Abstract
Pleurotus florida is an edible mushroom with various therapeutic uses. Recently, it has been shown to modulate the immune responses and inflammation. Macrophages have been shown to play an essential role as the first line of defence against microbial invaders and neoplasia and have intracellular killing capacities. After being stimulated, macrophages produce a number of inflammatory mediators. In this study, we evaluated the effect of P. florida on viability of macrophages and its nitric oxide (NO) production.
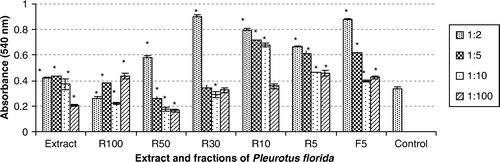
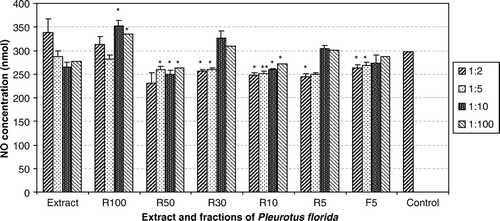
Cell viability of macrophages was altered by extract and fractions dose, dependently. R100, R50 and R30 fractions in some dilutions decreased and extract R10, R5 and F5 fractions increased cell viability. R50, R30, R10, R5 and F5 fractions cause significant reduction and R100 causes significant induction in NO production. Further studies are necessary to clarify the modulatory effects of P. florida mushroom on macrophage anti-inflammatory function.
Introduction
Macrophages have been shown to play an essential role as the first line of defence against microbial invaders and neoplasia and have intracellular killing capacities (Adams & Hamilton, Citation1984; An, Jeong, Um, Kim, & Hong, Citation2006). After being stimulated, macrophages produce a number of important inflammatory mediators, such as nitric oxide (NO), PGE2 and cytokines such as IL-1, TNF-alpha, IL-6, IL-10 (Hu et al., Citation2008).
NO is an inflammatory mediator significantly produced during infection and other inflammatory conditions (Fang & Vazquez-Torres, Citation2002; Park et al., Citation2005). The biological production of NO is also important for non-specific host defence (Pacher, Beckman, & Liaudet, Citation2007). In addition, it has either beneficial effects, e.g. micro-organism and tumour cell killing or harmful effects on the host organism, e.g. inflammation, genotoxicity or immunosuppression (Goldring, Reveneau, Algarte, & Jeannin, Citation1996). The enhanced formation of NO by the macrophage greatly contributes to tissue injury in human inflammatory arthritis (Lucas, Alves, del Olmo, San Feliciano, & Payá, Citation2003).
There is a growing interest in the consumption of mushrooms and mushroom extracts as dietary supplements promoting health (Borchers, Keen, & Gershwin, Citation2004). The reports showed that they have anti-tumour, immunomodulator, anti-coagulant, hypoglycemic and anti-viral properties (Ajith & Janardhanan, Citation2007; Rosado et al., Citation2003; Santos-Neves et al., Citation2008).
Pleurotus species are commonly called oyster mushrooms and considered as an edible mushroom (Rout, Mondal, Chakraborty, & Islam, 2006). It is also a delicious edible mushroom with many therapeutic uses, and is cultivated on a commercial scale in many parts of the world (Rout et al., Citation2006).
It is reported that a glucan isolated from the aqueous extract of the fruiting bodies of Pleurotus florida stimulates the phagocytic activity of macrophages (Rout, Mondal, Chakraborty, Pramanik, & Islam, 2005). The anti-inflammatory activity of P. florida suggests its potential ability to modulate inflammation which is increasingly implicated in cancer (Jose, Ajith, & Janardhanan, Citation2004).
The anti-inflammation and platelet aggregation inhibiting activities of the methanol extract of P. florida were studied, which suggested its potential therapeutic use against vascular disorders (Jose et al., 2004).
Therefore, this in vitro study was undertaken to determine whether aqueous extract of P. florida and its fractions have immunomodulatory properties on macrophages cell viability and NO production.
Materials and methods
Isolation of peritoneal macrophages
Peritoneal exudate cells were obtained from inbred female Balb/c mice aged 8–10 weeks obtained from the animal laboratory, Shahed University (Tehran, Iran). The mice were killed and peritoneal exudate cells were harvested by lavage using 5 ml of cold Roswell Park Memorial Institute (RPMI) medium with 5% FCS and 100 units heparin, and washed three times with RPMI medium as previously described (Ghazanfari, Hassan, & Khamesipour, 2006).
Preparation of extract and fractions
For the preparation of florida extract: 42.5 g of florida was mixed with 155 cc of deionised and distilled water. The solution was centrifuged. Florida extract is fractionated to R100 (>100 KD), R50 (100 KD > R50 > 50 KD), R30 (50 KD > R30 > 30 KD), R10 (30 KD > R10 > 10 KD), R5 (10 KD > R5 > 5 KD) and F5 (<5 KD) by amicon ultrafiltration system based on molecular weights according to our previous work (Ghazanfari et al., Citation2006).
Cell culture
Peritoneal macrophages (106 cell/ml) were cultivated in complete RPMI medium in 96 well plates. The P. florida extract and its fractions were added to the wells at different concentrations at that time. The cells were cultured for 24 h at 37°C in a humidified 5% CO2 incubator.
Nitrite assay
Production of NO was estimated 18 hours after treatment, by measuring the nitrite levels in the cell supernatant with the Griess reaction (Abad et al., Citation2006). Equal volumes of Griess reagent (1:1 of 0.1% in N-(1-Naphtyl)-ethylendiamindihydrochloride (NEDA) solution (Merck, Germany), in 5% phosphoric acid and 1% sulfanilamide in 5% phosphoric acid) and sample cell supernatant or NaNO2 standards were incubated together at room temperature for 10 min. Absorbance was read at 540 nm.
Cell viability
We used MTT (3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide) reduction assay for evaluating cells viability. In this assay, living cells reduce MTT tetrazolium salt into formazan crystals. MTT (Merck, Germany) powder was dissolved in PBS (5 mg/ml), filtered and stored at −20°C until use. Followed by the appropriate exposure of cells to P. florida with formyl methionyl leucyl phenylalanine (FMLP) mitogen, the cells were incubated for 4 h with MTT solution and formazan crystals were extracted in acidic isopropanol (0.04 N HCL). Measurement of optical density was performed at 540 nm with a Titertek microplate reader, using acidic isopropanol as blank reference.
Statistical analysis
Data were presented as mean + / − SEM. Comparisons between drug groups and control group were performed with Students’ t-test. Differences were considered significant at p<0.05.
Results
Effect of Pleurotus florida on cell viability
The viability of macrophages treated with serial dilutions of P. florida extract and its fractions were measured by MTT assay. Cell viability of macrophages was altered by extract and fractions doses, dependently. R100, R50, R30 fractions in 1/5, 1/10, 1/100 dilutions decreased and in 1/2 dilutions increased cell viability. Extract, R10, R5 and F5 fractions increased cell viability ().
Nitric oxide (NO) production by macrophages
As our results show, cells that treated with P. florida extract, has not shown any significant differences compared with control group. Whereas some doses of R50, R30, R10, R5 and F5 fractions cause significant reduction and R100 cause significant induction in NO production versus control group ().
Discussion
Pleurotus ostreatus (florida) is an edible mushroom that occupies the second most important position in the world mushroom market (Larraya, Pérez, Ritter, Pisabarro, & Ramirez, Citation2000; Rout et al., Citation2006). P. florida mushroom has been shown to modulate the immune system (Jose et al., 2004). In this study, the effect of P. florida on cell viability of macrophages and macrophage NO production has been investigated.
Macrophages are crucial cells against invading pathogen and malignancies and are very important in innate and adaptive immune responsces. NO plays a key role in the activation of macrophages and cellular defence (Durner, Gow, Stamler, & Glazebrook, Citation1999; Moncada, Citation1999). NO is produced in large quantities and is probably an important mediator of the acute and chronic signs of inflammation.
As the results show, cell viability of macrophages and NO production were altered by extract and fractions dose and fraction, dependently. Some fractions decreased and some increased cell viability or NO production. Our results indicated that P. florida, which is used as food in most parts of world, could be useful as an immunomodulatory or anti-inflammatory agent. Rout et al. (Citation2005) reported that a 40 KD polysaccharide isolated from P. florida cause an increase in NO production. In our study, molecules with this molecular weight were isolated in R30 fraction, but here this fraction decreased NO production. And only R100 fraction, including molecules over 100 KD, causes an increase in NO production. Higher doses which used in our study are the other explanation for this difference. The other difference is the procedure of fraction preparations. There is no study on macrophage viability and no more study on NO production with P. florida. Further studies must be planned to define the exact mechanisms of P. florida and its components effect on immune and inflammatory responses.
Acknowledgements
This study was performed by Immunoregulation Research Center of Shahed University, and was supported by Medical Research Center, Shahed University.
References
- Abad , M.J. , Bessa , A.L. , Ballarin , B. , Aragón , O. , Gonzales , E. and Bermejo , P. 2006 . Anti-inflammatory activity of four Bolivian Baccharis species (Compositae) . Journal of Ethnopharmacology , 103 : 338 – 344 .
- Adams , D.O. and Hamilton , T.A. 1984 . The cell biology of macrophage activation . Annual Review of Immunology , 2 : 283 – 318 .
- Ajith , T.K. and Janardhanan , K. 2007 . Indian medicinal mushrooms as a source of antioxidant and antitumor agents . Journal of Clinical Biochemistry and Nutrition , 40 : 157 – 162 .
- An , H.J. , Jeong , H.J. , Um , J.Y. , Kim , H.M. and Hong , S.H. 2006 . Glechoma hederacea inhibits inflammatory mediator release in IFN-( and LPS-stimulated mouse peritoneal macrophages . Journal of Ethnopharmacology , 106 : 418 – 424 .
- Borchers , A.T. , Keen , C.L. and Gershwin , M.E. 2004 . Mushrooms, tumors, and immunity: An update . Experimental Biology and Medicine , 229 : 393 – 406 .
- Durner , J. , Gow , A.J. , Stamler , J.S. and Glazebrook , J. 1999 . Ancient origins of nitric oxide signaling in biological systems . Proceedings of the National Academy of Sciences of the United States of America , 96 : 14206 – 14207 .
- Fang , F.C. and Vazquez-Torres , A. 2002 . Nitric oxide production by human macrophages: There's no doubt about it. American Journal of Physiology . Lung Cellular and Molecular Physiology , 282 : L941 – L943 .
- Ghazanfari , T. , Hassan , Z.M. and Khamesipour , A. 2006 . Enhancement of peritoneal macrophage phagocytic activity against Leishmania major by garlic (Allium sativum) treatment . Journal of Ethnopharmacology , 103 : 333 – 337 .
- Goldring , C.E. , Reveneau , S. , Algarte , M. and Jeannin , J.F. 1996 . In vivo footprinting of the mouse inducible nitric oxide synthase gene: Inducible protein occupation of numerous sites including Oct and NF-IL6 . Nucleic Acids Research , 24 : 1682 – 1687 .
- Hu , X.D. , Yang , Y. , Zhong , X.G. , Zhang , X.H. , Zhang , Y.N. Zheng , Z.P. 2008 . Anti-inflammatory effects of Z23 on LPS-induced inflammatory responses in RAW264.7 macrophages . Journal of Ethnopharmacology , 120 : 447 – 451 .
- Jose , N. , Ajith , T.A. and Janardhanan , K.K. 2004 . Methanol extract of the oyster mushroom, Pleurotus florida, inhibits inflammation and platelet aggregation . Phytotherapy Research , 18 : 43 – 46 .
- Larraya , L.M. , Pérez , G. , Ritter , E. , Pisabarro , A.G. and Ramirez , L. 2000 . Genetic linkage map of the edible basidiomycete Pleurotus ostreatus . Applied and Environmental Microbiology , 66 : 5290 – 5300 .
- Lucas , R. , Alves , M. , del Olmo , E. , San Feliciano , A. and Payá , M. 2003 . LAAE-14, a new in vitro inhibitor of intracellular calcium mobilization, modulates acute and chronic inflammation . Biochemical Pharmacology , 65 : 1539 – 1549 .
- Moncada , S. 1999 . Nitric oxide: Discovery and impact on clinical medicine . Journal of the Royal Society of Medicine , 92 : 164 – 169 .
- Pacher , P. , Beckman , J.S. and Liaudet , L. 2007 . Nitric oxide and peroxynitrite in health and disease . Physiological Reviews , 87 : 315 – 424 .
- Park , Y.M. , Won , J.H. , Kim , Y.H. , Choi , J.W. , Park , H.J. and Lee , K.T. 2005 . In vivo and in vitro anti-inflammatory and anti-nociceptive effects of the methanol extract of Inonotus obliquus . Journal of Ethnopharmacology , 101 : 120 – 128 .
- Rosado , F.R. , Carbonero , E.R. , Claudino , R.F. , Tischer , C.A. , Kemmelmeier , C. and Iacomini , M. 2003 . The presence of partially 3-O-methylated mannogalactan from the fruit bodies of edible basidiomycetes Pleurotus ostreatus ‘florida’ Berk and Pleurotus ostreatoroseus Sing . FEMS Microbiology Letters , 221 : 119 – 124 .
- Rout , D. , Mondal , S. , Chakraborty , I. and Islam , S.S. 2006 . The structure of a polysaccharide from fraction-II of an edible mushroom, Pleurotus florida . Carbohydrate Research , 341 : 995 – 1002 .
- Rout , D. , Mondal , S. , Chakraborty , I. , Pramanik , M. and Islam , S.S. 2005 . Chemical analysis of a new (1→3), (1→6)-branched glucan from an edible mushroom, Pleurotus florida . Carbohydrate Research , 340 : 2533 – 2539 .
- Santos-Neves , J.C. , Pereira , M.I. , Carbonero , E.R. , Gracher , A.H. , Gorin , P.A. Sassaki , G.L. 2008 . A gel-forming beta-glucan isolated from the fruit bodies of the edible mushroom Pleurotus florida . Carbohydrate Research , 343 : 1456 – 1462 .