Abstract
Crude extracts from traditional herbs have long been used in the treatment of a variety of acute and/or chronic diseases in many countries. Zingiber zerumbet Smith extract has been reported to have anti-inflammatory potential, especially in asthmatic patients. The aim of this study is to examine the capacity of water extract of Zingiber zerumbet (CC-ZWE) in protecting the lungs by inhibiting the release of inflammatory mediators during short-term treatment, and modulating cytokine gene expressions during long-term treatment. BALB/c mice were sensitised and challenged with ovalbumin to induce anaphylaxis. The anti-hypersensitive and anti-inflammatory potentials of water extract of Zingiber zerumbet Smith (ZZ-CWE) were evaluated using both in vitro and in vivo systems. ZZ-CWE decreased the release of tumour necrosis factor-alpha and interleukin-4 (IL-4) in vitro and effectively suppressed LTC4 release from lung tissue in vivo (p<0.05). Additionally, animals treated with ZZ-CWE expressed higher ratios of interferon-gamma/IL-4 mRNA in their splenocytes than that of the control group (p<0.05). Our results showed that ZZ-CWE should have beneficial effects for the treatment of asthmatic patients through its capabilities to inhibit the synthesis of LTC4 and through the immunomodulation of Th1/Th2 cytokine production.
Introduction
Crude extracts from traditional herbs have long been used in the treatment of a variety of acute and/or chronic diseases in many countries. Zingiber zerumbet Smith (ZZ), a tropical ginger, has been used as a traditional herbal remedy for a spectrum of ailments in Thailand and other countries throughout Southeast Asia (Farnsworth & Bunyapraphatsara, Citation1992). The main bioactive component in this ginger, known as Zerumbone, is a sesquiterpene, which has been shown to be a distinct tumour suppressor in 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced Epstein–Barr virus, early antigen activation in Raji cells (Vimala, Norhanom, & Yadav, Citation1999), and also activate phase II drug metabolising enzymes (Nakamura et al., Citation2004). Zerumbone also markedly diminish inducible nitric oxide synthase and cyclooxygenase (COX)-2, together with the release of tumour necrosis factor-alpha (TNF-α), in mouse macrophages (Murakami et al., Citation2002). Zingiber zerumbet Smith extract has been reported to have anti-inflammatory potential (Murakami et al., Citation2002), especially in asthmatic patients. However, the mechanism of its action has not been fully elucidated.
Leukotrienes (LT), specifically the cysteinyl LTs (LTC4, LTD4 and LTE4), are well known for their powerful roles in allergic diseases, including asthma (Flamand, Mancuso, Serezani, & Brock, Citation2007; Wenzel, Citation2003). LTC4, LTD4 and LTE4 are formed from cells commonly associated with asthma (Aizawa, Tamura, Ohtsu, & Takishima, Citation1990) and are known potent bronchoconstrictors (Leff, Citation2001). In recent years, leukotriene receptor antagonists have been widely targeted in the treatment of bronchial asthmatic inflammation. However, hepatotoxicity resulting from the use of this novel class of leukotriene receptor agonists in therapies has been noted (Davern & Bass, Citation2003). Thus, potential herbal treatment options for the long-term therapy of asthma have received wide interest. The peripheral blood T cell cytokines have been found to correlate with asthma severity. The binding of interleukin-4 (IL-4) to its receptor (IL-4R) is necessary for the development of airway inflammation in asthma (Beghe et al., Citation2003). A significantly lower ratio of interferon-gamma (IFN-γ)/IL-4 in CD4+ T cells has been found in patients with atopic asthma compared to normal subjects. Interestedly, these patients exhibited a significantly higher proportion of IL-4-producing CD4+ T cells than normal control subjects (Shirai et al., Citation2003). Furthermore, acute asthmatics had significantly increased levels of circulating IL-4 and IL-5, while IFN-γ serum levels differences were of borderline significance between asthmatics and the control subjects (Y. Lee, K. Lee, H. Lee, & Rhee, Citation2001). Thus, the immunomodulatory activities that influence the ratio of IL-4 to IFN-γ could be a useful indicator in the evaluation of a potential therapy. The aim of the present study is to examine the capacity of water extract of Zingiber zerumbet (CC-ZWE) in protecting the lungs by inhibiting the release of inflammatory mediators during short-term treatment, and modulating cytokine gene expressions during long-term treatment.
Methods and materials
Plant material
Zingiber zerumbet Smith (ZZ) was obtained from the Wholesome Life Science Co., Ltd (Taipei, Taiwan). The botanical identity of the sample and the structure of active component in ZZ extract were confirmed by Industrial Technology Research Institute of Taiwan (ITRI), Republic of China.
Preparation of water extract of Zingiber zerumbet Smith
The dry powders of the plant were boiled in water (1:10, w/v) for four hours. After cooling, the water extract of Zingiber zerumbet Smith (ZZ-CWE) was filtered through four layers of sterilised cotton gauze and dried to yield 3.15% (w/s) powders. It was stored at −20oC until use.
TNF-α release from murine peritoneal macrophages
The peritoneal macrophages were elicited by intraperitoneally (ip) injection of 1 ml of 4% Brewer's thioglycolate medium (Sigma; St. Louis, MO, USA) into the peritoneal cavities of male BALB/c mice (aged 6–8 weeks). Peritoneal cells were obtained by peritoneal lavage with ice-cold RPMI-1640 medium seven days after injection; cells were seeded in 60×15-mm tissue culture dishes at a density of 2×106 cells/ml of RPMI-1640 medium (Invitrogen (Gibco), Grand Island, NY, USA) supplemented with 10% heat-inactivated fetal calf serum (FCS), 5 mg/ml Gentamycine, 1 mg/ml kanamycine, 1.2 mM sodium pyruvate, 0.2 mM β-mercaptoethanol, and 2 g/l sodium bicarbonate. The cells were incubated at 37oC overnight, at which point non-adherent cells were removed by repeated washing with RPMI-1640 medium and then incubated in RPMI-1640 medium with 10% FCS and finally plated at a density of 1×105 cells/100 µl/well in 96-well plates. Cells were treated with ZZ-CWE, at the final concentrations of 0, 0.5, 5, 50, 500 µg/ml for 20 minutes, and doses were assayed in triplicate. Cells were stimulated with 1.5 µg/ml of lipopolysaccharides (LPS E. coli 0127:B8) (Sigma) for an additional 22 hours. TNF-α levels in the cultured medium were assayed using a commercially available ELISA kit (mouse TNF-α, DuoSet Systems, R&D Systems, Inc., Minneapolis, MN, USA) following the protocol outlined by the manufacturer. Briefly, the 96-well plates, pre-coated with the capture antibody, were washed three times with washing buffer prior to the application of 100 µl of test or standard samples to each well. Following sample application, plates were incubated with detection antibody solution (biotinylated goat anti-mouse TNF-α) at room temperature for 2 h and then washed twice. Then, 100 µl of streptavidin-HRP conjugated antibody solution was added and then incubated at room temperature for an additional 20 minutes. For signal detection, plates were washed and 100 µl of TMB was added; the reaction was stopped by adding 50 µl of 1 N HCl to each well. The optical density (OD) of each well was measured at 450 nm using a microplate reader (Thermo, Multiskan Spectrum, Vantaa, Finland). The inhibition percentage of TNF-α release in each treated-cell group (TNF-αt) was calculated using the following formula:
IL-4 release from EL-4 lymphocytes
EL-4 cells (1×105 cells/100 µl/well) in RPMI-1640 medium with 10% FCS were treated with 0, 0.5, 5, 50, 500 µg/ml of ZZ-CWE for two hours at 37oC and then stimulated with 1.5 ng/well of phorbol-12-myristate-13-acetate (PMA) and 15 ng/well of A23187 at 37oC for additional 22 h. Levels of IL-4 in cell-free supernatants were assayed using a commercially available ELISA kit (mouse IL-4, DuoSet Systems, R&D Systems, Inc.; Minneapolis, MN, USA), following manufacturer protocol. The inhibition percentage of IL-4 release in each treated-cell group (IL-4t) was calculated using following formula:
Animals
Female specific-pathogen-free (SPF) ICR mice, 4-weeks-old, were obtained from the National Laboratory Animal Center in Taiwan, and maintained on standard animal chow diet and water ad libitum. Permission and approval for animal studies was obtained from the Center for Research Animal Care and Use Committee of the National Pingtung University of Science & Technology. All animal experiments in this study were conducted in accordance with the accepted principles for laboratory animal use and care as described in the US guidelines (NIH publication #85-23, revised in 1985).
Anti-pulmonary inflammatory activities in vivo
Mice were housed under SPF conditions and were randomised into two groups for experimentation (the ZZ-CWE-treated and the control groups). Experimental mice were orally administrated with ZZ-CWE at a dosage of 100 mg/kg body weight per day for 28 days consecutively. At the conclusion of 28 days, all mice were anaesthetised with sodium pentobarbital and a blood sample was taken from each mouse, after which 20 ml of Tyrode's buffer (consisting of 130 mmol/l NaCl, 5 mmol/l KCl, 2.5 mmol/l CaCl2, 1.75 mmol/l NaKHPO4, 1.2 mmol/l MgSO4, 24 mmol/l NaHCO3, and 10 mmol/l glucose) per animal was immediately used to perfuse the lung. After perfusion, 0.5 g of lung tissue per animal was taken from the same portion of each lung. The tissue was minced (using a No. 11 surgical blade, chopping 100 times in 30 seconds) and then incubated in 10 ml Tyrode's buffer with 95% O2 at 37oC for 45 minutes. At this point, LTC4 in the medium was purified with a C18 cartridge (Waters, Milford, MA) and then quantified by the LTC4 EIA kit (Cayman Chemical Company, Ann Arbor, Michigan, USA). Levels of IL-1α in serum were assayed utilising the IL-1α ELISA kit (Cayman Chemical Company). Serum levels of AST, ALT, BUN and creatinine were examined using commercial diagnostic reagents obtained from Sigma (St. Louis, MO, USA).
Anti-hypersensitive activities in vivo
Mice were housed under SPF conditions and for experimentation were randomised into three groups: ZZ-CWE group, negative control (NC) group, and parallel control (PC) group. In the NC group, mice remained untreated (i.e. no oral administration or treatment for induction of an asthmatic reaction). Mice in the ZZ-CWE-treated group were orally administrated 100 mg/kg body weight of ZZ-CWE per day for 56 consecutive days. Animals in PC and ZZ-CWE groups were subjected to treatment inducing an asthmatic reaction. Namely, mice were sensitised with ip administration of 100 µl of 0.9% saline with ovalbumin (20 µg/mouse) and aluminum hydroxide (2 mg/mouse) starting on the 42nd day for three consecutive days; the asthmatic reaction was induced on the 56th day by administration of 10 ml of aerosol with 1% ovalbumin into the trachea 20 minutes before animals were sacrificed. Splenocytes were isolated by mechanical disruption of the spleen immediately followed by the hypotonic lysis of erythrocytes in suspension. Splenocytes were then cultured in RPMI-1640 medium supplemented with 10% FCS, with or without the addition of 10 µg ConA/2×106 cells/ml/well (Sigma) in a 24-well plate and incubated at 37oC for 24 h. After incubation, total RNA from cultured splenocytes was extracted using Trizol with manufacturer protocol (Invitrogen-Gibco) and immediately converted into cDNA with MMLV reverse transcriptase using a RT kit (Promega Corp., Sydney, Australia). Primers specific to mouse were designed for use in cloning the standard and competitor plasmids of IFN-γ and IL-4 (). The 5′ forward competitor primers for IFN-γ and IL-4 were designed as a 36-bp fragment containing the 5′ forward standard primer (20 bp) followed by a 16-bp fragment reading from the shortening 100 bp of 5′ sequences of standard plasmids of IFN-γ and IL-4. The 3′ reverse primers were the same as those used for making standard plasmids. Then, the standard/competitor plasmids of IFN-γ and IL-4 were cloned, and their PCR insertions were amplified and confirmed by sequencing. In this qc-RT-PCR assay, a constant amount of standard plasmids were competing with the competitor plasmids in depletion of the standard primers quantity per reaction, to amplify their own (standard) PCR products. Competitor and standard PCR products, differing in size by 100 bp (standard product longer), can be distinguished with ethidium-bromide staining bands on a 2% agarose gel, of which intensity of optic density (IOD) of each band was analysed using the Image Quant Densitometer (Molecular Dynamics) to yield a standard curve. Splenocytes gene expression levels of IFN-γ and IL-4 were evaluated using the described qc-RT-PCR assay. Serum levels of AST, ALT, BUN, creatinine, IgG2a and IgE were determined by the use of commercial diagnostic reagents (Sigma and Cayman Chemical Company, respectively).
Table 1. Primer design for qc-RT-PCR.
Evaluation of hepatotoxicity
Mice were treated with various doses of ZZ-CWE (0, 10, 100, 1000 mg/kg body weight per day) for 60 days and then sacrificed. Serum levels of AST, ALT, BUN and creatinine were determined, and histopathological changes in liver and kidney tissue were also examined.
Statistical analysis
Data obtained from the study were analysed using the general linear model (GLM) procedures of the SAS program (SAS Institute Inc.), and the Duncan's multiple range tests were used to compare the differences of means between groups. Values for p<0.05 were considered significant.
Results
Anti-inflammatory activities of ZZ-CWE in vitro
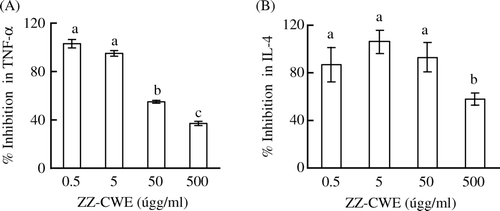
The treatment of 50 and 500 µg/ml of ZZ-CWE significantly inhibited the release of TNF-α of murine peritoneal macrophages in response to LPS stimulation (). The dose-dependent suppression of TNF-α release by ZZ-CWE was observed. In addition, the least effective dosage of ZZ-CWE to inhibit IL-4 production in EL-4 lymphocytes was 500 µg/ml ().
Figure 1. Inhibition of TNF-α and IL-4 production in cells treated with ZZ-CWE. Cells (1×105 cells/100 µl/well) were treated with various doses (0, 0.5, 5, 50 and 500 µg/ml) of ZZ-CWE in triplicate as detailed in the Methods and materials section. Data are presented as mean±SEM. Means annotated with different superscripts were significantly different (p<0.05).
Anti-pulmonary inflammatory activities in vivo
The lung tissue of ZZ-CWE-treated mice released significantly lower amounts of LTC4 than did that of control animals (429±69 versus 261±23 pg/lung) (). Serum levels of AST and ALT in mice treated with ZZ-CWE for four weeks did not differ significantly from control serum levels. Despite elevated serum creatinine level of ZZ-CWE-treated mice, the group showed no pathological renal disorders. The serum BUN levels of control and ZZ-CWE-treated groups were not significantly different, which further indicated normal renal function of mice treated with ZZ-CWE (). Furthermore, serum IL-1α levels of ZZ-CWE-treated mice did not differ from those of controls. These observations indicated that ZZ-CWE administration is not detrimental to liver or to renal function and also that no inflammation was caused by the long-term oral administration of ZZ-CWE.
Figure 2. Serum IL-1α and LTC4 release from lung tissue of ZZ-CWE-treated and untreated mice. *p<0.05 compared to controls (n=10).
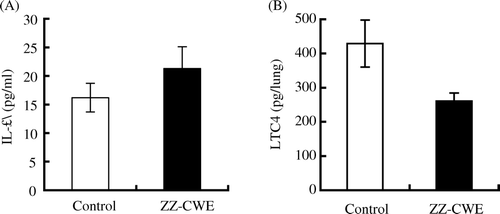
Table 2. Serum levels of AST, ALT, BUN and creatinine in ZZ-CWE-treated mice and control groups.
Anti-hypersensitive activities in vivo
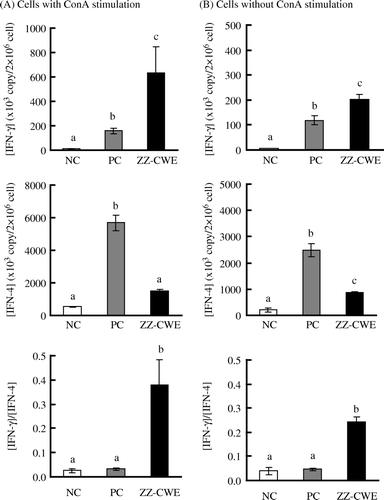
High regression coefficients for IFN-γ and IL-4 standard curves were obtained in the qc-RT-PCR assays ( and ). The sensitised mice in PC and ZZ-CWE groups with induction of allergic inflammation yielded significantly higher mRNA expressions of both IL-4 and IFN-γ in their splenocytes when compared to those in the NC group (p<0.05) (). In the ConA-stimulated splenocytes, PC gene expressions of IFN-γ and IL-4 were 12 and 11 times, respectively, of NC expression levels; this trend increased to IFN-γ and IL-4 levels that were 50 and 3 times, respectively, in ZZ-CWE-treated mice compared to those of the NC group. IFN-γ and IL-4 gene expression in splenocytes without ConA stimulation was, respectively, increased about 30 and 4-fold in ZZ-CWE-treated mice relative to those in NC, and increased about 20 and 12-fold in PC mice relative to the NC (). Thus, the 5- and 12-fold increments in IFN-γ/IL-4 ratios were observed in splenocytes stimulated with ConA or without ConA, respectively, in ZZ-CWE-treated mice as compared with those in PC group. Similar ratios of IFN-γ/IL-4 gene expression in splenocytes were observed between NC and PC groups.
Figure 3. Agarose gel electrophoresis of PCR productions of IFN-γ (A) and IL-4 (B), amplified from the standard or competitor plasmids in qc-RT-PCR assays. M indicates marker with a 100-bp interval between each band. In the qc-RT-PCR assay, a constant amount of standard plasmids was in competition with competitor plasmid for depletion of standard primers for amplification of their own PCR products.
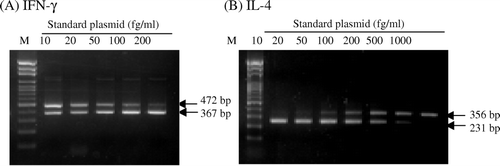
Figure 4. The IFN-γ(A) and IL-4 (B) standard curves in qc-RT-PCR assays. The intensity of optic density (IOD) was analysed using the Image Quant Densitometer. The standard curve was formulated by a linear regression with common logarithmic concentrations of standard plasmids of IFN-γ as the X-axis and natural logarithmic ratios of IODstandard to IODcompetitor as the Y-axis.
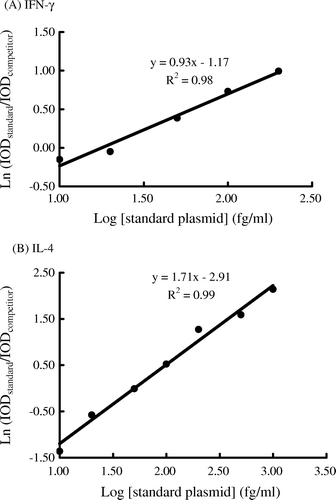
Figure 5. Splenocyte gene expression of IFN-γ and IL-4 in hypersensitised versus non-hypersensitised mice. Ten mice per group in each of ZZ-CWE-treated, negative control (NC) and parallel control (PC) groups were treated as described in the Methods and materials section. Splenocytes were cultured with or without the addition of 10 µg ConA/2×106 cells/ml/well and incubated at 37oC for 24 h. Means annotated with different superscripts were significantly different (p<0.05).
Evaluation of hepatotoxicity
No significant differences in serum AST and ALT levels among NC, PC and ZZ-CWE groups were observed (). Elevated serum creatinine levels were observed in the ZZ-CWE-treated animals, while serum BUN levels again remained unchanged with ZZ-CWE treatment relative to NC and PC groups.
Table 3. Serum levels of AST, ALT, BUN, creatinine, IgG2a and IgE in hypersensitised ZZ-CWE-treated, hypersensitised untreated versus non-hypersensitised mice.
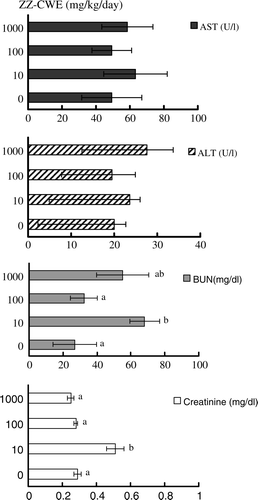
No hepatotoxicity was observed in mice treated with various doses of ZZ-CWE, inferred after assay quantification of serum levels of AST and ALT (). Elevated BUN and creatinine levels were observed in the mice treated with 10 mg/kg body weight per day for 60 days; interestingly, no differences in serum levels of BUN and creatinine were observed in mice treated with 100 or 1000 mg/kg body weight per day (both comparisons made relative to no ZZ-CWE-treatment control group) ().
Figure 6. Effects of different ZZ-CWE dosages on serum levels of AST, ALT, BUN and creatinine in mice. Mice were treated with various doses (0, 10, 100, 100 mg/kg body weight per day) of ZZ-CWE for 60 days consecutively (four animals per group). Means annotated with different superscripts were significantly different (p<0.05).
Discussion
In the present study, significant TNF-α inhibition (55 and 38%) was observed in ZZ-CWE-treated macrophages at the doses of 50 and 500 µg/ml. This data confirmed that ZZ-CWE contains the anti-inflammatory component. Zerumbone from ZZ has been known to possess anti-tumour activities (Huang, Chien, Chen, & Wang, Citation2005; Sharifah-Sakinah, Tri-Handayani, & Azimahtol-Hawariah, Citation2007; Takada, Murakami, & Aggarwal, Citation2005). It can also effectively decrease prostaglandin E2 release from HL-60 cells (Murakami et al., Citation2002). However, this known bioactive compound of ZZ is hardly dissolved in water-soluble fractions. Some other biologically active compounds should therefore be present in the ZZ-CWE. Recent studies indicate that 6-gingerol, a compound present in the extracts of various ginger-containing products (Schwertner & Rios, Citation2007), inhibits TNF-α and IL-1β productions from LPS-stimulated macrophages and prevents ROS productions and COX-2 expression (J. Kim, Y. Kim, Na, Surh, & Kim, Citation2007; Tripathi, Maier, Bruch, & Kittur, Citation2007). In addition, kaempferol glycosides and kaempferol derivatives in the water extracts of ZZ have been shown to significantly inhibit CYP3A4 and CYP2D6 activities (Usia et al., Citation2004; Usia, Watabe, Kadota, & Tezuka, Citation2005). Another possible biologically active compound, 5,7-dihydroxy-2-(4-hydroxy-phenyl)-3-methoxy-chromen-4-one, which significantly inhibits TNF-α and LTC4 release in vitro (Chaung, Huang, Lee, & Lin, Citation2007), has been identified in the ZZ-CWE extract in our study. However, it still needs more in vivo studies to confirm whether gingerols, kaempferols or chromenone are responsible bioactive compounds in ZZ-CWE.
LTC4 has been implicated in playing a significant role in the pathophysiology of asthma (Piper, Citation1983). Many novel drugs designed to inhibit the synthesis of LTC4 have been developed for the treatment of asthma (Ramires, Caiaffa, Tursi, Haeggstrom, & Macchia, Citation2004). In our study, the 39% reduction of LTC4 in ZZ-CWE-treated mice and the serum IL-1α levels remained unchanged in ZZ-CWE-treated mice compared to levels of controls () suggests that the medicinal components in ZZ-CWE could be effective in improving symptoms of asthmatic patients but without exacerbating inflammatory reactions. This result further supports the feasibility of using ZZ-CWE for the long-term treatment of asthmatic patients.
In addition to the villainous roles LTC4 plays during anaphylaxis, evidence has demonstrated that excess production of IL-4, IL-5, and IFN-γ of airway CD4+ and CD8+ T cells, is also involved in regulating the immune response in human asthmatic attacks (Cho, Stanciu, Holgate, & Johnston, Citation2005). In our study, ZZ-CWE significantly increased splenocyte gene expression of IFN-γ (p<0.05) and reduced gene expression of IL-4 (p<0.05) (), findings which were consistent with observations of increased IL-4 and IFN-γ productions in subjects with asthma. Currently, allergen-specific immunotherapy targets other mechanisms in addition to the inflammatory reactions of an asthmatic attack, such as ‘blunting off’ the allergen-specific IgE, altering the Th1/Th2 balance and down-regulating Th2 cytokine production in response to allergen (Erin et al., Citation2005). It has been well documented that the balance between IL-4 and IFN-γ plays a major role in regulating IgE synthesis in asthmatic patients (Stokes & Casale, Citation2004). And thus, most newly developed drugs act to inhibit the production of inflammatory mediators and Th2 cytokines in response to allergens for the treatment of asthma (Poff & Balazy, Citation2004). In our study, the ratios of splenocyte IFN-γ/IL-4 gene expression in ZZ-CWE-treated mice increased 5- and 12-fold, respectively, compared to PC group ratios, which may eliminate allergic asthmatic reactions through long-term up-regulation of the Th1/Th2 response in ZZ-CWE-treated group. Thus, these results indicated that ZZ-CWE has the potential to be a functional food in diminishing hypersensitive reactions by modulating the cytokine gene expression in immune cells after oral administration of ZZ-CWE.
Although mice in both PC and ZZ-CWE groups were sensitised in order to be induced an allergic asthmatic attack, no differences were observed in serum levels of AST and ALT compared to levels of the NC group (). Therefore, no hepatotoxicity was observed in mice having been treated with ZZ-CWE for as long as 60 days. In additional study yielding conflicting data regarding increased BUN and creatinine levels in mice treated with 10, but not with 100 or 1000 mg/kg body weight per day for 60 days, we examined the histopathological changes in three pieces of liver, kidney and lung tissues from each mouse. No pathological alterations or problems were found in any tissue (unpublished data).
In summary, ZZ-CWE may have beneficial effect for the treatment of asthmatic patients through its capabilities to inhibit the synthesis of LTC4 and through the immunomodulation of Th1/Th2 cytokine production, but without any hepatotoxicity complications.
Acknowledgements
This work was supported by a grant from Wholesome Life Science Co., Ltd (Taipei, Taiwan).
References
- Aizawa , T. , Tamura , G. , Ohtsu , H. and Takishima , T. 1990 . Eosinophil and neutrophil production of leukotriene C4 and B4: Comparison of cells from asthmatic subjects and healthy donors . Annals of Allergy , 64 : 287 – 292 .
- Beghe , B. , Barton , S. , Rorke , S. , Peng , Q. , Sayers , I. Gaunt , T. 2003 . Polymorphisms in the interleukin-4 and interleukin-4 receptor alpha chain genes confer susceptibility to asthma and atopy in a Caucasian population . Clinical and Experimental Allergy , 33 : 1111 – 1117 .
- Chaung , H.C. , Huang , C.S. , Lee , F.Y. , & Lin , T.C. 2007 . Anti-hypersensitive inflammation and anti-allergy activities of Zingiber zerumbet (L.) Smith . Taiwan Patent No. I282281 .
- Cho , S.H. , Stanciu , L.A. , Holgate , S.T. and Johnston , S.L. 2005 . Increased interleukin-4, interleukin-5, and interferon-gamma in airway CD4+ and CD8+ T cells in atopic asthma . American Journal of Respiratory and Critical Care Medicine , 171 : 224 – 230 .
- Davern , T.J. and Bass , N.M. 2003 . Leukotriene antagonists . Clinics in Liver Disease , 7 : 501 – 512 .
- Erin , E.M. , Zacharasiewicz , A.S. , Nicholson , G.C. , Tan , A.J. , Higgins , L.A. Williams , T.J. 2005 . Topical corticosteroid inhibits interleukin-4, -5 and -13 in nasal secretions following allergen challenge . Clinical and Experimental Allergy , 35 : 1608 – 1614 .
- Farnsworth , N.R. and Bunyapraphatsara , N. 1992 . Thai medicinal plants , Bangkok, , Thailand : Prachachon .
- Flamand , N. , Mancuso , P. , Serezani , C.H. and Brock , T.G. 2007 . Leukotrienes mediators that have been typecast as villains . Cellular and Molecular Life Sciences , 64 : 2657 – 2670 .
- Huang , G.C. , Chien , T.Y. , Chen , L.G. and Wang , C.C. 2005 . Antitumor effects of zerumbone from Zingiber zerumbet in P-388D1 cells in vitro and in vivo . Planta Medica , 71 : 219 – 224 .
- Kim , J.K. , Kim , Y. , Na , K.M. , Surh , Y.J. and Kim , T.Y. 2007 . [6]-Gingerol prevents UVB-induced ROS production and COX-2 expression in vitro and in vivo . Free Radical Research , 41 : 603 – 614 .
- Lee , Y.C. , Lee , K.H. , Lee , H.B. and Rhee , Y.K. 2001 . Serum levels of interleukins (IL)-4, IL-5, IL-13, and interferon-gamma in acute asthma . Journal of Asthma , 38 : 665 – 671 .
- Leff , A.R. 2001 . Regulation of leukotrienes in the management of asthma: Biology and clinical therapy . Annual Review of Medicine , 52 : 1 – 14 .
- Murakami , A. , Takahashi , D. , Kinoshita , T. , Koshimizu , K. , Kim , H.W. Yoshihiro , A. 2002 . Zerumbone, a Southeast Asian ginger sesquiterpene, markedly suppresses free radical generation, proinflammatory protein production, and cancer cell proliferation accompanied by apoptosis: The alpha, beta-unsaturated carbonyl group is a prerequisite . Carcinogenesis , 23 : 795 – 802 .
- Nakamura , Y. , Yoshida , C. , Murakami , A. , Ohigashi , H. , Osawa , T. and Uchida , K. 2004 . Zerumbone, a tropical ginger sesquiterpene, activates phase II drug metabolizing enzymes . FEBS Letters , 572 : 245 – 250 .
- Piper , P.J. 1983 . Leukotrienes: Possible mediators in bronchial asthma . European Journal of Respiratory Diseases , 129 (Suppl.) : 45 – 64 .
- Poff , C.D. and Balazy , M. 2004 . Drugs that target lipoxygenases and leukotrienes as emerging therapies for asthma and cancer . Current Drug Targets. Inflammation and Allergy , 3 : 19 – 33 .
- Ramires , R. , Caiaffa , M.F. , Tursi , A. , Haeggstrom , J.Z. and Macchia , L. 2004 . Novel inhibitory effect on 5-lipoxygenase activity by the anti-asthma drug montelukast . Biochemical and Biophysical Research Communications , 324 : 815 – 821 .
- Schwertner , H.A. and Rios , D.C. 2007 . High-performance liquid chromatographic analysis of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol in ginger-containing dietary supplements, spices, teas, and beverages . Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences , 856 : 41 – 47 .
- Sharifah-Sakinah , S. , Tri-Handayani , S. and Azimahtol-Hawariah , L. 2007 . Zerumbone induced apoptosis in liver cancer cells via modulation of Bax/Bcl-2 ratio . Cancer Cell International , 7 : 4 – 14 .
- Shirai , T. , Suzuki , K. , Inui , N. , Suda , T. , Chida , K. and Nakamura , H. 2003 . Th1/Th2 profile in peripheral blood in atopic cough and atopic asthma . Clinical and Experimental Allergy , 33 : 84 – 89 .
- Stokes , J. and Casale , T.B. 2004 . Rationale for new treatments aimed at IgE immunomodulation . Annals of Allergy, Asthma & Immunology , 93 : 212 – 217 .
- Takada , Y. , Murakami , A. and Aggarwal , B.B. 2005 . Zerumbone abolishes NF-kappaB and IkappaBalpha kinase activation leading to suppression of antiapoptotic and metastatic gene expression, upregulation of apoptosis, and downregulation of invasion . Oncogene , 24 : 6957 – 6969 .
- Tripathi , S. , Maier , K.G. , Bruch , D. and Kittur , D.S. 2007 . Effect of 6-gingerol on pro-inflammatory cytokine production and costimulatory molecule expression in murine peritoneal macrophages . Journal of Surgical Research , 138 : 209 – 213 .
- Usia , T. , Iwata , H. , Hiratsuka , A. , Watabe , T. , Kadota , S. and Tezuka , Y. 2004 . Sesquiterpenes and flavonol glycosides from Zingiber aromaticum and their CYP3A4 and CYP2D6 inhibitory activities . Journal of Natural Products , 67 : 1079 – 1083 .
- Usia , T. , Watabe , T. , Kadota , S. and Tezuka , Y. 2005 . Mechanism-based inhibition of CYP3A4 by constituents of Zingiber aromaticum . Biological & Pharmaceutical Bulletin , 28 : 495 – 499 .
- Vimala , S. , Norhanom , A.W. and Yadav , M. 1999 . Anti-tumour promoter activity in Malaysian ginger rhizobia used in traditional medicine . British Journal of Cancer , 80 : 110 – 116 .
- Wenzel , S.E. 2003 . The role of leukotrienes in asthma . Prostaglandins, Leukotrienes and Essential Fatty Acids , 69 : 145 – 155 .