Abstract
The present study examined the effects of a kiwifruit (Actinidia chinensis and Actinidia deliciosa) extract on immune response in BALB/c mice. The effects were investigated using cholera vaccine (11 days duration) and diphtheria/tetanus toxoid vaccine (29 days duration) models. Mice were given either test (standard diet incorporated with kiwifruit extract) or control diets ad libitum throughout the respective experimental periods. At the end, blood, spleen and intestinal fluids were collected for determination of cell proliferation, specific antibody responses, cytokine production, phagocytosis, and natural killer cell activity.
The kiwifruit extract significantly enhanced (p<0.05) specific intestinal mucosal and serum antibody responses to the vaccines and promoted interferon-γ and natural killer cell activitiy. No significant (p>0.05) improvement was observed in proliferative cell response, phagocytic activity or interleukin-4 production. The overall results of the present study demonstrate the ability of a kiwifruit extract to enhance markers of innate and acquired immunity in the tested murine model.
Introduction
A functional immune system is essential for the host's continued survival against the daily onslaught of foreign organisms and pathogens (Danielle, Silva, & Palmer, Citation2007). Nutrition plays a critical role in the proper functioning of the immune system and in eliciting immune responses. Malnutrition is the most common cause of immunodeficiency worldwide. The micronutrients, zinc, selenium, iron, copper, vitamins A, C, E, and B-6, and folic acid have important influences on immune responses. In addition to nutrient deficient diets, over-nutrition and obesity have also been linked to reduction in immune function. In the elderly, impaired immunity can be enhanced by modest amounts of a combination of micronutrients. These findings have considerable practical and public health significance (Chandra, Citation1997).
Kiwifruit is the most well-known crop in the genus Actinidia and is a commonly eaten fruit in many countries. Actinidia deliciosa is native to East Asia and was previously known as the Chinese gooseberry. Its commercial planting in New Zealand began in the 1930s and today New Zealand kiwifruit is globally marketed under the ZESPRITM brand (Jung et al., Citation2005; McGhie & Ainge, 2002; Nishiyama, 2007). Kiwifruit has been shown to contain many nutritionally important compounds such as vitamin C, carotenoids, polyphenols, flavonoids, fibre, minerals, and others, although the levels vary among the different genotypes of Actinidia fruits (Frenich, Torres, Vega, Vidal, & Bolaños, Citation2005; Jung et al., Citation2005; McGhie & Ainge, Citation2002; Nishiyama, Fukuda, & Oota, Citation2005; Nishiyama et al., Citation2004; Wills, Lim, & Greenfield, Citation1986).
Recent studies have demonstrated the health benefits of kiwifruit extracts in relation to markers of a range of cancer types (Ben et al., Citation2001; Motohashi et al., Citation2001, Citation2002; Ruan, Lai, & Zhou, Citation2006). For example, one study showed that a kiwifruit extract reduced the nitrosation reactions by nitrile scavenging activity as a result of the high level of ascorbic acid present in the kiwifruit extract (Normington et al., Citation1986). Furthermore, kiwifruit has been shown to inhibit the growth of cultured human hepatoma cells (BEL-7404) (Yang, Shen, Sun, Han, & Xu, Citation1985), Ehrlich ascites carcinoma and HeLa cells (Motohashi et al., Citation2002). Kiwifruit extracts have been demonstrated to have a significant protective effect against oxidative DNA damage (Ben et al., Citation2001), selective cytotoxic activity against human oral tumour cell lines and display elevated anti-HIV activity, radical generation and O2 − scavenging activity, with potential cardiovascular protective properties due to its cytotoxic and antioxidant activities (Jung et al., Citation2005). Rush, Patel, Plank, and Ferguson (Citation2002) reported the ability of kiwifruit to promote laxation in the elderly. Recently, this effect was supported by the work of Chan, Leung, Tong, and Wong (Citation2007) who hypothesised that the fibre content in kiwifruit was responsible for the laxation effect. Recent studies have reported the ability of Actinidia macrosperma and Actinidia arguta to increase the overall immune function in mice (Block, Patterson, & Subar, Citation1992; Lu et al., Citation2007).
It has been widely documented that the consumption of fruit-derived antioxidants can enhance immune function and protect against certain cancers and cardiovascular disease (Block et al. Citation1992; Hertog, Feskens, Hollman, Katan, & Kromhout, Citation1993; Miller & Rice-Evans, Citation1997). Consumption of certain berries and fruits such as blueberries, mixed grape and kiwifruit, was associated with increased plasma antioxidant capacity in the postprandial state (Prior et al., Citation2007). Furthermore, the consumption of high antioxidant foods with each meal has been recommended to prevent periods of postprandial oxidative stress (Prior et al., Citation2007). An optimally functioning immune system is essential for defence against invading pathogens and spontaneously developing cancers (Gill, Rutherford, Prasad, & Gopal, Citation2000). However, there are few published studies where the effects of kiwifruit have been considered on a broad spectrum of host, innate and acquired immune responses (Roitt, Brostoff, & Male, Citation1989; Zinkernagal & Hengartner, Citation1997). The main objective of this research was to investigate the potential of a kiwifruit extract administered through dietary supplementation, to beneficially modulate innate and acquired immune responses, using a vaccine-challenged murine model.
Materials and methods
Animals
The animal procedures were approved by the University of Auckland Animal Ethics Committee (c/496 in 2006). Equal numbers of male and female 6–7 week BALB/c mice were selected and transferred from the Animal Resources Unit, Faculty of Medical and Health Sciences, University of Auckland, New Zealand to the small animal facility at Bioactive Research, Auckland, New Zealand. Animals were housed under standard animal housing conditions (12 h light and 12 h dark period, temperature 21±1°C and relative humidity 50–60%).
Preparation of the kiwifruit extract
A combination of aqueous and supercritical fluid extracts of ZESPRITM GOLD kiwifruit (Actinidia chinensis cv. Hort16A) and Green kiwifruit (Actinidia deliciosa cv. Hayward) were combined in the final kiwifruit extract formulation. The aqueous extracts were prepared using whole fresh fruit. For each extract, whole fruit was homogenised and the resultant pulp was treated with a cellulase-based enzyme for 30 minutes at 50°C, at the natural pH of the kiwifruit (approximately pH 3.5). Following enzyme treatment, the pulp was pressed to produce juice. The pressed residue was further extracted by mixing with an equal volume of water (50°C) for 30 minutes, followed by pressing to remove insoluble material. The extracted juice from each press was mixed, resulting in a final juice with a total soluble solids content of 13%. This juice was then frozen, freeze-dried, and ground to produce a fine powder.
Freeze-dried dices of green and gold kiwifruit were processed, separately, for the supercritical fluid extractions. The dices were comminuted to a powder with an average particle size of approximately 1 mm. Extraction of the powders was completed on pilot-scale facilities (Industrial Research Ltd, NZ) using supercritical CO2 at 300 bar and 40°C to produce viscous oily extracts.
The different extracts were mixed together using a Waring blender to produce a final powder ready for incorporation into the animal feed. The final mix of the individual kiwifruit extracts produced a powder with the following characteristics: vitamin C 2.3 mg/g, vitamin E 374 µg/g, folate 1.1 ?µg/g, β-carotene 4.4 µ?g/g, chlorophyll 3.5 ?µg/g, water-soluble polysaccharides 68.4 mg/g, α-linolenic acid 33.8 mg/g, FRAP 260 mm/l, and ORAC 81 µmol TE.
Diets
A standard rodent diet was fed to all animals during the 12-day acclimatisation period and this was continued to be fed to the control group animals. Feed and water were available ad libitum to all animals throughout the study. The test group animals were fed a standard rodent chow incorporated with kiwifruit extract at a rate of 2.53 g/kg which is an estimated equivalent dose of 375 mg/kg body weight/day for the duration of the experiments. All food pellets were formulated and supplied by the Small Animal Production Unit, Massey University, New Zealand.
Experimental design
Following the 12-day acclimatisation period, the mice (72) were randomly divided into two experimental groups (36 each), each containing 18 female and 18 male mice.
Experiment 1: cholera vaccine model
Animals were divided into kiwifruit and control groups (n=18), each containing an equal number of male and female mice. The kiwifruit and control groups were fed the standard diet with and without kiwifruit extract, respectively. Animals were orally immunised with 10 µg of cholera vaccine-Dukoral preparation (SBL Vaccine AB, Stockholm, Sweden) in 50 µl of 0.1 M NaH2CO3 per mouse on days 0 and 7, followed by overnight fasting on day 10. All mice were housed separately in a cage, and their body weight, water and food intake were recorded periodically until the end of each experimental period. The mice were closely monitored daily for any overt signs of clinical toxicity.
The mice were humanely sacrificed on day 11 with an overdose of ketamine/xylazine (s/c) enabling collection of peritoneal macrophages, blood, spleens and intestinal washings (Gill et al., Citation2000; Shu & Gill, Citation2001, Citation2002; Shu et al., Citation1999; Shu et al., Citation2000).
Experiment 2: tetanus/diphtheria vaccine model.
The same protocols as Experiment 1 were applied, however, in this study the mice were immunised with 50 µl of ADT-tetanus/diphtheria vaccine (CSL, Parkville, Victoria, Australia) on days 7 and 21 (s/c), followed by fasting overnight on day 28 and were humanely sacrificed on day 29 with an overdose of ketamine/xylazine (s/c) enabling collection of peritoneal macrophages, blood, spleens and intestinal washings (Gill et al., Citation2000; Shu & Gill, Citation2001, Citation2002; Shu et al., Citation1999; Shu et al., Citation2000).
Collection of blood samples
Immediately following anaesthesia, blood samples were withdrawn by cardiac puncture into heparinsed tubes and Eppendorf tubes using a hypodermic needle and syringe. The blood in the Eppendorf tubes was allowed to clot, centrifuged at 3000 rpm for 10 minutes. The separated sera were held at −20°C until analysis. The fresh blood samples were used for the determination of phagocytic capacity (Shu & Gill Citation2002).
Preparation of peritoneal macrophages
Resident peritoneal cells were collected by washing the peritoneal cavity of each mouse with 5 ml of RPMI-1640 medium (Sigma), complete RPMI medium and re-suspended in fresh medium at 106 cells/mL (Shu & Gill, Citation2001, Citation2002).
Preparation of spleen cell suspensions
Spleens were aseptically removed and placed in 2 ml of complete RPMI-1640 medium. Single cell suspensions were prepared by chopping the spleens into small pieces with sterile scissors and then forcing the spleen tissues up and down through a 1-ml syringe. The resulting suspension was transferred to a tube containing 5 ml of RPMI-1640 and centrifuged at 1000 rpm for 10 minutes and discarded the supernatant. The cell pellet was re-suspended in ACK lysis buffer (Tris–NH4Cl) and incubated for 5 minutes at room temperature (RT) with occasional mixing to lyse the erythrocytes. After washing twice in complete RPMI-1640, and re-suspending in ml of RPMI the cell viability was determined by flow cytometry (transferred 100 µl of diluted cells to flow cytometer tube containing 400 µl of PBS and 2 µl of propidium iodide) and was found to be greater than 95% in all cases. The cells were adjusted to a final concentration of 2×106 cells/ml in complete RPMI-1640 medium (Gill et al., Citation2000).
Assay procedures
Proliferation assay
In vitro proliferation responses of spleen cells to mitogens were determined using a commercial MTT cell proliferation kit (Roche diagnostics) (Gill et al., 2000). A 100 µl a liquot of the cell suspension (105 spleen cells in complete RPMI-1640 medium) in the presence or absence of T- and B-cell mitogens, were added into triplicate wells of a 96-well flat-bottomed plate. Mitogens (100 µl/well), concanavalin A (2.5 µg/ml; Sigma) and lipopolysaccharide (5 µg/ml; Sigma) were added. Negative control wells received RPMI and mitogens, while the positive control well received spleen cell suspension without mitogens. The cells were cultured at 37°C for 48 h in a CO2 incubator and cell proliferation was determined by MTT assay; briefly, added 10 µl/well of MTT and incubated at 37°C for 4 h in a CO2 incubator followed by addition of solubilisation agent (100 µl/well) and incubating overnight in a CO2 incubator at 37°C. The absorbance of each well was read at 550 nm using an enzyme-linked immunosorbent assay (ELISA) reader (Metertech E960).
Estimation of antibody responses
An ELISA was used to determine antibody responses to cholera toxin (CT) and tetanus and diphtheria toxoid (ADT). The wells of a 96-well microtitre plate were coated with CT or ADT antigen in 0.05 M carbonate/bicarbonate buffer (pH 9.6) by incubating overnight at 4°C. The wells were washed three times with PBS containing 0.05% Tween-20 (PBST). Serum or intestinal fluid samples diluted in PBS were added to duplicate wells (100 µl/well) and the plates incubated overnight at 37°C. The plates were washed with PBST and 100 µl of alkaline phosphatase-conjugated sheep anti-mouse Ig (Serotec, USA) diluted in sample buffer was added to each well. After incubation for 1 h at 37°C and washing three times with PBST, 100 µl/well of alkaline phosphatase substrate (BioRad, USA) was added. The absorbance was read at 405 nm using a microtitre plate reader (Thermal Electron Corp: Mulitskan EX) (Gill et al., Citation2000).
Cytokines
Spleen cell suspensions (2 ml) were added to each well of a 24-well plate and cultured in the presence of ConA for 48 h at 37°C. The presence of interleukin-4 (IL-4) and interferon-γ (IFN-γ) were determined using rat anti-mouse IL-4 and rat anti-mouse IFN-γ antibodies (BD Chemicals) on a BD FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA) (Gill et al., Citation2000).
Phagocytic capacity
The level of phagocytic capacity was assessed by flow cytometry (Shu et al., Citation1999). Whole blood (100 µl) and 5µl of fluorescein isothiocyanate (FITC) labelled Escherichia coli (stored at –86°C) were added on ice, mixed thoroughly and incubated for 30 minutes at 37°C. To fix the blood cells, 100 µl of 8% formaldehyde was added and the mixture was incubated for 1 minute. Erythrocyte lysis was achieved by promptly adding 1 ml of ice-cold water, mixing thoroughly and left for 10 minutes. The samples were then centrifuged at 4000 rpm for 10 minutes, and the pellet was re-suspended in 0.5 ml of PBS and 50 µl of Trypan blue. Using a BD FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA) the level of phagocytic activity was determined.
Natural killer cell activity
Established flow cytometry methods were used to assess NK cell activity (Gill et al., Citation2000). Target cells (1×106 cells/ml) from the mouse Moloney leukaemia cell line (YAC-1) were labelled by overnight incubation at 37°C with D275 (Sigma) (1 µl/ml). The YAC-1 target cells were washed twice in RPMI (1000 rpm×10 minutes) and re-suspended to give a cell concentration of 1×106 cells/ml. To ensure the correct ratio of effector:target cells (40:1) lymphocyte counts were determined on the haemocytometer under the microscope using Trypan blue staining. Live and dead cell numbers were recorded and the percentage of dead cells in the population was determined (ideally 5–10%). The concentration of cells in the stock solution was also determined to enable correction for any large variances of the number of dead cells in the sample population. Using a 96-well cytotoxicity plate (N163320), duplicate 25 µl aliquots of the target cells were first added to the plate wells, then the spleen lymphocyte effector cells (1.5×106 cells/well) from the cell proliferation stocks. The total well volume was made up to 100 µl with RPMI media. Control wells contained effector cells and target cells only. All samples were centrifuged at 1000 rpm (or 240 g) for 1 minute then incubated at 37°C for three hours. To label the dead cells, 3 µl of propidium iodide was added to each well 15 minutes prior to the end of the incubation period. Following incubation, samples and controls were transferred to flow cytometer tubes containing 150 µl of PBS, and the level of target cell lysis was measured using a BD FACScan flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA, USA). NK cell activity was determined as the percentage of effector cell-specific lysis (Gill et al., Citation2000).
Statistical analysis
Data were analysed using t-test by employing Genstat statistical package. Antibody titres were transformed by log 10 prior to statistical analysis. Values of p<0.05 were considered significant.
Results
All the experimental animals remained healthy throughout the respective treatment periods except for one mouse that died on day 19 in Experiment 2. Examination of that mouse provided no indications of morbidity. All mice were anatomised at the end of the experiments. No visible pathological changes were observed, suggesting that treatment with the kiwifruit extract did not exert any clinical toxicity at the dosage administered in this study.
Body weight
All animals gained weight throughout the respective experimental periods. Consumption of the kiwifruit extract did not exert a significant effect (p>0.05) on the body weight of mice in either Experiment 1 (11 days) or Experiment 2 (29 days) ().
Table 1. Body weight (g) of mice in the kiwifruit extract and control groups on day 0 and day 11 or 29.
Cell proliferation
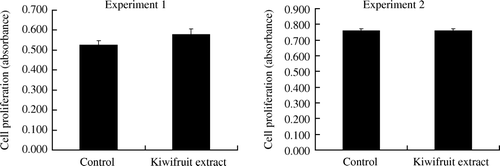
Proliferation response in spleen cells to lipopolysaccharides (LPS) using MTT assay did not reveal a significant difference (p>0.05) between animal groups treated with kiwifruit extract as compared to the respective control groups. The results are shown in .
Figure 1. Proliferation response in spleen cells to lipopolysaccharide in mice on days 11 (Experiment 1) and 29 (Experiment 2), respectively. Values are shown as means with the standard error bars. Statistical significance was not observed between the treatment groups (n=18, except there were only 17 mice in the kiwifruit extract group in Experiment 2) (*p>0.05).
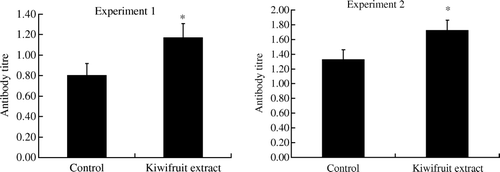
Antibody responses
The effect of feeding kiwifruit extract on specific antibody responses to CT and ATD is shown in . Mice fed with the kiwifruit extract for 10 days produced significantly higher levels of specific mucosal antibody responses to CT than in the control mice (p<0.05). Serum antibody responses to ATD were also significantly greater in mice fed with kiwifruit extract for 28 days compared with the responses in control mice (p<0.05).
Figure 2. Experiment 1: mucosal antibody response to cholera toxin vaccine (CT) in mice fed with kiwifruit extract or standard diet (control) for 11 days. Experiment 2: serum antibody response to diphtheria/tetanus toxoid (TD) in mice fed with kiwifruit extract or standard diet (control) for 29 days. Values are shown as means with the standard error bars. n=18 mice in each group, except n=17 in kiwifruit extract in Experiment 2, *p<0.05.
Cytokine production
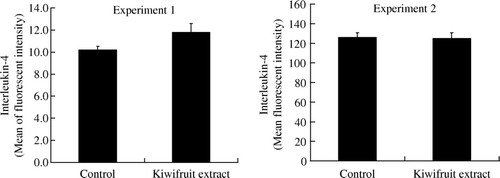
IFN-γ production was significantly higher (p<0.05) in spleen cells from CT-treated mice fed with the kiwifruit extract for 10 days. Whilst the kiwifruit extract did cause an elevation in the production of IFN-γ in ATD-treated mice, it failed to reach the set level of statistical significance level. No significant change in IL-4 production, either in CT-treated or ATD-treated mice, was observed. The results are shown in and .
Figure 3. Production of interferon-γ by spleen cells from mice fed with kiwifruit extract and standard diet (control) on day 11 (Experiment 1) and day 29 (Experiment 2). Values are shown as means with the standard error bars. n=18 mice in each group, except n=17 in kiwifruit extract in Experiment 2, *p<0.05.
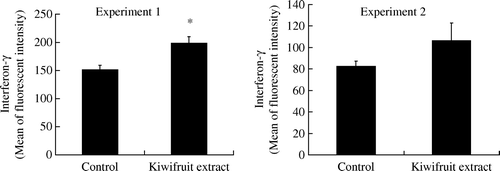
Figure 4. Production of interleukin-4 by spleen cells from mice fed with kiwifruit extract and non-kiwifruit extract control diet on day 11 (Experiment 1) and day 29 (Experiment 2). Values are shown as means with the standard error bars. Statistical significance was not observed between the treatment groups (n=18, except there were only 17 mice in the kiwifruit extract group in Experiment 2) (*p>0.05).
Phagocytic function of peritoneal macrophages
Peritoneal macrophages from mice fed with the kiwifruit extract for 10 or 28 days did not exhibit significantly (p>0.05) greater phagocytic activities than cells from the controls mice ( and ).
Table 2. Phagocytic activity in the peripheral blood and peritoneal cell preparation of mice in kiwifruit extract and control groups on day 11.
Table 3. Phagocytic activity in the peripheral blood and peritoneal cell preparation of mice in kiwifruit extract and control groups on day 29.
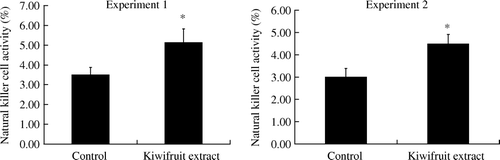
NK cell activity
As shown in , dietary supplementation with the kiwifruit extract had a stimulatory effect on NK cell activity of splenocytes. Spleen cells from mice fed with the kiwifruit extract displayed higher NK cell cytotoxic activity against the target cells, YAC-1, compared to that of control mice (p<0.05).
Figure 5. Natural killer cell activity in mice fed with kiwifruit extract and non-kiwifruit extract control diet on day 11 (Experiment 1) and day 29 (Experiment 2). Values are shown as means with the standard error bars. n=18 mice in each group, except n=17 in kiwifruit extract group in Experiment 2, *p<0.05.
Discussion
Kiwifruit (Actinidia species) is reputed to possess a host of valuable nutrients which are important in alleviating certain disease conditions and enhancing body power as immuno-modulating agents. Actinidia chinensis (gold kiwifruit) and Actinidia deliciosa (green kiwifruit) are the dominant varieties that are being commercially marketed worldwide as fruits of this genus (Nishiyama, Citation2007).
In the present study, several key measures of both innate and adaptive immunity were estimated in the respective groups of mice, including phagocytic activity and natural killer cell activity which are major effectors of innate immunity (De Simone et al., Citation1991; Gill et al., Citation2000).
NK cells, a form of cytotoxic lymphocyte, constitute a major component of the innate immune system where they play a role in the host-rejection of both tumours and virally infected cells. Recognition and elimination of cells undergoing carcinogenesis is one of the more challenging tasks that the immune system faces, and the defence against tumours is complex. Carcinogenesis can be mediated early by the innate immune system where phagocytes, NK cells, NKT cells, cytokines and complement proteins come into action (Bhardwaj, Citation2007). Supplementation with the kiwifruit extract was shown to stimulate the NK cell activity of splenocytes, suggesting that kiwifruit is able to modulate the immune responses to combat tumours and associated carcinogenic effects. However, it is not known whether the increase in NK cell activity reflects an increase in the percentage of NK cells or functional enhancement at a cellular level. Traditionally, Actinidia species have been employed to treat cancer in Chinese herbal medicine where the anti-cancer effects are brought about mainly by inducing apoptosis and differentiation, enhancing immune system, inhibiting angiogenesis and reversing multi-drug resistance, which would improve survival, increase tumour response, improve quality of life or reduce chemotherapy toxicity (Ruan et al., Citation2006).
Whilst phagocytosis is the primary immune system mechanism for the removal of pathogens and cell debris, in this study, the consumption of the kiwifruit extract did not change the level of phagocytic activity.
Lymphocyte proliferation responses to mitogens are widely used to assess T-cell and B-cell function (Gill et al., Citation2000). In the present study, proliferation responses to the B-cell mitogen, lipopolysaccharide, were evaluated and did not yield any significant difference between mice fed with and without the kiwifruit extract. In contrast, using a dermatological application model, it has been demonstrated that kiwifruit (Actinidia chinensis L.) polysaccharides exert a stimulating effect on cell proliferation via enhanced growth factor receptors, energy production, and collagen synthesis of human keratinocytes, fibroblasts, and skin equivalents (Deters, Schröder, & Hensel, Citation2005).
T cells, the main effectors and regulators of cell-mediated immunity (Gill et al. Citation2000; Roitt et al., Citation1989), can be subdivided into two functional types, Th1 and Th2, based on their cytokine profile (Mosmann & Coffman, Citation1989). Th1 cells produce IL-2, IFN-γ and tumour necrosis factor (TNF) and are vital for cell-mediated immunity. Th2 cells predominantly produce IL-4, IL-5 and IL-10 and are associated with humoral immunity and allergic responses. In the present study, IFN-γ production in kiwifruit extract-fed mice was significantly greater following stimulation with ConA in both experiments, but IL-4 production was not. This indicates that the kiwifruit extract had the ability to selectively activate Th1 cells rather than both types of T cells.
IFN-γ can inhibit viral replication, enhance antigen-presentation function of macrophages, modify the expression of major histocompatability complex antigens, enhance T-cell function, activate macrophages and improve the effectiveness of vaccines (Gill et al., Citation2000; Murray, Citation1988). IL-4 regulates the production of immunoglobulin E (IgE) and an elevated level of IgE is an important indicator of allergy (Mekori, Citation1996; Mosmann & Coffman, Citation1989; Ryan, Citation1997). In contrast, IFN-γ inhibits IL-4 secretion, and increased levels of IFN-γ suppress IgE production (Bhardwaj, 2007; Thyphronitis, Tsokos, June, Levine, & Finkelmen, Citation1989). The results of the present study therefore suggested that kiwifruit extract may inhibit IgE-mediated allergic responses through selective stimulation of Th1 cells.
Actinidia polygama is one commonly used herb in oriental medicine for treatment of anti-inflammatory and many allergic diseases. Its anti-asthmatic effects were investigated recently in a murine model of asthma (Lee, Kim, Seo, Roh, & Lee, Citation2005). The results showed that Actinidia polygama extract had a deep inhibitory effect on airway inflammation and hyper-responsiveness and played a crucial role as an immunomodulator which possessed anti-inflammatory and anti-asthmatic properties by modulating the relationship between Th1/Th2 cytokine imbalances. In particular, suppression of Th2 cytokines and a reduction in IgE was observed (Lee et al., Citation2005). Furthermore, Park et al. (Citation2005) demonstrated that PG102T and PG102E fractions isolated from Actinidia arguta had great potential as orally active immune modulators for the therapy of various allergic diseases by controlling IgE, and selective Th1 and Th2 cytokines.
Active immunity can also be generated artificially, through vaccination. The principle behind vaccination is to introduce an antigen from a pathogen in order to stimulate the immune system and develop specific immunity against that particular pathogen without causing disease associated with that organism. This deliberate induction of an immune response is successful because it exploits the natural specificity of the immune system, as well as its inducibility. With infectious disease remaining one of the leading causes of death in the human population, vaccination represents the most effective manipulation of the immune system mankind has developed. In the present investigation, mucosal and serum antibody responses in the kiwifruit extract-fed mice were increased as compared to those of control mice. Increased IFN-γ production can also be an accompanying factor for this. This is of significant importance to special populations of the community. In particular, the immune responses to pathogens are diminished in young children (Chandra, Citation1997).
Vaccination in special situations, such as, immunosuppression, prematurity, pregnancy and exposure to infectious diseases increases the risk of diseases or adverse post-vaccination events. In these situations, special vaccines or special vaccination schedules are indicated, or vaccines should be postponed or withheld. For immunosuppressed patients, in accordance with the type of immunosuppression, live virus or bacterial vaccines should be avoided, because of the risk of vaccine agent spread (Succi & Farhat, Citation2006). In such instances, it would be of great importance if previous immunological memory could be enhanced via dietary manipulations and immune nutrition could be introduced as an alternative. Additionally, immunonutrition confers an additive benefit when compared with standard enteral and parenteral nutrient preparations in the management of perioperative malnourished patients (O'Callaghan & Beale, Citation2003). According to the results of the present study, kiwifruit extract could be identified as a potential candidate for further enhancing immunity in vaccinated individuals especially young children, and also in special circumstances.
Overall, the results of the present study indicate the ability of a kiwifruit extract to modulate both innate and acquired immunity in a beneficial manner. Dietary supplementation with kiwifruit extracts may assist in optimising and/or enhancing immunocompetence, which may be beneficial in healthy, immunosuppressed, or immunocompromised individuals. Further research on the exploitation of the active ingredients in kiwifruit, to develop nutraceutical or pharmaceutical formulations to serve the worldwide population at large, is warranted.
Acknowledgements
We thank the staff at the Animal Research Unit of the University of Auckland, New Zealand. This work was supported by Zespri International Ltd, Mt. Maunganui, New Zealand.
References
- Ben , H. , Collins , B.H. , Horská , A. , Hotten , M.P. , Riddoch , C. and Collins , A.R. 2001 . Kiwifruit protects against oxidative DNA damage in human cells and in vitro . Nutrition and Cancer , 39 : 148 – 153 .
- Bhardwaj , N. 2007 . Harnessing the immune system to treat cancer . Journal of Clinical Investigation , 17 ( 5 ) : 1130 – 1136 .
- Block , G. , Patterson , B. and Subar , A. 1992 . Fruit, vegetables and cancer prevention: A review of the epidemiological evidence . Nutrition and Cancer , 18 : 1 – 29 .
- Chan , O.O.C. , Leung , G. , Tong , T. and Wong , N.Y.C. 2007 . Increasing dietary fibre intake in terms of kiwifruit improves constipation in Chinese patients . World Journal of Gastroenterology , 13 ( 35 ) : 4771 – 4775 .
- Chandra , R.K. 1997 . Nutrition and the immune system: An introduction . American Journal of Clinical Nutrition, S , 66 : 460S – 463 .
- Danielle , A.W. , Silva , A.B. and Palmer , D.B. 2007 . Immunosenescence: Emerging challenges for an ageing population . Immunology , 120 ( 4 ) : 435 – 446 .
- De Simone , C. , Rosati , E. , Moretti , S. , Bianchi Salvadori , B. , Vesely , R. and Jirillo , E. 1991 . Probiotics and stimulation of the immune response . European Journal of Clinical Nutrition , 45 ( Suppl ) : 32 – 34 .
- Deters , A.M. , Schröder , K.R. and Hensel , A. 2005 . Kiwi fruit (Actinidia chinensis L.) polysaccharides exert stimulating effects on cell proliferation via enhanced growth factor receptors, energy production, and collagen synthesis of human keratinocytes, fibroblasts, and skin equivalents . Journal of Cellular Physiology , 202 ( 3 ) : 717 – 722 .
- Frenich , A.G. , Torres , M.E. , Vega , A.B. , Vidal , J.L. and Bolaños , P.P. 2005 . Determination of ascorbic acid and carotenoids in food commodities by liquid chromatography with mass spectrometry detection . Journal of Agricultural and Food Chemistry , 53 ( 19 ) : 7371 – 7376 .
- Gill , H.S. , Rutherfurd , K.J. , Prasad , J. and Gopal , P.K. 2000 . Enhancement of natural and acquired immunity by Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (HN017) and Bifidobacterium lactis (HN019) . British Journal of Nutrition , 83 : 167 – 176 .
- Hertog , M.G.L. , Feskens , E.J.M. , Hollman , P.C.H. , Katan , M.B. and Kromhout , D. 1993 . Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly . Lancet , 23 : 1007 – 1011 .
- Jung , K.A. , Song , T.C. , Han , D. , Kim , I.H. , Kim , Y.E. and Lee , C.H. 2005 . Cardiovascular protective properties of kiwifruit extracts in vitro . Biological & Pharmaceutical Bulletin , 28 : 1782 – 1785 .
- Lee , Y.C. , Kim , S.H. , Seo , Y.B. , Roh , S.S. and Lee , J.C. 2005 . Inhibitory effects of Actinidia polygama extract and cyclosporine A on OVA-induced eosinophilia and bronchial hyperresponsiveness in a murine model of asthma . International Immunopharmacology , 6 ( 4 ) : 703 – 713 .
- Lu , Y. , Fan , J. , Zhao , Y. , Chen , S. , Zheng , X. Yin , Y. 2007 . Immunomodulatory activity of aqueous extract of Actinidia macrosperma . Asia Pacific Journal of Clinical Nutrition , 16 ( Suppl. 1 ) : 261 – 265 .
- McGhie , T.K. and Ainge , G.D. 2002 . Color in fruit of the genus actinidia: Carotenoid and chlorophyll compositions . Journal of Agricultural and Food Chemistry , 50 ( 1 ) : 117 – 121 .
- Mekori , Y.A. 1996 . Introduction to allergic diseases . Critical Reviews in Food Science and Nutrition , 36 : S1 – S18 .
- Miller , N.J. and Rice-Evans , C.A. 1997 . The relative contributions of ascorbic acid and phenolic antioxidants to the total antioxidant activity of orange and apple fruit juices and blackcurrant drink . Food Chemistry , 60 : 331 – 337 .
- Mosmann , T.R. and Coffman , R.L. 1989 . Th1 and Th2 cells: Different patterns of lymphokine secretion lead to different functional properties . Annual Review of Immunology , 7 : 145 – 173 .
- Motohashi , N. , Shirataki , Y. , Kawase , M. , Tani , S. , Sakagami , H. Satoh , K. 2001 . Biological activity of kiwifruit peel extracts . Phytotherapy Research , 15 : 337 – 343 .
- Motohashi , N. , Shiratake , Y. , Kawase , M. , Tani , S. , Sakagami , H. Satoh , K. 2002 . Cancer prevention and therapy with kiwifruit in Chinese folklore medicine: A study of kiwifruit extracts . Journal of Ethnopharmacology , 81 : 357 – 364 .
- Murray , H.W. 1988 . Interferon-gamma, the activated macrophages, and host defense against microbial challenge . Annals of Internal Medicine , 108 : 595 – 608 .
- Nishiyama , I. 2007 . Fruits of the actinidia genus . Advances in Food and Nutrition Research , 52 : 293 – 324 .
- Nishiyama , I. , Fukuda , T. and Oota , T. 2005 . Genotypic differences in chlorophyll, lutein, and beta-carotene contents in the fruits of actinidia species . Journal of Agricultural and Food Chemistry , 53 ( 16 ) : 6403 – 6407 .
- Nishiyama , I. , Yamashita , Y. , Yamanaka , M. , Shimohashi , A. , Fukuda , T. and Oota , T. 2004 . Varietal difference in vitamin C content in the fruit of kiwifruit and other Actinidia species . Journal of Agricultural and Food Chemistry , 52 ( 17 ) : 5472 – 5475 .
- Normington , C.W. , Baker , I. , Molina , M. , Wishnok , J.S. , Tannenbaum , S.R. and Puju , S. 1986 . Characterization of a nitrile scavenger, 3-hydroxy-2-pyranone, from Chinese wild plum juice . Journal of Agricultural and Food Chemistry , 34 : 215 – 217 .
- O'Callaghan , G. and Beale , R.J. 2003 . The role of immune-enhancing diets in the management of perioperative patients . Critical Care and Resuscitation , 5 ( 4 ) : 246 – 247 .
- Park , E.J. , Kim , B. , Eo , H. , Park , K. , Kim , Y. Lee , H.J. 2005 . Control of IgE and selective T(H)1 and T(H)2 cytokines by PG102 isolated from Actinidia arguta . Journal of Allergy and Clinical Immunology , 116 ( 5 ) : 1151 – 1157 .
- Prior , R.L. , Gu , L. , Wu , X. , Jacob , R.A. , Sotoudeh , G. Kader , A.A. 2007 . Plasma antioxidant capacity changes following a meal as a measure of the ability of a food to alter in vivo antioxidant status . Journal of the American College of Nutrition , 26 ( 2 ) : 170 – 181 .
- Roitt , I. , Brostoff , J. and Male , D. 1989 . Immunology , (2nd ed.) , London : Gower Medical .
- Ruan , W.J. , Lai , M.D. and Zhou , J.G. 2006 . Anticancer effects of Chinese herbal medicine, science or myth . Journal of Zhejiang University Science B , 7 ( 12 ) : 1006 – 1014 .
- Rush , E.C. , Patel , M. , Plank , L.D. and Ferguson , L.R. 2002 . Kiwifruit promotes laxation in the elderly . Asia Pacific Journal of Clinical Nutrition , 11 : 164 – 168 .
- Ryan , J.J. 1997 . Interleukin-4 and its receptors: Essential mediators of the allergic response . Journal of Allergy and Clinical Immunology , 99 : 1 – 6 .
- Shu , Q. and Gill , H.S. 2001 . A dietary probiotic (Bifidobacterium lactis HN019) reduces the risk of Escherichia coli O157: H7 infection in mice . Medical Microbiology and Immunology , 189 : 147 – 152 .
- Shu , Q. and Gill , H.S. 2002 . Immune protection mediated by the probiotic Lactobacillus rhamnosus HN001(DR20) against Escherichia coli O157:H7 infection in mice . FEMS Immunology and Medical Microbiology , 34 ( 1 ) : 59 – 64 .
- Shu , Q. , Lin , H. , Rutherfurd , K.J. , Fenwick , S.G. , Prasad , J. Gopal , P.K. 2000 . Dietary Bifidobacterium lactis (HN019) enhances resistance to oral Salmonella typhimurium infection in mice . Microbiology and Immunology , 44 : 213 – 222 .
- Shu , Q. , Zhou , J.S. , Rutherfurd , K.J. , Birtles , M.J. , Prasad , J. Gopal , P.K. 1999 . Probiotic lactic acid bacteria (Lactobacillus acidophilus HN017, Lactobacillus rhamnosus HN001 and Bifidobacterium lactis HN019) have no adverse effects on the health of mice . International Dairy Journal , 9 ( 11 ) : 831 – 836 .
- Succi , R.C. and Farhat , C.K. 2006 . Vaccination in special situations . Jornal de Pediatria , 82 ( Suppl. 3 ) : S91 – S100 .
- Thyphronitis , G. , Tsokos , G.C. , June , C.H. , Levine , A.D. and Finkelmen , F.D. 1989 . IgE secretion by Epstein-Bar virus-infected purified human B lymphocytes is stimulated by interleukin-4 and suppressed by interferon g . Proceedings of the National Academy of Sciences of the United States of America , 86 : 5580 – 5584 .
- Wills , R.B.H. , Lim , J.S.K. and Greenfield , H. 1986 . Composition of Australian foods: Tropical and subtropical fruits . Food Technology in Australia , 38P : 118 – 123 .
- Yang , J.L. , Shen , Z.M. , Sun , Y.F. , Han , J.X. and Xu , B. 1985 . Cultured human hepatoma cells (BEL-7404) for anticancer drugs screening . Acta Pharmacologica Sinica , 6 : 144 – 148 .
- Zinkernagal , R.M. and Hengartner , H. 1997 . Antiviral immunity . Immunology Today , 18 : 258 – 259 .