Abstract
Two ciprofloxacin (CF)–bovine serum albumin conjugates with oppositely orientated hapten and gemifloxacin (GF)–glucose oxidase synthesised by activated esters method, carbodiimide condensation and glutaraldehyde linking, respectively, were used as immunogens. A wide spectrum of immobilised antigens different in carrier, type of hapten (CF, GF, sparfloxacin), hapten load and conjugating method were prepared. Thirteen variants of competitive indirect enzyme-linked immunosorbent assay (ELISA) using three rabbit antisera and solid-phase conjugates were developed to detect fluoroquinolones (FQs). The immunoreagent combinations allowed to determine CF and GF selectively, to carry out the quantification of CF, enrofloxacin, norfloxacin, pefloxacin and lomefloxacin practically with equal efficacy. Under the conditions of group-specific ELISAs the immunochemical activity of 9–10 FQ representatives differed only 10–20 times. The possibility of efficient influence on ELISA specificity and sensitivity with especially selected immobilised antigens was demonstrated.
Introduction
Fluoroquinolones/quinolones (FQs) are a numerous group of antibacterial synthetic agent derivates of 3-carboxyquinolone or 3-carboxynaphthyridone. Its inhibition effect on DNA gyrase and topoisomerase IV followed by failure of DNA replication and protein synthesis in microorganism results in a broad spectrum of bactericidal action. Because of its activity with respect to aerobic, anaerobic, gram-negative and gram-positive bacteria, mycobacteria, chlamydia, mycoplasma, rickettsia, borrelia, as well as some protozoa FQ come to be in much need for treatment of the infections both in veterinary practice and medicine.
The potential contamination of animal origin foodstuffs with FQs made it necessary for monitoring the quality of these materials and to develop a method to screen for the possible presence of the drug residues. The maximum residue levels (MRLs) established by European Union for various FQs in meat products, poultry, fish and milk range from 10–1900 µg/kg (CR ECC, Citation1990).
A series of works concerning antibody production and immunoassay developing of ciprofloxacin (CF) (Duan & Yuan, Citation2001), sarafloxacin (Holtzapple, Buckley, & Stanker, Citation1997), enrofloxacin (EF) (Watanabe, Satake, Kido, & Tsuji, Citation2002), flumequin (Van Coillie, De Block, & Reybroeck, Citation2004), and pefloxacin (Lu et al., Citation2006) were based on using conjugates of the above substances synthesised by the activated esters method or carbodiimide condensation as a result of interaction between quinolone carboxyl at C3 and protein amine group.
However, expanding the number of agents used make to focus on developing more efficient assays with group specificity (Loomans, van Wiltenburg, Koets, & van Amerongen, Citation2003; Spinks, Wyatt, Lee, & Morgan, Citation1999; Usleber, Litz, & Martlbauer, Citation1998). For better presentation of common FQs epitopes on the immunogen surface which is the region of quinolone ring with carboxyl radical at C3 position the different steric orientation of the hapten molecule on the carrier was proposed. Hence, the secondary amine in piperazine of norfloxacin (NF) (Bucknall, Silverlight, Coldham, Thorne, & Jackman, Citation2003) and carboxyl derivate in sarafloxacin piperazine cycle (Huet et al., Citation2006) were involved as reacting groups to produce immunogens. These approaches permitted to raise antibodies and develop the assay for determination of a wide spectrum of representatives of the FQ group.
This paper describes the preparation of immunoreagents based on gemifloxacin (GF), CF and sparfloxacin (SF) and estimation of its usage in indirect enzyme-linked immunosorbent assay (ELISA) for selective or group determination of FQs.
Materials and methods
Chemicals and reagents
CF, ofloxacin (OF) and pefloxacin (PF) were from Sintez Ltd (Moscow, Russia). GF from LG Life Sciences (Seoul, Korea), EF from Bayer AG (Leverkusen, Germany), NF and nalidixic acid (NA) were obtained from KRKA (Moscow, Russia). Lomefloxacin (LoF) and SF from Ipca Labs Ltd (Moscow, Russia), and levofloxacin (LeF) from Hoechst Marion Roussel (Frankfort-am-Main, Germany). Glucose oxidase (GO, (EC 1.1.3.4)), N-hydroxysuccinimide, 1-ethyl-3-(3-dimethyl-aminopropyl) carbodiimide (EDC), glutaraldehyde, and o-phenylenediamine were purchased from Sigma (St. Louis, MO). Peroxidase conjugate of donkey antiserum to rabbit IgG, bovine serum albumin (BSA), ovalbumin (OVA) and gelatine (Gel) were from Farmateh (Moscow, Russia).
To prepare standards the doses of weighed substances were poured with estimated volumes of dimethylformamide and stirred intensively for 2 days. Prepared solutions were diluted 100 times with water and analysed spectrophotometrically using a Hitachi-557 device (Japan). The concentration of solutions was determined by the optical density at maximum absorption range from 270 to 300 nm and molar extinction coefficients indicated in the works (Clark, Citation1986; Titov, Dorofeev, & Arzamastsev, Citation2004).
Conjugated antigens synthesis
BSA–CF×100(ae)
N-hydroxysuccinimide (10 mg, 45 µmol) and 1-ethyl-3-(3-dimethyl-aminopropyl) carbodiimide (EDC, 9 mg, 45 µmol) were added to 10 mg of CF (30 µmol) in 1.0 mL DMF. After 1-, 10- and 24-h stirring at room temperature 0.33 mL of each reaction mixture was added dropwise to BSA solutions (7 mg, 0.1 µmol) in 2 mL of 0.05 M carbonate–bicarbonate buffer (CBB, pH 9.5), incubated during 16 h at room temperature and dialysed against 0.5% NaCl.
Gel-CF(ae)
Under the same conditions of 24-h incubation gel was conjugated with CF taken in 5-, 10- and 25-fold molar excess over protein.
BSA-CF(f)
Three mixtures of BSA (5 mg, 0.07 µmol) in 1.5 mL of water, CF (0.7, 2.1 and 7 µmol) and 0.3 ml of 37% formaldehyde solution (3690 µmol) were stirred at 30°C for 16 h and then dialysed three times against 1000 volumes of 0.5% NaCl.
OVA-SF(ga), Gel-SF(ga)
To 1 ml solutions containing OVA (3 mg, 0.07 µmol) or Gel (3 mg, 0.025 µmol) in CBB 100- and 300-fold molar excess of SF (2.6, 7.9 mg, and 1.0, 2.9 mg, respectively) was added together with 30 µl freshly prepared 2.5% solution of glutaraldehyde. Followed by 2-h stirring at room temperature each reaction mixture was supplemented with 0.1 ml of aqueous solution of sodium borohydride (1 mg/ml), maintained in a refrigerator for 1 h with periodic stirring and dialysed against 2×5 L 0.15 M phosphate-buffered saline (PBS, pH 7.4) for 2 days.
GO-GF(ga), Gel-GF(ga)
In the same manner GO (8 mg, 0.05 µmol) and Gel (4 mg, 0.033 µmol) were conjugated with GF taken in 75- and 10-, 30-, and 100-fold molar excess (3.75 and 0.16, 0.5, 1.6 mg, respectively).
BSA-CF(edc), OVA-CF(edc)
The above conjugates were synthesised by carbodiimide condensation. For this purpose to two portions of BSA (5 mg, 0.07 µmol) and OVA (4 mg, 0.1 µmol) in 2 mL of water were added CF (2, 4 and 2 mg–6, 12 and 6 µmol) and EDC (30 and 25 mg–156 and 130 µmol). Reaction mixtures were stood for 1–4 h at room temperature and then another 16 h at 4°C. After this incubation exhaustive dialysis of each conjugate in 0.2% sodium chloride was carried out.
All the prepared conjugates were supplemented with equal volumes of glycerol and stored at −10°? as solutions with concentration of 1 mg/ml.
The linkage formation between hapten and carrier was proved by registration of specific changes in UV spectrum of conjugate in comparison with carrier spectrum and/or by interaction with antibodies in ELISA. UV spectra were recorded on a Hitachi-557 device (Japan).
Immunisation and antisera preparation
BSA-CF(ae), BSA-CF(edc), and GO-GF(ga) conjugates were chosen for immunisations realised identically. The rabbits (2.5–3 kg) were injected subcutaneously (into 15–20 loci on the back) with FQ-conjugate, emulsified in complete Freund's adjuvant (0.1 mg/animal). Then the same immunogen doses were administered in physiological saline at 4-week intervals. One week after the second and subsequent injections, blood samples were taken from the ear marginal vein, the serum was separated, supplemented with an equal volume of glycerol and stored until utilisation at –10 to –15°C.
ELISAs
To analyse the development of animal immune response each sample of antiserum was tested under conditions of indirect enzyme immunoassay in 96-well plates (Costar). Then the immunoreagent optimal ratio was chosen and used in competitive indirect ELISA. For this purpose, the plate wells were filled with 0.2 ml of synthesised conjugates in several concentrations (0.05–0.5 mg/ml) in CBB and incubated at 4°C for 16 h. The wells were washed 3–5 times with PBS containing 0.05% Tween 20 (PBS-t), and 0.1 ml of antiserum serial dilutions in PBS-t with 1% BSA and standard solutions of CF (10,000–0.1 ng/ml) (B) and 0 ng/ml (B 0) were added to each well. The plates were left in a humid chamber at room temperature for 1 h and washed again; the wells were filled with 0.2 ml solution of anti-rabbit IgG antibodies conjugated with horseradish peroxidase. After 1-h incubation and washing, the wells were filled with 0.2 ml of the substrate solution containing 0.4 mg/ml o-phenylenediamine and 0.005% hydrogen peroxide in 0.15 M citrate–phosphate buffer (pH 5.0). The enzymatic reaction was stopped after 45 min with 50 µl of 4 M sulphuric acid with 0.1 M Na2SO3 per well. The optical density was measured at 492 nm using a Dynatech MR 5000 (Germany) reader.
The colour reaction in wells with null CF concentration (B 0) was taken as 100% antibody binding level. The extent of antibody binding in the presence of each CF standards was normalised to 100% control, and antibody binding rate was calculated as
The sensitivity of assay was evaluated as 20% inhibition or 80% antibody binding level. The specificity of antibodies was assessed under optimal ELISA conditions. Calibration curves were plotted for each substance, and the concentration that induced 50% inhibition of antibody binding to the solid phase (IC50) was calculated. Cross-reactivity was calculated as the ratio of IC50 for CF to the corresponding EF, NF, PF, OF, LeF, LoF, SF, GF and NA concentrations.
Results and discussion
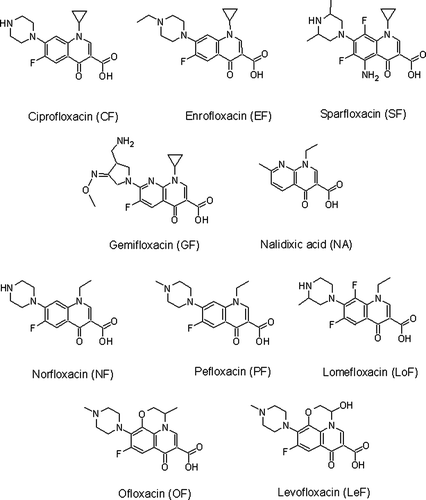
The UV spectra shown in (a)–(c) demonstrate the most evident changes in protein spectra, as a result of the addition of hapten molecules. The pattern of these changes are the following: peaks gain at λ 275 and 325 nm that agrees with maximum absorption for FQ, the content of its expression depending on the amount of hapten taken in synthesis proved conjugate formation. Using BSA-CF×100(ae)-24h and GO-GF×75(ga) antigens with opposite spatial hapten arrangement on the carrier as immunogens were aimed to present different regions of FQ molecule for recognising immunocompetent cells – more individual epitopes in the case of CF or group-specific determinants of GF.
The immunisation with the third antigen BSA-CF×85(edc) was realised to reproduce carbodiimide condensation involving the secondary amine of piperazine and results were compared with described ones (Bucknall et al., Citation2003). In spite of spectrophotometric data supporting the evidence of conjugate formation () it was not clear what functional groups were involved in reaction: the secondary amine of CF and carboxyl of BSA, or alternatively, the COOH-group of CF with protein amines.
The additional control experiments were carried out. Two compounds with the secondary amines in their structure – 7-nitro-5-phenyl-1,3-dihydro-2H-1,4-benzodiazepin-2-one (nitrazepamum) and l-1-phenyl-2-methylamino-propanol-1 (ephedrine) were conjugated accordingly by the same procedure. However, there were no typical changes in UV spectra of reaction products indicative of compound linking with albumin. Thus, the nature of ‘BSA–CF’ bond remained to be cleared up according to the specificity of antiserum, raised against this conjugate.
The conjugation procedure by activated esters method was found to have a special feature. The successful synthesis depended on preparing hapten (prolonged C3-carboxyl activation) rather than hapten excess. The influence of time factor (1-, 10- and 24-h activation) on the degree of coupling of CF with BSA at the same molar ratio (100:1) is demonstrated in (b). The similar conditions were used elsewhere (Duan & Yuan, Citation2001).
Figure 2. UV-spectrum of FQ-conjugates: (a) 1 – BSA, 2 – BSA-CF×85(edc), 3 – BSA-CF×170(edc); (b) BSA-CF×100(ae) 1 – 1 h, 2 – 10 h, 3 – 24 h, 4 – CF, 10 µg/ml, H2O; (c) 1 – GO, 2 – GO-GF×75(ga), 3 – GF, 10 µg/ml, H2O.
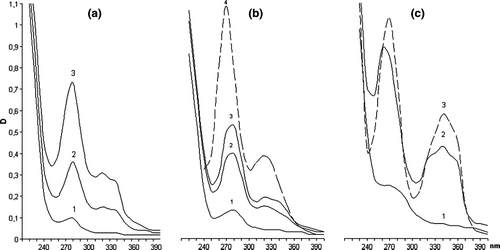
Figure 3. Determination of fluoroquinolones in competitive ELISA variant ‘Anti-BSA-CF(edc)–BSA-CF×100(f)’. (♦), CF; (○), GF; (×), EF; (▴), NF; (+), PF; (▪), LoF; (□), NA; (•), OF; (⋄), LeF; (▵), SF. Each value shows the mean of B/B 0 (n=3).
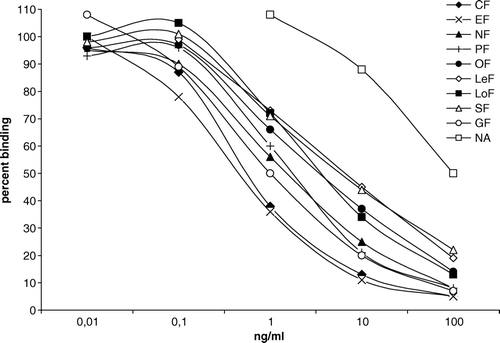
The criterion for successful synthesis of other conjugated antigens immobilised on the plates was not only the similar spectrophotometric parameters but also its interaction with antisera and inhibition of this interaction with free hapten.
Such experiments allowed us to choose among the whole panel of prepared conjugates a group of five – Gel-CF×25(ae), BSA-CF×100(f), OVA-SF×300(ga), Gel-GF×10(ga), OVA-CF×60(edc) – differed structurally in the type of FQ hapten and the manner of its linking to protein, and determine an optimal concentration for using as solid-phase antigens. The magnitude of molar excess hapten over the protein indicated for conjugates, provided more sensitive CF detection in comparison with the homologous antigens on the base of another protein carrier or different hapten load.
Antisera from rabbits immunised with BSA-CF(ae), BSA-CF(edc) and GO-GF(ga) were tested on binding with selected antigens; its optimal dilution was determined and these parameters were used for a specificity study.
The prominent heterologous property of SF-based solid-phase antigen made anti-GO-GF(ga) antibodies incapable of interacting with this conjugate (). On the contrary, binding affinity between antiserum to BSA-CF(edc) and homologous OVA-CF×60(edc) was so strong that no inhibition by free FQ was caused taken in high concentration (1000 ng/ml). Thus, out of 15 possible pairs of immunoreagents (three antisera and five antigens), 13 variants of immunoassay were examined.
Table 1. Comparison of fluoroquinolone IC50 values, cross-reactivity profiles and group recognition coefficients for ELISAs developed.
The IC50 values and cross-reaction rates for CF, GF, EF, NF, PF, LoF, NA, OF, LeF, and SF calculated in all the variants ‘antiserum–solid-phase antigen’ allowed us to characterise the assay specificity with respect to individual members or the group as a whole. The limit of detection was on the average of 1/20 of the concentration that induced 50% inhibition of antibody binding to the solid phase.
BSA-CF(ae) immunogen masked common-group epitopes as a result of conjugation and presented CF-specific determinants induced the antibody with preferred recognition of CF that is in complete agreement with published data (Duan & Yuan, Citation2001; Watanabe et al., Citation2002). Hence, the ELISA using BSA-CF×100(f) appeared to be the most selective for CF – the recognition of the nearest competitor, EF, was three times lower and the next FQs – more than 10 times lower. Another immobilised antigen modified the assay specificity in such a manner that the recognition of remaining FQs increased markedly. The inhibiting effect of GF and EF on antibody binding with Gel-GF×10(ga) and OVA-SF×300(ga) was higher than 100% CF level.
The polyclonal antibodies to immunogen GO-GF(ga) were the best for the determination of GF. The selectivity of GF detection (10 times more sensitive than the rest FQs) appeared to be the highest possible for homologous system (Gel-GF×10(ga)).
The group specificity should be interpreted as the capability of antibodies to recognise structurally relative substances with equal or near efficacy. It could be demonstrated with an example of interaction of anti-BSA-CF(edc) with EF and its metabolite CF, as well as anti-GO-GF(ga) activity towards CF, EF, NF, PF and LoF.
However, ‘group-specific assay’ means quite often that the assay permits to measure the concentrations at a certain rate (e.g. MRL) for the series of analytes. At the same time, the lack of equivalent recognition may be compensated; firstly, high sensitivity that increases the probability of immunochemically minor substances to be detected at the required rate, and secondly, wide operating range capable of overlapping threshold levels for both major and minor analytes.
The content (MRLs) in foodstuffs of animal origin for several FQs used in this work was not established; therefore, it seemed impossible to evaluate specificity at this criterion.
The highest sensitive variant for the most substances appeared to be the system ‘anti-BSA-CF(edc)–BSA-CF×100(f)’ revealing the following rate of concentrations: 0.08 ng/ml EF, 0.15 – CF, 0.2 – GF and NF, 0.3 – PF, 0.4 – OF, 0.6-LoF, LeF, SF and 15 ng/ml NA (). The near detection rate can be reached using immobilised Gel-CF×25(ae). The same threshold of sensitivity for GF was obtained in ‘anti-GO-GF(ga)–BSA-CF×100(f)’. The assay format ‘anti-BSA-CF(ae)–OVA-SF×300(ga)’ was the best sensitive in regard to NA (3 ng/ml) and SF (0.5 ng/ml).
To estimate the width of specificity spectrum or the extent of group recognition we use an approach described previously (Burkin & Smirnov, Citation2004). For each of the 13 ELISAs, the coefficients k 10 and k 9 were calculated as a ratio of IC50-index for inhibitor with the poorest activity (IC50 MAX-10) or the next after it (IC50 MAX-9) to the concentration of FQ with minimum IC50 value (IC50 MIN) (). The greatest k 10 and k 9 values, corresponding to the maximum range between extreme IC50 magnitudes in case of anti-BSA-CF(ae) can be explained by its selective specificity. The anti-GO-GF(ga) serum demonstrated the similar features: the highest k 10=100 corresponds to the best GF-selectivity.
The closely grouped standard curves pointed to a generic assay and little differences between ICs50 resulted in the least value of k. ‘Anti-BSA-CF(ae)–OVA-SF×300(ga)’ variant possessing the minimal k 10 value showed the best correlation with the definition of ‘group assay’ or ‘assay with broad specificity’. Under the conditions of this system the immunochemical activity of 10 FQ representatives was distinguished by a factor of 21. Whereas, for the described group specificity, ELISAs possessed a range for 10 cross-reacted FQs (from 143 to 1%) (Bucknall et al., Citation2003) and for 15 substances – from 105 to 1% (Huet et al., Citation2006).
The broad specificity of antibodies to BSA-CF(edc) must be noted. Weak recognition of NA due to structural differences of this substance from the remaining FQ had an effect on k 10 though the spread in IC50 values for nine specimens (k 9), excluding NA appeared to be quite insignificant (12–14 times). Moreover, because of high sensitivity the following variants of assay, ‘anti-BSA-CF(edc)–BSA-CF×100(f)’ and ‘anti-BSA-CF(edc)–Gel-GF×10(ga)’, may be accepted as the best for group determination of CF, EF, NF, PF, OF, LeF, LoF, SF and GF.
The group specificity properties of anti-GO–GF(ga) may be found when GF was excluded. The other nine FQs differed by their binding no more than 10–15 times.
Furthermore, the present study confirmed the earlier observations (Burkin, Kononenko, & Soboleva, Citation2002) that group recognition properties of antibodies depend on either ‘correct’ design of immunogen or well-selected solid-phase antigen. The latter could affect sufficiently on some analytes to be revealed. For example, the detection limits of NA and SF were reduced by an order and more by using anti-BSA-CF(ae) and OVA-SF×300(ga). Moreover, this conjugate being immobilised on the plate modified critically the assay properties from selective (BSA-CF×100(f)) to group specificity.
Anti-BSA-CF(edc) serum preferred to bind EF rather than CF and PF than NF that presented a contrast to antiserum against BSA-CF(ae). These data indicate on ethyl (EF) or methyl (PF) masking that it is possible when BSA reacts with the secondary amino group of CF piperazinyl ring to produce BSA–CF(edc) immunogen. In addition, group-specific antibodies raised to this conjugate may confirm such a mode of carbodiimide condensation (Bucknall et al., Citation2003), in spite of negative results in control experiments with nitrazepamum and ephedrine.
Thus, the possibility of influence on specificity and sensitivity of indirect ELISA with especially selected immobilised antigen was demonstrated in the present work and may be very useful for the future development of test systems for different purposes.
References
- Bucknall , S. , Silverlight , J. , Coldham , N. , Thorne , L. and Jackman , R. 2003 . Antibodies to the quinolones and fluoroquinolones for the development of generic and specific immunoassays for detection of these residues in animal products . Food Additives and Contaminants , 20 : 221 – 228 .
- Burkin , A.A. , Kononenko , G.P. and Soboleva , N.A. 2002 . Group-specific antibodies against zearalenone and its metabolites and synthetic analogues . Applied Biochemistry and Microbiology , 38 : 169 – 176 .
- Burkin , A.A. and Smirnov , A.V. 2004 . ELISA of drugs and their metabolites. Part 3. 1,4-Benzodiazepines . Pharmaceutical Chemistry Journal , 38 : 573 – 577 .
- Clark , E.G.C. 1986 . Isolation and identification of drugs , London : The Pharmaceutical Press .
- Council Regulation (EEC) N 2377/90 . The establishment of maximum residue limits of veterinary medicinal products in foodstuffs of animal origin .
- Duan , J. and Yuan , Z. 2001 . Development of an indirect competitive ELISA for ciprofloxacin residues in food animal edible tissues . Journal of Agricultural and Food Chemistry , 49 : 1087 – 1089 .
- Holtzapple , C.K. , Buckley , S.A. and Stanker , L.H. 1997 . Production and characterization of monoclonal antibodies against sarafloxacin and cross-reactivity studies of related fluoroquinolones . Journal of Agricultural and Food Chemistry , 45 : 1984 – 1990 .
- Huet , A. , Charlier , C. , Titlemier , S. , Singh , G. , Benrejeb , S. and Delahaut , P. 2006 . Simultaneous determination of (fluoro)quinolone antibiotics in kidney, marine products, eggs, and muscle by enzyme-linked immunosorbent assay (ELISA) . Journal of Agricultural and Food Chemistry , 54 : 2822 – 2827 .
- Loomans , E.M.G. , van Wiltenburg , J. , Koets , M. and van Amerongen , A. 2003 . Neamin as an immunogen for the development of a generic ELISA detecting gentamicin, kanamycin, and neomycin in milk . Journal of Agricultural and Food Chemistry , 51 : 587 – 593 .
- Lu , S. , Zhang , Y. , Liu , J. , Zhao , C. , Liu , W. and Xi , R. 2006 . Preparation of anti-pefloxacin antibody and development of an indirect competitive enzyme-linked immunosorbent assay for detection of pefloxacin residue in chicken liver . Journal of Agricultural and Food Chemistry , 54 : 6995 – 7000 .
- Spinks , C.A. , Wyatt , G.M. , Lee , H.A. and Morgan , M.R.A. 1999 . Molecular modelling of hapten structure and relevance to broad specificity immunoassay of sulfonamide antibiotics . Bioconjugate Chemistry , 10 : 583 – 588 .
- Titov , I.V. , Dorofeev , V.L. and Arzamastsev , A.P. 2004 . The using of UV-spectroscopy method to authenticate fluoroquinolone drugs. Vestnik VGU. Seria: Chimia. Biologia . Farmazia , 2 : 264 – 269 .
- Usleber , E. , Litz , S. and Martlbauer , E. 1998 . Production and characterization of group-specific antibodies against penicillin antibiotics . Food and Agricultural Immunology , 10 : 317 – 324 .
- Van Coillie , E. , De Block , J. and Reybroeck , W. 2004 . Development of an indirect competitive ELISA for flumequine residues in raw milk using chicken egg yolk antibodies . Journal of Agricultural and Food Chemistry , 52 : 4975 – 4978 .
- Watanabe , H. , Satake , A. , Kido , Y. and Tsuji , A. 2002 . Monoclonal-based enzyme-linked immunosorbent assay and immunochromatographic assay for enrofloxacin in biological matrices . Analyst , 127 : 98 – 103 .