Abstract
Whole wheat and refined wheat differ substantially for dietary fibre and polyphenol contents, however, the exact relationship between the in vitro contents and in vivo functions have not been well established. Two groups of growing rats were fed for 6 weeks with diets containing 53% of whole durum and refined durum wheat flours. In plasma and in mesenteric lymphocytes parameters of redox status and in lymphocytes the rate of cell proliferation and the type of immune response were measured. Plasma antioxidant activity showed that whole wheat was able to increase antioxidative status with respect to refined wheat and to reduce the carbonyl content. The diets rich in whole wheat can improve proliferative responses with respect to refined wheat. The results indicated that a constant intake of whole wheat may have important implications for health, by acting as modulator of immune function and redox status.
Keywords:
Introduction
Wheat is the most widespread cereal in the Mediterranean diet and, with rice and maize, has the highest worldwide production. Wheat consumed in developed countries is subjected to refining processes, which deplete the flour of many important nutrients such as dietary fibre, oligosaccharides, vitamins, minerals and of antioxidants such as polyphenols. Such compounds are concentrated in the outer part of kernel (bran and germ) that is separated from the starchy endosperm in the refining processes.
Whole grains in general are important foods from a nutritional and heath point of view because of their elevated content of carbohydrates, fibre and phytochemicals. Epidemiological and experimental studies have shown that consumption of whole cereals is associated with a reduced risk of cancer, cardiovascular diseases and diabetes (Anderson, Citation2003; Slavin, Citation2000; Slavin, Martini, Jacobs, & Marquart, Citation1999) thanks to the presence of several bioactive compounds, nutrients (vitamins and minerals) and non-nutrients (lignans, sterols and polyphenols). Different mechanisms have been indicated for the protectiveness of whole grains in relation to single components, but generally the protectiveness against a wide range of chronic disease is greater than that seen with individual ingredients (Pereira, Pins, Jacobs, Marquart, & Keenan, Citation2001). Among the possible mechanisms related to the protective role of whole grains, particular relevance has been assigned to the antioxidant activity of their phytochemical compounds. Whole wheat naturally contains a wide variety of polyphenols such as phytic acids, phenolic acids and flavonoids (Carrat[ugrave] & Sanzini, Citation2005). These bioactive compounds induce a marked antioxidant activity with values about double with respect to refined wheat products (Slavin, Citation2003).
Oxidative stress may result from the imbalance in the body between oxidants and antioxidants due to a decrease in natural cell antioxidant capacity or to an increase in the amount of reactive oxygen species (ROS) produced. Consumption of foods rich in antioxidants may lead to scavenge free radicals and ROS that could cause oxidative damage to biomolecules such as lipids, proteins and nucleic acids. An improvement of the redox state is associated with a positive modulation of many biological functions, among which, of particular relevance to the immune functions. There is a close relation between oxidative stress and immune system: generation of ROS is a key part of normal immune system function. ROS are important in both natural and acquired immunity. Macrophage and neutrophil phagocytosis stimulate various cellular processes including the ‘respiratory burst’ whereby increased cellular oxygen uptake results in the production of the potent oxidant bactericidal agents, hypochlorous acid and hydroxyl radical. Conversely, oxidative stress may be detrimental in acquired immunity by activation of nuclear factor kappa B, which governs gene expression involving various cytokines, chemokines and cell adhesion molecules, among others. Antioxidant supplementation essentially reverses several age-associated immune deficiencies, resulting in increased levels of interleukin-2, elevated numbers of total lymphocytes and T-cell subsets, enhanced mitogen responsiveness, increased killer cell activity, augmented antibody response to antigen stimulation, decreased lipid peroxidation and decreased prostaglandin synthesis (Knight, Citation2000).
Thus, it is important to maintain a balance between ROS generation and antioxidant defences within immune cells to ensure a correct functioning of the immune system and help to prevent the onset of chronic diseases (Alvarez, Alvarado, Mathieu, Jimenez, & De la Fuente, Citation2006; Alvarez, Alvarado, Puerto et al., Citation2006). Indeed few in vivo experiments have been conducted with whole grains and whole wheat in particular, so little is known about the precise relationships between the in vitro contents and in vivo responses. It is known that the level of antioxidant activity in blood increases after the assumption of individual antioxidants (Rondini et al., Citation2004), but the physiologic effects of diets high in whole wheat have not been investigated. The aim of this work was to study the ‘in vivo’ activity of diets rich in whole wheat compared with diets rich in refined wheat in the modulation of redox status and immune responses in experimental rats.
Materials and methods
Animals and diets
Two groups of six growing male Sprague Dowley rats, weighing about 100 grams have been fed for six weeks with experimental diets.
The care and use of rats were approved by the Animal Care and Ethics Committee of University of Tuscia. The animals were housed in single boxes at a constant temperature (22±2°C) in reversed light/dark cycle. Food intake and body weights were determined twice every week. The two experimental diets were prepared freshly every week using the various ingredients according to the composition reported in .
Table 1. Composition of experimental diets (g/100g).
Analysis of flours
The flours from whole and refined durum wheat (obtained from the University of Molise) were analysed for basic chemical composition (humidity, ash, proteins, fats, according to the official methods AOAC soluble and insoluble fibres according to the method of Prosky) (Prosky, Asp, Schweizer, Devries, & Furda, Citation1988) and for total phenolic content. For total phenolic determination 1 g of flour, was extracted with 80% aqueous methanol (1/10 g/ml), shaking for 2 hours at room temperature, and centrifuging at 12,000 g at 4°C. A 0.25 ml aliquot of the methanol extract was mixed with 0.25 ml of Folin-Ciocalteau reagent (previously diluted with water, 1:1 v/v), 0.5 ml of saturated sodium carbonate solution and 4 ml of water. The mixture was allowed to stand at room temperature for 25 min, and then it was centrifuged at 2100 g for 10 min. Supernatant absorbance was measured at 765 nm in spectrophotometer and using a ferulic acid as standard. The results have been expressed as mg of ferulic acid/g (Shahidi & Naczk, Citation1995).
Preparation of plasma
Blood was drawn into syringes from heart of rat, placed in plastic tube containing heparin and centrifuged at 1500 g for 15 min at 4°C. After centrifugation plasma was collected in aliquots, immediately frozen for analyses of total polyphenols, antioxidant activity and protein carbonyl.
Analysis of plasma total polyphenols
The amount of total phenolic compounds in plasma was determined by the Folin-Ciocalteau method. To 500 µl of plasma were added 70 µl of HCl 1 N (pH 1.6) and 300 mg of NaCl. The sample was mixed and extracted four times with 1 ml ethyl ether for 4 min by vortex and centrifuged for 2 min at 15,000 g. After each extraction, the extracts were collected in glass tube on anhydrous sodium sulfate and dried under nitrogen flow. The residue was suspended in 100 µl of 80% methanol. The total phenolic concentration was determined measuring absorbance at 765 nm, using as standard ferulic acid. The results have been expressed as ferulic acid (mg/ml) of plasma.
Determination of plasma total antioxidant activity by ferric reducing antioxidant power (FRAP)
FRAP assay was carried-out by measuring the ability of plasma samples to reduce the colourless ferric-2,4,6-tripyridyl-s-triazine complex (TPTZ-Fe3 + ) to its ferrous coloured form (TPTZ-Fe2 + ) strain (Benzie & Strain, Citation1999). About 800 µl of FRAP reagent, prepared daily (acetate buffer 0.3 M pH 3.6, 10 mM TPTZ in 40 mM HCl and 20 mM FeCl3 6H2O) was mixed with 50 µl of plasma diluted 1:2 with acetate buffer. The reagents mixed and the absorbance at 593 nm was recorded after 30 min incubation at 37°C. FRAP values were obtained using a standard curve with various amounts of 2 mM FeSO4. The results are expressed as µmoles Fe+ + /l.
Analysis of plasma carbonyl content
Two aliquots of 1 ml of plasma were taken: one was marked as ‘test’ and the other as ‘control’. About 4 ml of 10 mM 2,4-dinitrophenylhydrazine (DNPH) prepared in 2 mM HCl was added to the test sample and 4 ml of 2 M HCl alone was added to the control sample. The tubes were left in the dark for 1 h at room temperature and were mixed by vortex every 15 min. About 5 ml of 20% trichloroacetic acid solution was then added to both tubes for a 10 min incubation on ice, after which the tubes were centrifuged at 3000 g, for 5 min at 4°C. The supernatant fluid was discarded and another wash was performed by using 4 ml of 10% trichloroacetic acid. Finally the precipitates were washed three times with 4 ml of ethanol:ethyl acetate (1:1) to remove unreacted DNPH and lipid remnants. The final protein precipitate was dissolved in 2 ml of 6 M guanidine hydrochloride and incubated at 37°C for 10 min. Each test sample was read against the control sample. The carbonyl content was calculated from the peak absorption at 370 nm using an absorption coefficient of 22,000 M–1 cm–1. The protein carbonyl content was expressed as nmole/mg protein. Protein content was determined on the HCl blank pellets using BSA standard curve in guanidine hydrochloride and reading the absorbance at 280 nm (Reznich & Packer, Citation1994).
Preparation of lymphocytes from mesenteric lymph nodes
Lymphocytes were isolated from mesenteric lymph nodes (MLN), suspended in 10 ml of phosphate buffer saline (PBS), layered onto 5 ml of Histopaque-1083 (Sigma) and centrifuged at 720 g for 40 min. The lymphocytes were removed from the PBS–Histopaque interface and washed in PBS centrifuging for 5 min at 720 g. The lymphocytes were counted in Neubauer cell counter and diluted to optimal concentration in RPMI 1640 culture medium containing 5% antibiotics (penicillin and streptomycin), and 5% l-glutamine.
Analysis of lymphocytes proliferation
Lymphocytes proliferation was assayed by measuring [H3]-thymidine incorporation, after stimulation with the T-cell mitogen concanavalin A (ConA). Lymphocytes were seeded at 3×105 cells/well in 96-well plates in complete RPMI 1640 culture medium with and without ConA (concentration range: 0–5 µg/ml) and incubated at 37°C in 5% CO2 for 24 and 48 hours. [H3]-thymidine (0.1 µCi) was added to each sample 18 h before harvesting. After incubation with ConA, the proliferative response was determined by radiochemical counting of each culture well. The cells were harvested on glass filter discs with an automated cell harvester. The filter discs were dried, transferred to scintillation vials and counted in a liquid scintillation counter. All assays were performed in triplicate. Lymphocytes proliferation is expressed as stimulation index (cpm of stimulated/cpm of unstimulated cells).
T-cell subpopulation analysis
The CD4+ and CD8+ T-cells were assayed by cytometry analysis. For this analysis 1×106 mesenteric lymphocytes were fixed with 200 µl of 4% paraformaldheyde for 20 min at 4°C. After washing with 1 ml of PBS, T-cells were stained with 50 µl of monoclonal fluorescein-labelled mouse anti-CD4 at concentration of 1 µg/ml, and incubated for 40 min at 4°C. After incubation and washing to remove non-binding antibody, lymphocytes were stained with 50 µl of monoclonal phycoerythrin labelled anti-CD8 at concentration of 1 µg/ml, and incubated for a further 40 min. In the end, after another washing with 1 ml of PBS, samples were analysed with flow cytometer (Becton-Dickinson, San Josè, CA). Each analysis was based on at least 104 events and the results were expressed as CD4/CD8 ratios.
Statistical analysis
Statistical analysis was performed using one factor analysis of variance (ANOVA), and two-tailed t-test.
Results
Chemical composition of flours
The flours have been characterised for their content in humidity, ash, proteins, fats, total, insoluble and soluble fibre ().
Table 2. Chemical composition of flour (percentage (%) of dry matter).
The chemical composition analysis showed elevated differences in fibre content between the refined and whole flour, especially due to the soluble fibre fraction. Furthermore, whole wheat is richer in total fenolic compounds than the refined wheat ().
Table 3. Total phenolic content in flours (ferulic acid (mg/g)).
Growth rate of rats
No significant differences in feed consumption and body weight among the experimental groups were observed during the period of the study (data not shown).
Plasma polyphenols
Results showed that diets rich in whole wheat determined a plasma polyphenol concentration higher than diets rich in refined wheat ().
Table 4. Redox status of plasma of rats fed with experimental diets.
Plasma total antioxidant activity
Plasma analysis with the method ‘ferric reducing antioxidant power’ showed that whole wheat induces a higher plasma antioxidant activity than refined wheat ().
The data indicated that at more elevated value of plasma total polyphenols of rats fed with diets rich in whole wheat corresponded an increase of in plasma antioxidant activity.
Plasma carbonyl content
The data showed that diet rich in whole wheat determined a reduction of carbonyl content in plasma of rats with respect to the diet rich in refined wheat ().
Immunological parameters
Lymphocytes proliferation
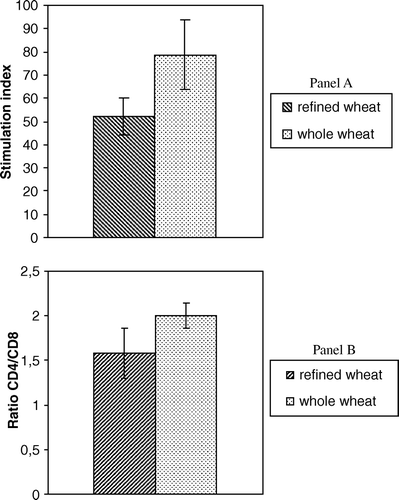
The proliferative responses of lymphocyte isolated from mesenteric lymph nodes of rats fed with diets containing 53% of refined and 53% of whole durum wheat flours, alternatively, are reported in (panel A). The results showed that whole wheat induced a significant increase of stimulation index of lymphocytes of rats fed whole wheat in comparison with those fed with refined wheat.
T-cell subpopulation
The results of the proportion of CD4+ and CD8+ T-cell in MLN lymphocytes, measured by cytometry analysis, are reported in (panel B). Results showed that the ratio of CD4+/CD8+ cells was higher in rats fed with the diet rich in whole wheat than in rats fed with diet rich in refined wheat.
Discussion
In the present work we have analysed the biological effects in vivo of a diet rich in whole wheat in comparison to refined wheat. Generally, grains consumed in developed countries are subjected to some type of processing as milling, where the bran and the germ are separated from the starchy endosperm and the latter is ground into flour. Many phytonutrients are present in higher concentration in the outer part of the grain, so refining process has a significant effect on their content in flour (Slavin et al., 1999). Bran is a very important source of fibre and polyphenols: which have important functional properties and exert health benefits in humans through various mechanisms, including antioxidant activity. The primary protective function of antioxidants in the body is their reaction with free radicals. Free radicals attack DNA, lipids and proteins and are the initiating factor for several chronic diseases, also contributing to general inflammatory response and tissue damage. Polyphenols are receiving increasing interest from consumers and food manufacturers because, together with other dietary antioxidants such as vitamin C, vitamin E and carotenoids, they are believed to protect the body's tissue against oxidative stress (Charalampopoulos, Wang, Pandiella, & Webb, Citation2002; Zdunczyk et al., Citation2006). Various studies have demonstrated the relation between the ‘in vitro’ antioxidant activity and the different composition in phenolic compounds and in other phytochemicals of vegetal products and cereals (Velioglu, Mazza, Gao, & Oomah, Citation1998). Furthermore, ‘in vivo’ experiments have showed that these compounds, as supplements to the diet, may be absorbed, transported and metabolised in the organism with biological effects on the antioxidative defence, but few works have studied the exact relation between ‘in vitro’ antioxidant content and effective absorption after intake of complete food such as grain flour. In this work we have determined the effects of diets nutritionally balanced, in which the wheat flour is the major constituent (53%). After a long experimental period of feeding (6 weeks), it can be observed that the major content of polyphenols in whole wheat flour respect to refined wheat flour is reflected on plasma polyphenols content. This result indicates that the phenolic compounds present in whole wheat are available for absorption, probably binding phenolics associated with cell wall materials, surviving the upper gastrointestinal digestion condition and reaching the colon where they are digested by intestinal microflora, where they release the bulk of bound phytochemicals (Adom & Lui, Citation2002; Kern, Bennett, Mellon, Kroon, & Garcia-Conesa, Citation2003). The health beneficial phytochemicals of wheat are not evenly distributed because these compounds are concentrated in outermost layer of kernel (the bran) mainly bound with cellulose and at other insoluble components. In particular phenolic acids, the major group of phenolic compounds present in cereals, may form both ester and ether linkages owing to their bifunctional nature through reactions involving their carboxylic and hydroxyl groups, respectively. This allows phenolic acids to form cross links with cell wall macromolecules. Various experimental works have demonstrated that the contribution of bound phenolics to the total phenolic content is significantly higher than that of free and esterified fraction (Liyana-Pathirana & Shahidi, Citation2006; Perez-Jimez & Saura, Citation2005). Other experiments have focussed attention on the antioxidant activity of milling fractions and their have shown that bran fraction exhibits the highest antioxidant activity (Adom, Sorrells, & Liu, Citation2005; Martinez-Tomè et al., Citation2004; Zielisńki & Kozłowska, Citation2000).
The improvement of plasma redox status of rats fed with diets rich of whole cereals may help to protect against oxidative stress, with resulting health promotion and disease prevention. Moreover, an adequate intake of these compounds in food can enhance certain aspects of immune response related to health. Several studies have in effect demonstrated that cereal-based dietary supplementation positively affects the immune function and cellular redox states (Alvarez, Alvarado, Mathieu et al., 2006; Alvarez, Alvarado, Puerto et al., 2006). Interactions between the immune system and nutrients are very complex and the mechanisms involved are not well known. In this work we have found that whole wheat positively affects immune response in rats: lymphocytes of rats fed with a diet rich in whole wheat have a stimulation index value higher than lymphocytes of rats fed with a diets rich in refined wheat. The same trend is obtained with the analysis of T-cell subpopulation: the CD4+/CD8+ ratio is higher in the rats fed with the whole cereals than those fed with the refined wheat. Increases in the proliferation index and in the proportion of CD4+ T-cells, are parameters that denote an immunostimulating effect. The increases of the lymphocytes proliferation and CD4+ T-cells ratio in response to an external stimulus indicate an improvement of the immune defense against exogenous and endogenous detrimental factors (Albers et al., Citation2005). It is well-known from the literature that, in addition to antioxidants, dietary fibre may influence the immune function: diets supplemented with fermentable dietary fibre as fructo-oligosacharides or β-glucan or other components with a prebiotic function, should have a immune-enhancing effects, like the increase of immunoglobulin A secretion and of CD4+/CD8+ ratio (Dongowski, Huth, Gebhardt, & Flamme, Citation2002; Lim et al., Citation1997; Merendino et al., Citation2006; Shley & Field, Citation2002). Furthermore, a supplementation of diets with different cereal fractions improves many parameters of leukocyte function such as chemotaxis capacity, microbicidal activity, lymphoproliferative response to mitogens and interleukin-2 (IL-2) (Alvarado, Alvarez, Jimenez, & De La Fuente, Citation2006; Alvarez, Alvarado, Mathieu et al., 2006).
The present work indicates that diet rich in whole wheat containing a complex natural mixture of substances of different size, polarity, solubility and bioavailability can act synergistically, affecting functions of great biological relevance. In conclusion, our results suggest that a good and constant intake of whole wheat may have important implications for health preservation, by acting as modulator of immune function and redox status.
Acknowledgements
We thank Professor Gianfranco Panfili of the University of Molise for providing the flours and for their basic chemical analysis.
References
- Adom , K.K. and Lui , R.H. 2002 . Antioxidant activity of grains . Journal of Agricultural and Food Chemistry , 50 ( 21 ) : 6182 – 6187 .
- Adom , K.K. , Sorrells , M.E. and Liu , R.H. 2005 . Phytochemicals and antioxidant activity of milled fractions of different wheat varieties . Journal of Agricultural and Food Chemistry , 53 ( 6 ) : 2297 – 2306 .
- Albers , R. , Antoine , J.M. , Bourdet-Sicard , R. , Calder , P.C. , Gleeson , M. Lesourd , B. 2005 . Markers to measure immunomodulation in human nutrition intervention studies . British Journal of Nutrition , 94 ( 3 ) : 452 – 481 .
- Alvarado , C. , Alvarez , P. , Jimenez , L. and De la Fuente , M. 2006 . Oxidative stress in leukocytes from young prematurely aging mice is reversed by supplementation with biscuits rich in antioxidant . Developmental and Comparative Immunology , 30 ( 12 ) : 1168 – 1180 .
- Alvarez , P. , Alvarado , C. , Mathieu , F. , Jimenez , L. and De la Fuente , M. 2006 . Diet supplementation for 5 weeks with polyphenol-rich cereals improves several functions and redox state of mouse leucocytes . European Journal of Nutrition , 45 ( 8 ) : 428 – 438 .
- Alvarez , P. , Alvarado , C. , Puerto , M. , Schlumberger , A. , Jimenez , L. and De la Fuente , M. 2006 . Improvement of leucocyte functions in prematurely ageing mice after five weeks of diets supplementation with polyphenol-rich cereals . Nutrition , 22 ( 9 ) : 913 – 921 .
- Anderson , J.W. 2003 . Whole grains protect against atherosclerotic cardiovascular disease . Proceedings of the Nutrition Society , 62 ( 1 ) : 135 – 142 .
- Benzie , I.F.F. and Strain , J.J. 1999 . Ferric reducing antioxidant power assay: Direct measure of total antioxidant activity of biological fluid and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration . Methods in Enzymology , 299 : 15 – 25 .
- Carrat[ugrave] , B. and Sanzini , E. 2005 . Sostanze biologicamente attive presenti negli alimenti di origine vegetale . Annali Istituto Superiori di Sanità , 410 : 7 – 16 .
- Charalampopoulos , D. , Wang , R. , Pandiella , S.S. and Webb , C. 2002 . Application of cereals and cereal components in functional foods: A review . International Journal of Food Microbiology , 79 ( 1–2 ) : 131 – 141 .
- Dongowski , G. , Huth , M. , Gebhardt , E. and Flamme , W. 2002 . Dietary fiber-rich barley products beneficially affect the intestinal tract of rats . Journal of Nutrition , 132 ( 12 ) : 3704 – 3714 .
- Kern , S.M. , Bennett , R.N. , Mellon , F.A. , Kroon , P.A. and Garcia-Conesa , M.T. 2003 . Absorption of hydroxycinnamates in humans after high-bran cereal consumption . Journal of Agricultural and Food Chemistry , 51 ( 20 ) : 6050 – 6055 .
- Knight , J.A. 2000 . Review: Free radicals, antioxidants, and the immune system . Annals of Clinical and Laboratory Science , 30 ( 2 ) : 145 – 157 .
- Lim , B.O. , Yamada , K. , Nonaka , M. , Kuramoto , Y. , Hung , P. and Sugano , M. 1997 . Dietary fiber modulate indices of intestinal immune function in rats . Journal of Nutrition , 127 ( 5 ) : 663 – 667 .
- Liyana-Pathirana , C.M. and Shahidi , F. 2006 . Importance of insoluble-bound phenolics to antioxidant properties of wheat . Journal of Agricultural and Food Chemistry , 54 ( 4 ) : 1256 – 1264 .
- Martinez-Tomè , M. , Murcia , A.M. , Frega , N. , Ruggirei , S. , Jmenez , A.M. Roses , F. 2004 . Evaluation of antioxidant capacity of cereal brans . Journal of Agricultural and Food Chemistry , 52 ( 15 ) : 4690 – 4699 .
- Merendino , N. , D'Aquino , M. , Molinari , R. , De Gara , L. , D'Egidio , M.G. Paradiso , A. 2006 . Chemical characterization and biological effects of immature durum wheat in rats . Journal of Cereal Science , 43 ( 2 ) : 129 – 136 .
- Pereira , M.A. , Pins , J.J. , Jacobs , D.R. , Marquart , L. and Keenan , J.M. 2001 . “ Whole grains, cereal fiber and chronic disease: Epidemiologic evidence ” . In CRC handbook of dietary fiber in human nutrition , Edited by: Spiller , G.A. 461 – 479 . Boca Raton, FL : CRC Press .
- Perez-Jimenez , J. and Saura , C.F. 2005 . Literature data may underestimate the actual antioxidant capacity of cereals . Journal of Agricultural and Food Chemistry , 53 ( 12 ) : 5036 – 5040 .
- Prosky , D.M. , Asp , N.G. , Schweizer , T. , Devries , J.W. and Furda , I. 1988 . Determination of insoluble, soluble and total dietary fiber in foods and food products: Interlaboratory study . Journal-Association of Official Analytical Chemists , 71 : 1017 – 1023 .
- Reznich , A.Z. and Packer , L. 1994 . Oxidative damage to proteins: Spectrophotometric method for carbonyl assay . Methods Enzymology , 233 : 257 – 263 .
- Rondini , L. , Peyrat-Maillard , M.N. , Marsset-Baglieri , A. , Fromentin , G. , Duran , P. Tomè , D. 2004 . Bound ferulic acid from bran is more bioavailable than the free compound in rat . Journal of Agricultural and Food Chemistry , 52 ( 13 ) : 4338 – 4343 .
- Shahidi , F. and Naczk , M. 1995 . “ Methods of analysis and quantification of phenolic compounds ” . In Food phenolic: Sources, chemistry, effects and applications , Edited by: Shahidi , F. and Naczk , M. 287 – 293 . Lancaster, PA : Technomic .
- Shley , P.D. and Field , C.J. 2002 . The immune-enhancing effects of dietary fibres and prebiotics . British Journal of Nutrition , 87 ( Suppl. 2 ) : S221 – S230 .
- Slavin , J.L. 2000 . Mechanisms for the impact of whole grain foods on cancer risk . Journal of the American College of Nutrition , 19 ( Suppl. 3 ) : 300S – 307S .
- Slavin , J.L. 2003 . Why whole grains are protective: Biological mechanisms . Proceedings of the Nutrition Society , 62 ( 1 ) : 129 – 134 .
- Slavin , J.L. , Martini , M.C. , Jacobs , D.R. Marquart , L. Jr . 1999 Plausible mechanisms for the protectiveness of whole grains . American Journal of Clinical Nutrition , 70 Suppl. 3 459S 463S .
- Velioglu , Y.S. , Mazza , G. , Gao , L. and Oomah , B.D. 1998 . Antioxidants activity and total phenolics in selected fruits, vegetables, and grain products . Journal of Agricultural and Food Chemistry , 46 : 4113 – 4117 .
- Zdunczyk , Z. , Flis , M. , Zielinski , H. , Wroblewska , M. , Antoszkiewicz , Z. and Juskiewicz , J. 2006 . In vitro antioxidant activities of barley, husked oat, naked oat, triticale, and buckwheat wastes and their influence on the growth and biomarkers of antioxidant status in rats . Journal of Agricultural and Food Chemistry , 54 ( 12 ) : 4168 – 4175 .
- Zieliński , H. and Kozłowska , H. 2000 . Antioxidant activity and total phenolics in selected cereal grains and their different morphological fraction . Journal of Agricultural and Food Chemistry , 48 ( 6 ) : 2008 – 2016 .