – 740 .
- Atanassova , V and Ring , C . 1999 . Prevalence of Campylobacter spp. in poultry and poultry meat in Germany . Int J Food Microbiol , 51 : 187 – 190 .
- Aydin , F , Atabay , HI and Akan , M . 2001 . The isolation and characterisation of Campylobacter jejuni subsp. jejuni from domestic geese (Anser anser) . J Appl Microbiol , 90 : 637 – 642 .
- Bastyns , K , Chapelle , S , Vandamme , P , Goossens , H and De Wachter , R . 1994 . Species-specific detection of Campylobacters important in veterinary medicine by PCR amplification of 32S rDNA areas . Syst Appl Microbiol , 17 : 563 – 568 .
- Berndtson , E , Danielsson-Tham , M-L and Engvall , A . 1996 . Campylobacter incidence on a chicken farm and spread of Campylobacter during the slaughter process . Int J Food Microbiol , 32 : 35 – 47 .
- Berzofsky , JA and Schechter , AN . 1981 . The concepts of crossreactivity and specificity in immunology . Mol Immunol , 18 : 751 – 763 .
- Borck , B , Stryhn , H , Ersb⊘ll , AK and Pedersen , K . 2002 . Thermophilic Campylobacter spp. in turkey samples: evaluation of two automated enzyme immunoassays and conventional microbiological techniques . J Appl Microbiol , 92 : 574 – 582 .
- Challacombe , SJ . 1988 . “ Application of ELISA to microbiology ” . In ELISA and other solid phase immunoassays. Theoretical and practical aspects , Edited by: Kemeny , DM and Challacombe , SJ . 319 – 342 . Chichester, , UK : John Wiley & Sons Ltd .
- Corry , JEL and Atabay , HI . 2001 . Poultry as a source of Campylobacter and related microorganisms . J Appl Microbiol , 90 : 96S – 114S .
- De Boer , E and Hahne , M . 1990 . Cross-contamination with Campylobacter jejuni and Salmonella spp. from raw chicken products during food preparation . J Food Protect , 53 : 1067 – 1068 .
- Denis , M , Refrégier-Petton , J , Laisney , M-J , Ermel , G and Salvat , G . 2001 . Campylobacter contamination in French chicken production from farm to consumers. Use of a PCR assay for detection and identification of Campylobacter jejuni and Campylobacter coli . J Appl Microbiol , 91 : 255 – 267 .
- Docherty , L , Adams , MR , Patel , P and McFadden , J . 1996 . The magnetic immuno-polymerase chain reaction assay for the detection of Campylobacter in milk and poultry . Lett Appl Microbiol , 22 : 288 – 292 .
- Domínguez , C , Gómez , I and Zumalacárregui , J . 2002 . Prevalence of Salmonella and Campylobacter in retail chicken meat in Spain . Int J Food Microbiol , 72 : 165 – 168 .
- Edmonds , P , Patton , CM , Griffin , MP , Barrett , TJ , Schmid , GP , Baker , CN , Lambert , MA and Brenner , DJ . 1987 . Campylobacter hyointestinalis associated with human gastrointestinal disease in the United States . J Clin Microbiol , 25 : 685 – 691 .
- Endtz , HP , Vligenthart , JS , Vandamme , P , Wevering , HW , van den Braak , NP , Verbrugh , HA and van Belkum , A . 1997 . Genotypic diversity of Campylobacter lari isolated from mussels and oysters in The Netherlands . Int J Food Microbiol , 34 : 79 – 88 .
- Fahey , T , Morgan , D , Gunneburg , C , Adak , GK , Majid , F and Kaczmarski , E . 1995 . An outbreak of Campylobacter jejuni enteritidis associated with failed milk pasteurisation . J Infect , 31 : 137 – 143 .
- Fennel , CL , Rompalo , AM , Totten , PA , Bruch , KI , Flores , BM and Stamm , WE . 1986 . Isolation of Campylobacter hyointestinalis from a human . J Clin Microbiol , 24 : 146 – 148 .
- Frost , JA , Oza , NA , Thwaites , RT and Rowe , B . 1998 . Serotyping scheme for Campylobacter jejuni and Campylobacter coli based on direct agglutination of heat-stable antigens . J Clin Microbiol , 36 : 335 – 339 .
- Harvey , SM and Greenwood , JR . 1983 . Probable Campylobacter fetus subsp. fetus gastroenteritidis . J Clin Microbiol , 18 : 1278 – 1279 .
- Hochel , I , Jeníková , G , Dursi , CF , Pazlarová , J , Girotti , S and Demnerová , K . 2001 . Application of mouse antibodies to somatic antigen for detection of Salmonella enteritidis by competitive ELISA . Food Agric Immunol , 13 : 115 – 126 .
- Hochel , I , Viochna , D , Škvor , J and Musil , M . 2004 . Development of an indirect competitive ELISA for detection of Campylobacter jejuni subsp. jejuni O:23 in foods . Folia Microbiol , 49 : 579 – 586 .
- Hoorfar , J , Nielsen , EM , Stryhn , H and Andersen , S . 1999 . Evaluation of two automated enzyme-immunoassays for detection of thermophylic Campylobacters in faecal samples from cattle and swine . J Microbiol Methods , 38 : 101 – 106 .
- Jacob , L , Schitt , E and Bruemmer , W . 1994 . Purification of antibodies by new chromatography techniques . Am Biotechnol Lab , 12 : 44 – 45 .
- Jacobs-Reitsma , WF , Maas , HME and Jansen , WH . 1995 . Penner serotyping of Campylobacter isolates from poultry, with adsorbed pooled antisera . J Appl Microbiol , 79 : 286 – 291 .
- Jones , IG and Roworth , M . 1996 . An outbreak of Escherichia coli O157 and campylobacteriosis associated with contamination of a drinking water supply . Publ Health , 110 : 277 – 282 .
- Jones , FT , Axtell , RC , Rives , DV , Schneideler , SE , Tarver , FR Jr , Walker , RL and Wineland , MJ . 1991 . A survey of Campylobacter jejuni contamination in modern broiler production and processing system . J Food Protect , 54 : 259 – 262 .
- Kahlich , R , Aldová , E , Paleček , A and Šourek , J . 1985 . Use of live cultures for serotyping Campylobacter jejuni . Syst Appl Microbiol , 6 : 82 – 85 .
- Kahlich , R , Aldová , E , Kušiak , I , Hausner , O , Roch , P , Potužník , V and Radovnický , V . 1989 . Use of live cultures for serotyping Campylobacter jejuni II. Further experience and use in an epidemiological study . Syst Appl Microbiol , 12 : 306 – 309 .
- Kapperud , G and Rosef , O . 1983 . Avian wildlife reservoir of Campylobacter fetus subsp. jejuni, Yersinia spp. and Salmonella spp. in Norway . Appl Environ Microbiol , 45 : 375 – 380 .
- Karpinski , KF . 1990 . Optimality assessment in the enzyme-linked immunosorbent assay (ELISA) . Biometrics , 46 : 381 – 390 .
- Kumar , A , Agarwal , RK , Bhilegaonkar , KN , Shome , BR and Bachhil , VN . 2001 . Occurrence of Campylobacter jejuni in vegetables . Int J Food Microbiol , 67 : 153 – 155 .
- Lacey , RW . 1993 . Food-borne bacterial infections . Parasitology , 107 : S75 – S93 .
- Lindblom , G , Sjögren , E , Hansson-Westerberg , J and Kaijser , B . 1995 . Campylobacter upsaliensis, C. sputorum sputorum and C. concisus as common causes of diarrhoea in Swedish children . Scand J Infect Dis , 27 : 187 – 188 .
- Lior , H , Woodward , DL , Edgar , JA , Laroche , LJ and Gill , P . 1982 . Serotyping of Campylobacter jejuni by slide agglutination based on heat-labile antigenic factors . J Clin Microbiol , 15 : 761 – 768 .
- Logue , CM , Sherwood , JS , Elijah , LM , Olah , PA and Dockter , MR . 2003 . The incidence of Campylobacter spp. on processed turkey from processing plants in the midwestern United States . J Appl Microbiol , 95 : 234 – 241 .
- Lu , P , Brooks , BW , Robertson , RH , Nielsen , KH and Garcia , MM . 1997 . Characterization of monoclonal antibodies for the rapid detection of foodborne Campylobacters . Int J Food Microbiol , 37 : 87 – 91 .
- Madden , RH , Moran , L and Scates , P . 1998 . Frequency of occurrence of Campylobacter spp. in red meats and poultry in Northern Ireland and their subsequent subtyping using polymerase chain reaction-restriction fragment length polymorphism and the random polymorphic DNA method . J Appl Microbiol , 84 : 703 – 708 .
- Maruyama , S , Tanaka , T , Katsube , Y , Nakamishi , H and Nukima , M . 1990 . Prevalence of thermophilic campylobacters in crows (Corvus levaillantii, Corvus corone), and serogroups of isolates . Jap J Vet Sci , 52 : 1237 – 1244 .
- Mishu , B and Blaser , MJ . 1993 . Role of infection due to Campylobacter jejuni in the initiation of Gullain-Barré Syndrome . Clin Infect Dis , 17 : 104 – 108 .
- Molnar , GK , Mertsola , J and Erkko , M . 1982 . Guillain-Barré syndrome associated with campylobacter infection . Br Med J , 285 : 652
- Moorhead , SM and Dykes , GA . 2002 . Survival of Campylobacter jejuni on beef trimmings during freezing and frozen storage . Lett Appl Microbiol , 34 : 72 – 76 .
- Nesbakken , T , Eckner , K , H⊘idal , HK and R⊘tterud , O-J . 2003 . Occurrence of Yersinia enterocolitica and Campylobacter spp. in slaughter pigs and consequences for meat inspection, slaughtering, and dressing procedures . Int J Food Microbiol , 80 : 231 – 240 .
- Ono , K and Yamamoto , K . 1999 . Contamination of meat with Campylobacter jejuni in Saitama, Japan . Int J Food Microbiol , 47 : 211 – 219 .
- Othsuka , K , Nakamura , Y , Hashimoto , M , Tagawa , Y , Takahashi , M , Saito , K and Yuki , N . 1988 . Fisher syndrome associated with IgG anti GQ1b antibody following infection by a specific serotype of Campylobacter jejuni . Ophthalmology , 105 : 1281 – 1285 .
- Pacha , RE , Clark , GW , Williams , EA and Carter , AM . 1988 . Migratory birds of central Washington as reservoir of Campylobacter jejuni . Can J Microbiol , 34 : 80 – 82 .
- Penner , JL and Hennessy , JN . 1980 . Passive hemaglutination technique for serotyping Campylobacter fetus subsp. jejuni on the basis of soluble heat-stable antigens . J Clin Microbiol , 12 : 732 – 737 .
- Peterson , MC . 1994 . Rheumatic manifestations of Campylobacter jejuni and C. fetus infection in adults . Scand J Rheumatol , 23 : 167 – 170 .
- Phillipps , CA . 1995 . Incidence, epidemiology and prevention of foodborne Campylobacter species . Trends Food Sci Technol , 6 : 83 – 87 .
- Pronovost , AD , Baumgarten , A and Hsiung , GD . 1981 . Sensitive chemiluminescent enzyme-linked immunosorbent assay for quantification of human immunoglobulin G and detection of herpes simplex virus . J Clin Microbiol , 13 : 97 – 101 .
- Rice , BE , Rollins , DM , Mallinson , ET , Carr , L and Joseph , SM . 1997 . Campylobacter jejuni in broiler chickens: colonization and humoral immunity following oral vaccination and experimental infection . Vaccine , 15 : 1922 – 1932 .
- Ridsdale , JA , Atabay , HI and Corry , JEL . 1998 . Prevalence of Campylobacters and Arcobacters in ducks at the abattoir . J Appl Microbiol , 85 : 567 – 573 .
- Sambrook J , Fritsch EF , Maniatis T. 1989 . Molecular cloning. A laboratory manual . New York, NY : Cold Spring Harbor Laboratory Press .
- Schaad , UB . 1982 . Reactive arthritis associated with Campylobacter enteritidis . Ped Infect Dis , 1 : 328 – 332 .
- Skirrow , MB . 1994 . Diseases due to Campylobacter, Helicobacter and related bacteria . J Comp Pathol , 111 : 113 – 149 .
- Skirrow , M and Blaser , M . 2000 . “ Clinical aspects of campylobacter infection ” . In Campylobacter , Edited by: Nachamkin , I and Blaser , M . 69 – 88 . Washington, DC : ASM Press .
- Stanley , KN , Wallace , JS , Currie , JE , Diggle , PJ and Jones , K . 1998 . Seasonal variation of thermophilic Campylobacters in lambs at slaughter . J Appl Microbiol , 84 : 1111 – 1116 .
- Steinhauserová , I , Fojtíková , K and Klimeš , J . 2000 . The incidence and PCR detection of Campylobacter upsaliensis in dogs and cats . Letts Appl Microbiol , 31 : 209 – 212 .
- Tauxe , RV , Patton , CM , Edmonds , P , Barett , TJ , Brenner , DJ and Blake , PA . 1985 . Illness associated with Campylobacter laridis, a newly recognized Campylobacter species . J Clin Microbiol , 21 : 222 – 225 .
- Whyte , P , McGill , K , Cowley , D , Madden , RH , Moran , L , Scates , P , Carroll , C , O'Leary , A , Fanning , S , Collins , JD , McNamara , E , Moore , JE and Cormican , M . 2004 . Occurrence of Campylobacter in retail foods in Ireland . Int J Food Microbiol , 95 : 111 – 118 .