Abstract
Characterisation of potential antigen(s) in the teliospore wall of Tilletia indica, the causal agent of Karnal bunt (KB) of wheat (Triticum aestivum) is necessary to generate specific immunoprobes for the development of reliable immunodiagnostic procedures. The intact teliospores of KB were used to generate anti-teliospore antibodies for the development of microtitre ELISA. The anti-teliospore antibodies cross-reacted with Tilletia foetida, Puccinia recondita and Puccinia striiformis suggesting that these species share one or more antigenic components. These antibodies recognised two unique immunoreactive bands of molecular weight ~34 kDa and ~15 kDa present in the spore wall fraction of T. indica after western blotting. The periodate treatment alone and sequential treatment of periodate followed by trypsin lost the immunoreactivity partially and completely, respectively. This suggests that cross-reactivity among bunt and rust of wheat fungal pathogens is most likely due to peptidal/oligosaccharide epitopes overlapping the spore wall's glycoprotein.
Introduction
Karnal bunt (KB) disease of wheat, incited by fungus Tilletia indica, is an economically important disease which hampers the international trade of wheat. The disease was reported not only from India but also from Mexico, USA, north-western Europe, Afghanistan, Iran, Iraq, Pakistan and Nepal (Gill, Sharma, & Aujla, Citation1993; Joshi, Singh, Srivastava, & Wilcoxon, Citation1983; Warham, Mujeeb-Kazim, & Rosas, Citation1986). The disease has gained significant importance not only because of its qualitative and quantitative losses (0.2–0.5%) but also for reluctance on the part of many countries to accept wheat from the Indian subcontinent (Munjal, Citation1975). After the reporting of KB at Arizona State in March 1996, USDA has designated T. indica as a quarantine pest. Hence, wheat movement into the USA and other KB free countries is regulated and subjected to quarantine (Ykema, Floyed, Palm, & Petrson, Citation1996). Other countries such as the USA, Canada, Russia and China, are also under the threat of spreading this disease (Prescott, Citation1986; Zhang, Lange, & Mathur, Citation1984).
The teliospore of KB is an infectious entity and can be detected in the grains by simple microscopic techniques, but the teliospores of this fungus closely resemble teliospores of fungi causing bunts in other crops such as rye and rice. These pathogens have similar teliospore texture but they differ in size. Similarity in teliospore configuration makes it difficult to differentiate KB teliospores from the teliospores of other bunt fungi. In order to determine the correct identity of contaminating fungi, it is essential to develop specific diagnostic probes, which could be used by the seed certification labs and plant quarantine departments.
Significant progress has been made in our lab to develop the rapid formats for immunodiagnosis of KB teliospores by using high titre polyclonal antibody probes raised against intact teliospores in New Zealand white rabbits (Gupta, Kumar, Lakhchaura, & Garg, Citation2001; Kumar, Singh, & Garg, Citation1998). The developed immunodiagnostic formats viz. Seed immunoblot binding assay (SIBA), indirect immunoflorescent staining tests, dyed latex bead agglutination assay and immuno-dipstick assay can be successfully employed for the sensitive detection of seed borne inoculum of the fungus present either in bunted seeds or as loose teliospores on the seed surface (Gupta, Kumar, Singh, & Garg, Citation2000; Kesari & Kumar, Citation2003; Kesari, Mishra, Garg, & Kumar, Citation2005; Kumar et al., Citation1998).
The immuno-dipstick assay developed in our lab is sensitive enough to detect the teliospore antigens as low as five teliospores (Kesari & Kumar, Citation2003). However, the major obstacle is the specific detection of KB teliospores due to cross-reactivity of anti-teliospore antibodies with other bunt and related fungal pathogens. The partial reactivity of anti-teliospore antibodies even after DE-52 ion exchange column purification with teliospores of Tilletia barclayana, a non-quarantined rice fungal pathogen, and also with spores of rust fungus Puccinia recondita indicates the common antigens or similarity in antigen configuration of these pathogens (Kesari & Kumar, Citation2003).
High-quality immunological reagents (specific immunoprobes against infectious entities) are required for the development of specific immunodiagnostic procedure which can be used for the management of a disease. Therefore, it is mandatory to characterise the antigenic configuration of teliospore walls of T. indica. Our present study focusses on characterisation of potential antigens residing in or on the spore wall of T. indica, in the anticipation that characterisation of such antigens could be useful in the development of specific diagnostic immunoprobes for designing, optimisation and refinement of immunodiagnostic kits for single step identification and differential diagnosis of T. indica. The present study has generated the anti-teliospore antibodies and standardised the optimum conditions for the development of microtitre ELISA for immunodetection and preliminary characterisation of potential antigen(s).
Materials and methods
Collection of teliospores/spores of pathogens
The teliospores of T. indica, Tilletia foetida and spores of Ustilago nudo tritici, P. recondita and Puccinia striiformis were extracted from seed, stem and leaf of wheat with the help of brush, needle, forceps and surgical blades. The spores of Fusarium spp., Helminthosporium sativum and Aspergillus niger were extracted from their respective culture plates. The teliospores were collected in a paper sheet and the debris was separated from the teliospores/spores by using a sieve.
Morphological study of fungal spores
Spores of U. nudo tritici, P. recondita, P. striiformis, Fusarium spp., H. sativum, A. niger, and teliospores of T. indica and T. foetida were observed under the Polyvar microscope at 20× and 40× magnifications. Photographs were taken and compared on the basis of shape and texture of fungal spores.
Preparation of polyclonal antibodies
Albino, New Zealand white rabbits procured from the Indian Veterinary Research Institute (IVRI), Bareilly were used for production of anti-teliospore antibodies. The rabbits were immunised, by administering five injections through subcutaneous, intramuscular and foot pad routes. The immunogen was prepared using intact teliospores in phosphate buffered saline (PBS, pH 7.4) and emulsified with an equal volume of either Freund's complete adjuvant (FCA) or Freund's incomplete adjuvant (FIA). The first injection was given with 25 mg of teliospore suspension emulsified in FCA followed by three injections at biweekly intervals of 10 mg of spores in FIA. After three booster doses, the rabbits were bled and antiserum was collected from one hyper-immunised rabbit and straw coloured clear supernatant was transferred into capped cryo-vials and stored at −20°C. The antibody reactivity with teliospores of KB was checked using indirect ELISA.
Development of microtitre ELISA
The ELISA was developed with minor modifications as described initially by Engvall and Perlmann (Citation1971) and modified in our lab. Microtitre plates were coated with teliospores (5–10,000) in coating buffer, i.e. 100 µl particulate antigen of fungal teliospore per well. The plates were incubated for 1 h at room temperature and then kept overnight at 4°C. The plates were washed with PBS + Tween-20 (0.01%). The wells were filled with PBS containing 5% skimmed milk for 2 h at room temperature to prevent adventitious binding, and were washed again with PBS + Tween-20 (0.01%) + 0.5% skimmed milk. The polyclonal antibodies were diluted in PBS + 0.5% skimmed milk. The primary antibody (100 µl) was added and incubated for 1 h at room temperature. Washing was done thrice with PBS + Tween-20 (0.01%) + 0.25% bovine serum albumin (BSA). Then 100 µl of alkaline phosphatase with conjugated secondary antibody (1:1000 dilutions) was incubated for 2 h at room temperature. The plate was washed with PBS + Tween-20 (0.01%) + 0.25% BSA three times. Alkaline phosphatase activity was assayed with the substrate solution (p-nitrophenyl phosphate sodium salt dissolved in di-ethanolamine buffer, 1.0 mg/µl). The plates were incubated for 30 min in the dark and the reaction was stopped with 100 µl of 1.5 M NaOH solution. Absorbance of the colour developed was determined at 405 nm in ELISA reader.
Isolation of spore wall proteins
The teliospores/spores (100 mg) were crushed with a pestle and mortar with liquid N2 and 4–5 ml protein extraction buffer (80 mM Tris-Cl, 10 mM ethylenediaminetetraacetic acid (EDTA), 1 mM phenylmethylsulfonyl fluoride (PMSF)). Approximately, 100% breakages of teliospores were seen under polyvar microscope. Broken spores were pelleted at 8450 g at 4°C for 10 min and supernatant was discarded. Spore wall pellet was washed with Tris buffered saline twice and pellets were mixed with 3.0 ml of equal parts of 0.1 M ammonium acetate saturated phenol and Tris-EDTA buffer. Spore wall fragments were pelleted from the extract by centrifugation at 8450 g for 10 min. The resulting supernatant was transferred to a clean tube and re-centrifuged to separate the aqueous and phenol phases. The phenol phase was recovered and washed three times with equal volumes of Tris EDTA (TE) and re-centrifuged at 8450 g as above to separate the aqueous and phenol phases. Washed phenol extract was transferred to 1.5 ml micro-centrifuge tubes. Proteins were precipitated from the washed phenol phase by addition of 5 Vol. of 0.1 M ammonium acetate in methanol with an overnight incubation at −20°C. Precipitate was collected by centrifugation at 8450 g for 15 min. Pellets were washed with ice-cold 95% ethanol twice and final phenol extract precipitate was re-suspended in 100 µl TE and stored at −20°C. The protein was estimated by using the method of Bradford (Citation1976).
Biochemical characterisation of antigen(s)
The preliminary characterisation of antigen(s) was done using chemical modifiers and enzyme treatment studies (Gupta et al., Citation2001). Teliospores of T. indica were washed three times with PBS and suspended in PBS at a concentration of 1 mg/ml in glass tubes. The glass tubes were kept in a boiling water bath for 15 min. The other tube was taken as control and kept at room temperature. After removing the tube from the water bath, it was cooled to room temperature and processed for ELISA test. To know the proteineous nature of antigen(s), intact teliospores were pelleted in 5 ml tubes and treated with trypsin in appropriate buffer (0.25% Trypsin in 40 mM Tris-HCl, pH 8.1, containing 50 mM CaCl2) at a concentration of 1.0 mg/ml. Incubation was done for 60 min at 37°C. For control, the pellets were suspended in 1 ml of buffer omitted with the enzyme and incubated under the same conditions. After washing the pellet for three times with PBS, the teliospores were processed for ELISA test.
Periodate treatment showed the carbohydrate nature of antigen. Intact teliospores were pelleted in 5 ml glass tubes and treated with periodate (50 mM sodium periodate in 50 mM sodium acetate, pH 4.5) at room temperature for 2 h. For control, the pellet was suspended in 1 ml of buffer omitted with sodium periodate and incubated under the same conditions. After washing the pellet for three times with PBS, the teliospores were processed for ELISA test.
Methanol treatment described the lipid nature of antigen. The teliospores were incubated with methanol and absolute ethanol for 60 min at 0–4°C and after washing three times with PBS, the pellet was processed for ELISA test.
To know the glycoprotein nature of antigen, sequential treatment of periodate and trypsin was done. Intact teliospores were pelleted in 5 ml glass tubes and treated with periodate (50 mM sodium periodate in 50 mM sodium acetate, pH 4.5) for 2 h. The teliospores were washed four times with PBS and subsequently treated with trypsin (0.25% in 40 mM Tris-HCl, pH 8.1 containing 50 mM CaCl2) for 1 h at 37°C. For control, the pellets were suspended in PBS. After washing the pellets for three times in PBS, the teliospores were processed for ELISA test.
Sodium dodecyl sulphate-polyacrylamide gel electrophroesis (SDS-PAGE) analysis of spore wall's proteins
Sodium dodecyl sulphate-polyacrylamide gel electrophroesis (SDS-PAGE) was done for analysing proteins of teliospore walls. After electrophoresis, the gel was fixed and stained with Coomassie brilliant blue for 4–6 hours. The gel was destained thoroughly by soaking it in the methanol/acetic acid solution, changing the destaining solution three or four times. After destaining, gel was stored indefinitely in water in a sealed plastic bag for documentation.
Western blot analysis of spore wall's proteins
The proteins were transferred from the gel to the nitrocellulose membrane (NC) with the help of wet electro-transfer assembly (BIO-RAD, Hercules, CA). The NC membrane was removed after transfer and was washed with sterile distilled water and semi-dried. The NC membrane was treated with 2% blocking solution overnight at 4°C. The NC membrane was washed thrice with PBS containing 0.5% skimmed milk for 5 min. Each was incubated with 1:500 dilutions of primary antibody in PBS containing 0.25% BSA for 1 h at room temperature. The NC membrane was washed in PBS containing 0.25% BSA three times with PBS for 5 min each. The membrane was incubated with alkaline phosphatase linked secondary antibodies (1:1000) and incubated for 1 h. Washing was done with PBS + 0.25% BSA thrice 5 min each. The membrane was then incubated with substrate solution (BCIP–NBT) for development of colour for 10 min. Reaction was stopped by adding distilled water.
Results
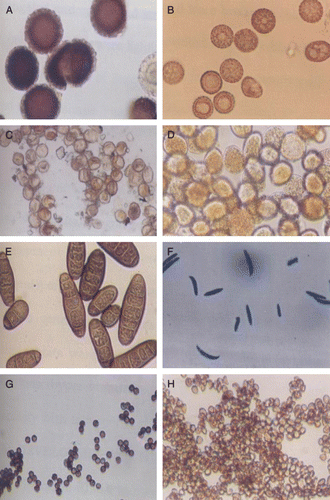
Morphological examination describes the large size of teliospores of Tilletia indica
The slide mounts of the pathogen spores and teliospores were prepared and observed under light microscope at 20× and 40× magnifications. The teliospores of KB appeared spherical or oval in shape and dark brown to yellow in colour, ranging in size from 40 to 60 microns in diameter. The immature teliospores were yellow and subhyaline, round, angular or lacrimiform and had thin limited walls. However, teliospores of T. foetida were irregular in shape and measures 17–22 microns in diameter. The wall is smooth. The spores of P. recondita were brown and spherical, 16–20 microns in diameter and with a minutely echinulate wall. The spores of P. striiformis were nearly round and yellow in colour ranging from 23 to 30 microns in diameter.
The spores of U. tritici were nearly round, olive brown, lighter on one side than other, echinulate and measure 5–9 microns in diameter. The spores of H. sativum showed elongated and cylindrical structure and were brown in colour. The spores of A. niger were smooth walled and dark blackish in colour. The spores of Fusarium spp. were colourless smooth walled and spindle shaped (). There are marked differences in surface topology of spores/teliospores of various fungal pathogens which make the basis of their differential diagnosis. However, teliospores of Tilletia spp. causing bunt diseases in other crops closely resemble with each other and hence immunodiagnosis is an important strategy for differential diagnosis of bunt diseases.
Polyclonal antibodies against intact teliospores of Tilletia indica leads to development of microtitre ELISA
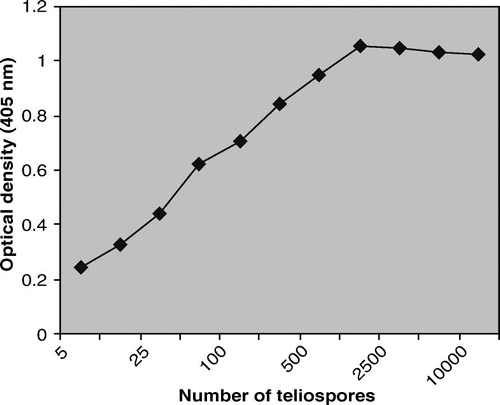
Intact teliospores were taken as immunogen for raising polyclonal anti-teliospore antibodies and development of immunoassay system in order to detect surface antigen(s) of teliospores/spores. Indirect ELISA was developed to check the reactivity and specificity of polyclonal antibodies generated against teliospores. Indirect ELISA was performed to establish optimal antigen concentration. For antigen preparation, teliospore suspension (1.0 mg of teliospores/ml of distilled water) was counted by haemocytometre and teliospore concentration was adjusted to ≈105 teliospores per ml. The wells of microtitre plate were coated with different number of teliospores ranging 5–10,000 as antigen in triplicate. For studying antigen concentration kinetics, primary and secondary antibodies were diluted at 1:500 and 1:1000, respectively. As evident from , the OD405 showed a linear increase up to 1000 teliospores after which the curve levelled off. In ELISA, highest value in linear relationship from the semi-log graph was recorded at 1000 teliospores and taken as optimum antigen concentration in indirect ELISA. As few as five teliospores were detected by ELISA which justifies the greater affinity of raised anti-teliospore antibodies for its antigen and higher sensitivity for detection of teliospores.
Figure 2. Determination of optimum number of teliospores by indirect ELISA using anti-teliospore antibodies.
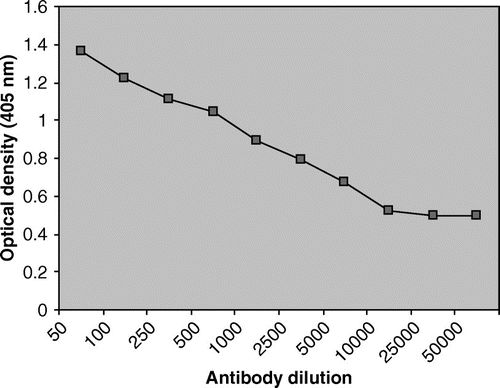
For determination of optimum dilution for antibodies, 1000 teliospores of antigen were coated in each well and different sets of primary antibody dilution (1:50, 1:100, 1:250, 1:500, 1:1000, 1:2500, 1:5000, 1:10,000, 1:25,000 and 1:50,000) were assigned a value of in triplicate. The OD405 showed a linear decrease on increasing dilutions. A mid point from the antibody dilution curve was selected (1:300) and 1:250 was taken as optimum dilution of anti-teliospore antibody for further study ().
Cross-reactivity study reveals the similarity in the antigenic epitopes of different fungal spores/teliospores
For immunodetection of teliospores of T. indica and making differential diagnosis with other seed borne and related fungal pathogens of wheat, cross-reactivity of anti-teliospore antibodies was checked using the teliospores/spores of Tillletia indica, P. recondita, P. striiformis, U. nudo tritici, Fusarium spp., H. sativum, A. niger as antigen. The reactivity of anti-teliospore antibodies to fungal spores of other pathogen was compared to the reactivity of teliospores of T. indica. The reactivity with teliospores of T. indica was taken as 100% for comparison of cross-reactivity of anti-teliospore antibodies with teliospores and spores of other fungal pathogens of wheat which varied from 5 to 70% (). Anti-teliospore antibodies showed cross-reactivity with T. foetida, P. recondita and P. striiformis, however, no cross-reactivity was observed with Fusarium spp., H. sativum, A niger and U. nudo tritici. The higher percentage of reactivity was observed with T. foetida (64%), P. recondita (55%) and P. striiformis (60%) which suggests similarities in the antigenic epitopes present in the spores and teliospore of bunt and rust fungal pathogens of wheat. However, morphologically spores/teliospores of these pathogens were quite distinct from each other.
Table 1. Cross-reactivity of anti-teliospore antibodies of Karnal bunt with other fungal spores.
Negligible or low cross-reactivity was observed with Ustilago nudo tritiici (15%), Fusarium (9%), Helminthosporium (6%) and A. niger (8%). It indicates minimal similarity of antigenic epitopes of teliospore/spore wall proteins of these pathogens with T. indica. They were also distinct in terms of their morphology. The cross-reactivity of anti-teliospore antibodies with only bunt and rust species suggests the specificity of these polyclonal antibodies against these wheat pathogens.
Preliminary characterisation shows the glycoproteineous nature of teliospore antigens of Tilletia indica
To study the nature of antigens recognised by the rabbit anti-teliospores antiserum, the effect of various chemical modifiers and enzymes on the reactivity of antibodies in indirect ELISA was investigated. The immunoreactivity of polyclonal antiserum with teliospores/spores of different fungal pathogens was not affected by the heat and methanol treatments. In both the cases, the loss in immunoreactivity was less than 12% (). The enzyme (trypsin) treatment only reduced the immunoreactivity of teliospores of T. indica 5%, however, teliospores/spores of P. recondita (37%), T. foetida (30%) and P. striiformis (42%) showed some loss of immunoreactivity.
Table 2. Effect of various treatments on immunoreactivity of teliospores and the spores of different fungal pathogens using anti-teliospore antibodies of T. indica.
Periodate treatment of teliospores and spores of different pathogens caused notable loss of immunoreactivity including 57% in T. indica, 60% in P. recondita, 62% in T. foetida and 69% in P. striiformis () but nearly complete loss of antigenicity of teliospores of T. indica (89%) and spores of P. recondita (95%), T. foetida (94%) and P. striiformis (93%) was observed after sequential treatment of periodate and trypsin. A particular polyclonal antibody may recognise a glycoprotein via either protein moiety or the carbohydrate prosthetic group and may be that the antigenic epitopes consists of both protein and carbohydrate entities.
These results suggest a glycoprotein nature of antigen recognised by polyclonal antiserum or could be due to loss of multiple polysaccharide and protein epitopes. shows the loss of reactivity of anti-teliospore antibodies with spores and teliospores of wheat fungal pathogens after treatment of different chemical modifier/enzyme.
Molecular characterisation of antigen(s) of teliospores of Tilletia indica reveals the presence of two unique proteins
The SDS-PAGE analysis of phenol extracted spore wall proteins showed distinct banding pattern for each species. Although, a 12% SDS-PAGE gel was used which is capable of resolving polypeptides and proteins ranging from 10 kDa to 100 kDa. Only proteins in the range of 14–85 kDa were detected in the spore and teliospore walls of Fusrium sp., H. sativum, T. indica, T. foetida, P. recondita, P. steriiformis, A. niger and U. nudotritici (A) by Coomassie staining. One unique band was detected in T. indica by SDS-PAGE analysis having molecular weight (MW) of 15 kDa which could be useful in making differential diagnosis with other fungal pathogens. Unique bands were also detected in H. sativum and P. striiformis with MWs of 76 kDa and 21 kDa, respectively.
Figure 4. (A). Protein profile analysis of spores/teliospores walls of different fungal pathogens of wheat by SDS-PAGE (12%). (B). Identification of shared immunoreactive epitopes in spores/teliospores walls of common fungal pathogens of wheat by western blotting. M – Marker, Lane 1 – Fusarium spp.; Lane 2 – Helminthosporium sativum; Lane 3 – Tilletia indica; Lane 4 – Tilletia foetida; Lane 5 – Puccinia recondita; Lane 6 – Puccinia striiformis; Lane 7 – Aspergillus niger; Lane 8 – Ustilago nuda tritici.
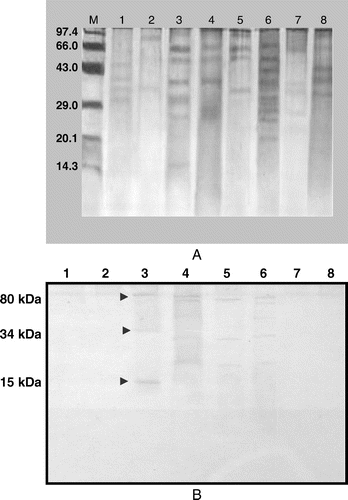
Many common bands were observed in bunt and rust pathogens in low, medium and high MW range, i.e. 25–35 kDa, 38–45 kDa and 60–80 kDa, respectively. The teliospore/spore wall protein extracts of T. indica, T. foetida, P. recondita and P. steriiformis showed six bands in low MW range, four in medium MW range and seven in high MW range (). The presence of many common bands suggests similarity in protein composition of spores/teliospores walls of bunt and rust fungal pathogens of wheat (A).
Table 3. Comparison of common bands identified by SDS-PAGE and identification of immuno-reactive proteins in spores/teliospores walls of different fungal pathogens using western blotting.
Crude anti-teliospore antibodies to T. indica recognised unique immunoreactive bands having MW ≈34 kDa and ≈15 kDa, however, these anti-teliospore antibodies also reacted with common wheat fungal pathogens T. foetida, P. recondita and P. steriiformis. One unique immunoreactive band was present in P. steriiformis having MW 63 kDa but no unique immunoreactive band was observed in T. foedita and P. recondita (B). The immunoreactivity of antisera against T. indica was not observed with spore wall proteins from Fusarium spp., H. sativum, A. niger and U. nudo tritici. The two bands in T. indica (34 kDa and 15 kDa) uniquely reacted with anti-teliospore antibody, however, a ≈80 kDa protein from T. foetida and P. recondita also reacted with antiserum ().
The aim of the present study was to characterise the potential antigen(s) for developing specific enzyme immunoassay for detection of teliospores of KB. Two unique immuno dominant bands of MW ≈34 and 15 kDa were found in the T. indica spore wall only and could be exploited for subsequent characterisation, isolation and development of specific immunoprobes for differential diagnosis of KB teliospores.
Discussion
Early detection, correct identification and differential diagnosis of fungal pathogens is of primary importance in determining the most effective course of treatment to prevent the spread of fungi causing plant disease and post-harvest storage rots. Correct diagnosis on the basis of symptoms alone, is difficult for many diseases. Identification and enumeration of spores of fungi is also important, especially for disease forecasting and development of control strategies. Microscopic methods used to identify and count air borne spores are slow, tedious and often there is need to validate visual identification. Immunodetection assays will never entirely replace classical methods but they do enable farmers and seed growers, who may have little mycological expertise, to screen large number of samples on the field site with minimum facilities.
It is well established that differentiation of fungal pathogen is dependent upon the qualitative and quantitative differences of specific markers present on the surface of pathogens. The number of these markers is very small compared to common structural components of two fungal pathogens. It, therefore, becomes extremely difficult to pinpoint these markers in fungal spores/teliospores by conventional, chemical and biochemical methods. Due to high specificity of antigen–antibody reaction, immunological approaches have great promise to study the structural components of teliospores and discriminate species. In the present investigation, an attempt was made to differentiate the spores/teliospores of various fungal pathogen of wheat using immunological test viz. indirect ELISA.
Immunodiagnostic tests are specific, sensitive and quick to detect small number of disease causing entities. The success of any newer diagnostic method developed for seed borne fungal pathogens is solely dependent on the effective removal of pathogen from wheat lots. The detergent washing test developed in our laboratory using ionic and non-ionic detergents was efficient for extraction of KB teliospores present as surface contaminants (Gupta, Kumar, Joshi, Agarwal, & Garg, Citation2002). The salvated pathogens can be identified and distinguished using indirect ELISA developed in the present study.
The present work primarily deals with the characterisation of potential antigen(s) for the development of specific enzyme immunoassay for detection of KB, a seed borne fungal pathogen of wheat. An attempt was made to generate anti-teliosporic antibodies against intact teliospores in New Zealand white rabbits. These polyclonal antibodies were also employed for characterising the potential antigen(s) of teliospore walls of T. indica for the development of indirect microtitre ELISA.
Most of the ELISA tests developed for fungi are simple indirect assays in which the microtitre wells are directly coated with the test sample or fungal antigen. Brill, McClary, and Sinclari (Citation1994) compared two ELISA formats for their sensitivity using polyclonal antibodies against mycelial extracts of Phomopher longicala. Currently, ELISA is employed for routine detection of fungal pathogens (Broggio & Bertocci, Citation1995). The development of microtitre ELISA included standardisation and optimisation of concentration of antigen and titre of anti-teliosporic antibodies by following antigen concentration kinetic and antibodies dilution curve analysis. The sensitivity and specificity of the indirect microtitre ELISA was also evaluated. The minimum sensitivity of indirect ELISA developed in our laboratory was as low as five teliospores to 1000 teliospores which gave linear relationship in antigen concentration kinetics. The titre of anti-teliospore antibodies was 1:10,000 which was of maximal dilution giving reactivity in ELISA. The specificity of antibodies was checked by cross-reactivity studies with other seed borne, leaf and foliar fungal pathogens and to utilise these antibodies in differential diagnosis. The anti-teliospore antibodies of T. indica were cross-reacting with spores/teliospores of T. foetida, P. recondita and P. striiformis, but there were no cross-reactivity with Fusarium sp., H. sativum, A. niger and U. nudo tritici. This showed the specificity of anti-teliospore antibodies of wheat fungal pathogens.
Efforts have been made to characterise the nature of antigen(s) of spore and teliospore walls using chemical modifiers and enzyme cleavage analysis through ELISA. The heat and methanol treatment did not affect the reactivity of antigen of spore/teliospore walls to anti-teliospore antibodies of T. indica (<12%). The complete loss of reactivity in the sequential treatment of periodate and trypsin in preliminary characterisation of spores/teliospores of different common fungal pathogens indicated the glycoprotein nature of antigen; however, the periodate treatment also caused the loss of reactivity of anti-teliospore antibodies to some extent (55–70%) showing the carbohydrate nature of immunodominant antigenic determinant in the spore/teliospore walls of common wheat pathogens, especially in the case of T. indica. Enzymatic cleavage affected the reactivity of other fungal pathogens viz. T. foetida, P. recondita and P. striiformis up to (30–45%) but not in T. indica (only 5%).
The detection of plant pathogenic fungi by immunodiagnosis has been slow due to the problems experienced in raising specific antisera against infectious entities. Most such antisera cross-react widely with both related and unrelated fungi and host tissues or extracts when tested by ELISA or related techniques yet appear species-specific when tested by immunodiffusion. Non-specific antigens may be common to both the insoluble and soluble fractions of fungal material (Chard, Gray, & Frankland, Citation1985a,Citationb), but few efforts to improve specificity by diluting out non-specific antibodies or cross-absorbing antisera with related fungi have proved effective (Kitagawa, Sakamoto, Furumi, & Ogwara, Citation1989). Antisera against protein precipitates from either culture filtrates or mycelial extracts (Barker & Pitt, Citation1988; Gerik, Lommel, & Huisman, Citation1987; Gleason, Gharbrial, & Ferries, Citation1987; Mohan, Citation1989) or specific fungal fractions, such as enzymes, toxins or soluble carbohydrates (Notermans & Kamphuis, Citation1990), are generally highly specific (Johnson, Pirone, Siegel, & Varney, Citation1982). Monoclonal antibodies that have been raised against fungi (Dewey, Citation1988; Dewey, McDonald, & Philips, Citation1989; Estrada-Garcia, Green, Booth, White, & Callow, Citation1989; Wong, White, & Wright, Citation1988) have shown great diagnostic ability including the detection of fungi in seeds (Mitchell & Sutherland, Citation1986). The main difficulty appears to be related to the presence of non-specific immunodominant carbohydrates or glycoproteins that induce non-T-cell stimulated responses. The site and nature of species and subspecies-specific antigens is not yet generally known, but specific antigens may often be present in the walls and cross walls of the hyphae but not the spores (Dewey et al., Citation1989; Gleason et al., Citation1987).
The molecular characterisation of spore and teliospore wall protein was evaluated using protein profile analysis and immunoreactivity of spore walls proteins through western blotting. The spores/teliospore wall proteins were extracted using both mechanical and chemical methods from different fugal pathogens; however, for molecular characterisation of antigenic proteins, the chemical method was preferred as it gives better yield of proteins. The protein extracts from whole teliospores analysed by Kawchuk, Kim, and Nielsen (Citation1988) and Weber and Schanz (Citation1985) possibly contained cellular proteins (Etten, Freer, & McCune, Citation1979). However, it should be noted that Banowetz, Trione, and Krygier (Citation1984) were unable to extract detectable amounts of protein antigens from intact teliospores of either T. controversa or T. tritici with a variety of solvents. The chemical method was preferred in the laboratory for protein extraction giving good yield of proteins (26.4 mg/100 mg teliospores) of T. indica, while in the mechanical method yield was 15.6 mg/100 mg teliospores of T. indica. In chemical methods, the reason for better protein yield was Tris buffer saline washed spore wall fraction consisted of >90% dark, melanin containing fragments. Neither lipid droplets (Stockwell & Trione, Citation1986) nor opalescent transparent particles that were observed in unwashed fractions were found in the saline washed spore wall fraction. It was assumed that teliospore cellular contents had been removed from the melanin containing teliospores wall fragments and hence, good recovery of spore wall proteins was observed in the present study.
The present study reveals the unique bands/proteins of some fungal pathogens including T. indica after SDS-PAGE and western blotting. In SDS-PAGE analysis, teliosporic wall proteins contained one unique 15 kDa band which was not detected in the spore wall proteins of other fungal pathogens. There was similarity in protein/bands in the range of 25–35 kDa, 38–45 kDa and 60–80 kDa in bunt and rust of wheat including T. foetida, T. indica, P. recondita and P. striiformis. The Western blot analysis showed the presence of two unique reactive bands of teliosporic wall proteins of T. indica having MWs 34 kDa and 15 kDa and also one unique reactive band of 63 kDa was detected in P. striiformis. A 34 kDa band was detected in the spore wall fraction of P. striiformis in SDS-PAGE analysis, but there was no immunoreactivity of 34 kDa band in P. striiformis during Western blot analysis. Therefore, the two unique immunoreactive bands of teliospore wall of T. indica 34 and 15 kDa band can be characterised as a potential antigenic epitope for the development of a specific enzyme assay for immunodetection of teliospores of KB.
In the present study, the western blotting analysis displayed the unique antigenic configuration of teliospores of T. indica; moreover, chemical treatments followed by ELISA of teliospores suggested a glycoprotein nature of the antigens present on the walls of teliospores of KB. Furthermore, specific monoclonal antibodies may be raised to potential antigens observed on SDS-PAGE and western blotting and, which can be used for immunodetection of KB infestation in wheat lots. A number of different Armilaria species have been separated in this manner (Lung-Escarmant & Dunez, Citation1979) and any unique protein band detected by PAGE may be cut out and used as immunogens to raise monoclonal antibodies. A consequence of this expansion should be a greater selection of antibodies that may be used to help in characterisation of potential antigens (Fox & Hahne, Citation1989; Martin, Fox, & Baldwin, Citation1992) and their role in spore dormancy, disease initiation, and the evolution of pathogenicity and even the basis of interspecies recognition.
References
- Banowetz , G.M. , Trione , E.J. and Krygier , B.B. 1984 . Immunological comparisons of teliospores of two wheat bunt fungi of Telletia species, using monoclonal antibodies and antisera . Mycologia , 76 : 51 – 62 .
- Barker , I. and Pitt , D. 1988 . Detection of leaf curl pathogen of anemones in corms by enzyme-immunosorbent assay (ELISA) . Plant Pathology , 37 : 417 – 422 .
- Bradford , M. 1976 . A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein dye binding . Analytical Biochemistry , 72 : 248 – 254 .
- Brill , L.M. , McClary , R.D. and Sinclari , J.B. 1994 . Analysis of two ELISA formats and antigen preparation using polyclonal antibodies directed against Phomosis longicolla . Phytopathology , 84 : 173 – 179 .
- Broggio , M. and Bertocci , F. 1995 . ELISA detection of potato early blight agents . Phytopathologia-Mediterranea , 34 : 173 – 179 .
- Chard , J.M. , Gray , T.R.G. and Frankland , J.C. 1985a . Purification of an antigen characteristic of Mycena galopus . Transactions of the British Mycological Society , 84 : 235 – 241 .
- Chard , J.M. , Gray , T.R.G. and Frankland , J.C. 1985b . Use of an anti-Mycena galopus serum as an immunofluorescent reagent . Transactions of the British Mycological Society , 84 : 243 – 249 .
- Dewey , F.M. 1988 . Development of immunodiagnostic assays for fungal plant pathogens . In Proceedings of the Brighton Crop Protection Conference Pest and Diseases 777 786 Thornton Heath : British Crop Protection Council .
- Dewey , F.H. , McDonald , M.M. and Philips , I. 1989 . Development of monoclonal antibody ELISA, dot blot and dipstick immunoassays for Humicola lanuginosa in rice . Journal of General Microbiology , 135 : 361 – 374 .
- Engvall , E. and Perlmann , P. 1971 . Enzyme linked immunosorbent assay (ELISA) quantitative assay of immunoglobulin G . Immunochemistry , 8 : 871 – 874 .
- Estrada-Garcia , M.T. , Green , J.R. , Booth , J.M. , White , G. and Callow , I.A. 1989 . Monoclonal antibodies to cell surface componenets of zoospores and cysts of the fungus Pythium aphanidermatum reveal species-specific antigens . Experimental Mycology , 13 : 348 – 356 .
- Etten , J.L. , Freer , S.N. and McCune , B.K. 1979 . Presence of a major storage protein in dormant spores of the fungus Botryodiploida theobromae . Journal of Bacteriology , 138 : 650 – 652 .
- Fox , R.T.V. , & Hahne , K. ( 1989 , July 11–13 ). Separation of the Armillaria complex using monoclonal antibodies. Abstracts obtained from The British Society for Plant Pathology/British Crop Protection Council . In Conference on Techniques for the Rapid Diagnosis of Plant Diseases 15 Norwich : University of East Anglia .
- Gerik , J.S. , Lommel , S.A. and Huisman , O.C. 1987 . A specific serolological staining procedure for Verticillium dehliae in cotton root tissue . Phytopathology , 77 : 261 – 266 .
- Gill , K.S. , Sharma , I. and Aujla , S.S. 1993 . Karnal bunt and wheat production , Ludhiana, , India : Punjab Agriculture University .
- Gleason , M.K. , Gharbrial , S.A. and Ferries , R.S. 1987 . Serological detection of Phomosis longicolla in soybean seeds . Phytopathology , 77 : 371 – 375 .
- Gupta , V. , Kumar , A. , Joshi , G.K. , Agarwal , V.K. and Garg , G.K. 2002 . Detergent washing technique for the efficient extraction of teliospores of Tilletia indica from contaminated wheat seeds . Seed Science and Technology , 31 : 95 – 101 .
- Gupta , V. , Kumar , A. , Lakhchaura , B.D. and Garg , G.K. 2001 . Generation of anti-teliospore antibodies for immunolocalization and characterization of antigenic epitopes of teliospores of Karnal bunt (Tilletia indica) of wheat . Indian Journal of Experimental Biology , 39 : 686 – 690 .
- Gupta , V. , Kumar , A. , Singh , A. and Garg , G.K. 2000 . Immunodetection of teliospores of Karnal bunt (Tilletia indica) of wheat using fluorescent staining tests . Plant Cell Biotechnology and Molecular Biology , 1 : 81 – 85 .
- Johnson , M.C. , Pirone , T.P. , Siegel , M.R. and Varney , D.R. 1982 . Detection of Epichloe typhina in tall fescue by means of enzyme-linked immunosorbent assay . Phytopathology , 72 : 647 – 650 .
- Joshi , L.M. , Singh , D.V. , Srivastava , K.D. and Wilcoxon , R.D. 1983 . Karnal bunt: A minor disease that is now a threat to wheat . The Botanical Review , 49 : 309 – 330 .
- Kawchuk , L.M. , Kim , W.K. and Nielsen , J. 1988 . A comparison of polypeptides from the wheat bunt fungi Tilletia laevis, T. tritici and T. controversa . The Canadian Journal of Botany , 66 : 2367 – 2376 .
- Kesari , R.E. and Kumar , A. 2003 . Design and optimization of novel immuno-dipstick test for detection of teliospores of Karnal bunt (Tilletia indica) of wheat . Indian Journal of Biotechnology , 2 : 603 – 606 .
- Kesari , R. , Mishra , D.P. , Garg , G.K. and Kumar , A. 2005 . Suitability of dyed latex bead agglutination test for immunodiagnosis of Karnal bunt (Tilletia indica) teliospores in a single seed of wheat . Food and Agricultural Immunology , 16 : 73 – 81 .
- Kitagawa , T. , Sakamoto , Y. , Furumi , K. and Ogwra , H. 1989 . Novel enzyme immunoassays for specific detection of various Fusarium species . Phytopathology , 78 : 127 – 130 .
- Kumar , A. , Singh , A. and Garg , G.K. 1998 . Development of seed immunoblot binding assay for the detection of Karnal bunt (Tilletia indica) of wheat . Journal of Plant Biochemistry and Biotechnology , 7 : 119 – 120 .
- Lung-Escarmant , B. and Dunez , J. 1979 . Differentiation of Armillariella and Clitocybe species by the use of immunoenzymatic ELISA procedure . Annales de phytopathologie , 11 : 515 – 518 .
- Martin , L.A. Fox , R.T.V. Baldwin , B.C. 1992 . Rapid methods for the detection of MBC resistance in fungi: Immunological approaches . In Proceedings of 10th International Reinhardsbrunn Symposium 209 218
- Mitchell , L.A. and Sutherland , J.K. 1986 . Detection of seed borne Sirococcus strobilinus with monoclonal antibodies in an enzyme-linked immmunosorbent assay . Canadian Journal of Forest Research , 16 : 945 – 948 .
- Mohan , S.B. 1989 . Cross-reactivity of antiserum raised against Phytophthora species and its evaluation as a genus detecting antiserum . Plant Pathology , 38 : 352 – 363 .
- Munjal , R.L. 1975 . Status of Karnal bunt (N. indica) of wheat in North India during 1968–69 to 1969–70 . Indian Journal of Mycology & Plant Pathology , 5 : 185 – 187 .
- Notermans , S. and Kamphuis , H. 1990 . Detection of moulds in foods by latex agglutination: A collaborative study . Food and Agricultural Immunology , 2 : 34 – 47 .
- Prescott , J.M. 1986 Sporidia trapping studies of Karnal bunt . In C. Obregon Proceedings of the 5th Biennial Smut Workshop 22 Senora, , Mexico : International Maize and Wheat Improvement Center .
- Stockwell , V.O. and Trione , E.J. 1986 . Distinguishing teliospores of T. controversa from those of T. caries by fluorescence microscopy . Plant Disease , 70 : 924 – 926 .
- Warham , E.J. , Mujeeb-Kazim , A. and Rosas , V. 1986 . Karnal bunt (Tilletia indica) resistance screening of Aegilops species and their practical utilization for Triticum aestivum improvement . The Canadian Journal of Plant Pathology , 8 : 65 – 70 .
- Weber , G. and Schauz , K. 1985 . Characterization of spore protein patters in Tellitia controversa and Tilletia caris with gel electrophoresis method . Z pflanzenkrankh und Pflanzenschutz , 92 : 600 – 605 .
- Wong , W.C. , White , M. and Wright , I.G. 1988 . Production of monoclonal antibodies to Fusarium oxysporium f.sp. cubense race 4 . Letters in Applied Microbiology , 6 : 39 – 42 .
- Ykema , R.E. , Floyed , J.P. , Palm , M.E. and Petrson , G.L. 1996 . First repot on Karnal bunt of wheat in the United States . Plant Disease , 80 : 1207
- Zhang , Z. , Lange , L. and Mathur , S.B. 1984 . Teliospore survival and plant quarantine significance of T. indica (causal agent of Karnal bunt) particularly in relation to China . The European Plant Protection Organization Bulletin , 14 : 119 – 126 .