Abstract
A synthetic hapten doxycycline (DOX) with a spacer-arm (para-aminobenzoic acid (PABA)) was attached to bovine serum albumin (BSA) or ovalbumin (OVA) by the diazonium coupling reaction and mixed anhydride methods. Then, DOX–PABA–OVA conjugate was used as a coating antigen in enzyme-linked immunosorbent assay (ELISA), while DOX–PABA–BSA was used as an immunogen to produce polyclonal antibodies. A reliable and sensitive indirect competitive enzyme-linked immunosorbent assay was developed and applied to the quantitative determination of DOX residue in muscle and liver samples. After the optimisation of the main parameters, 50% inhibition was 8.74 µg/l and the limit of detection was 1.96 µg/l. A weak cross-reactivity (CR) was also observed with other structurally related compounds, such as oxytetracycline (10.71%), tetracycline (4.10%) and chlortetracycline (1.89%). The CRs with other antibiotics were all below 0.1%. With the ELISA method, the recoveries were demonstrated to be from 80.19 to 89.41% in liver samples and 83.98–94.75% in muscle samples. The mean of the coefficients of variation with the intra-assay test were 5.75 and 7.53% in liver and muscle samples, respectively. For the inter-assay test, the average of the coefficients of variation was 5.92% in liver and 7.21% in muscle samples. The ELISA method is accurate and reliable for the detection of DOX residue in edible foods of animal origins.
Introduction
Doxycycline (DOX) is an important member of tetracycline (TC) which is often used to treat infectious diseases in animals. They are used for the treatment of diseases and as well as feed additives to gain weight. When the withdrawal periods are not respected, the antibiotic residues may remain in animal tissues or by-products, such as eggs or milk. It was raised that the presence of residues in animal food productions may lead to the increase of health threat in humans.
Many government authorities have established some control programmes for antibiotic determination in foods. For example, in China (Chinese Agriculture Ministry, Citation2002) and the European Union (EMEA/MRL/803/01-FINAL, Citation2001), the maximum residue limits (MRLs) for DOX was set at 100 µg/kg in muscle, 300 µg/kg in liver and 600 µg/kg in kidney. To ensure confidence in the animal productions and to limit the improper uses, the control of veterinary drug residues in animal tissues has considerable importance.
Several analytical methods have been described for the determination of DOX at trace levels, including liquid chromatography–mass spectrometry (LC–MS; Aga et al., Citation2005; Croubels et al., Citation1998; Hamscher, Sczesny, Hoper, & Nau, Citation2002; Lindsey, Meyer, & Thurman, Citation2001), HPTLC (Kay, Blackwell, & Boxall, Citation2004), capillary electrophoresis (Gil, Van Schepdael, Roets, & Hoogmartens, Citation2000), thin-layer chromatography (Choma, Grenda, Malinowska, & Suprynowicz, Citation1999), continuous-flow chemiluminometric (Syropoulos & Calokerinos, Citation1991), flow injection (Karlicek & Solich, Citation1994), HPLC (Axisa, Naylor, Bell, & Thompsion, Citation2000; Santos et al., Citation1996), spectrofluorimetric method (Jiang & Zhang, Citation2004), and time-resolved spectroscopy (Nadine & Joesph, Citation2001). Although, these instrumental methods have the advantage of determining individual DOX analogues with high sensitivity, but they are time consuming and also require expensive equipments. A rapid and inexpensive assay is needed to detect DOX in routine screening.
Enzyme-linked immunosorbent assay (ELISA) is an immunological technique with very suitable sensitivity and simplicity that include enzyme to detect the presence of an antibody or an antigen in sample. This technique can be applied in a qualitative and semi-quantitative design. Qualitative design provides a simple result, such as a positive or a negative sample. On the other hand, quantitative design provides results that translate the optical density (OD) or fluorescent unit into a standard curve which is typically a serial dilution of the analyte (Asensio, Gonzalez, Garca, & Martin, Citation2008). Thus, ELISA is suitable for screening a large number of samples. Recently, one paper has been published for the determination of TC by ELISA and DOX was involved in the experiment for the cross-reactivity (CR) study (Pastor-Navarro, Morais, Maquieira, & Puchades, Citation2007). Here, we reported the synthesis of haptens carrier for DOX by the diazonium coupling reaction method and mixed anhydride method and the development of an indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) method to quantify the analyte using polyclonal antibodies.
Materials and methods
Chemicals and instruments
DOX, oxytetracycline (OTC), TC, chlortetracycline (CTC), minocycline (MNC), methacycline (MTC), demeclocycline (DMC), Para-aminobenzoic acid (PABA), tributylamine, isobutyl chloroformate, N,N-Dimethylformamide (DMF), Freund's incomplete adjuvant (FIA), Freund's complete adjuvant (FCA), ovalbumin (OVA) and bovine serum albumin (BSA) were purchased from Sigma Chemical (St. Louis, MO, USA). 3,3’,5,5’-tetramethylbenzidine (TMB), horseradish peroxidase (HRP) and peroxidase labelled goat anti-rabbit immunoglobulins (GAR–HRP) were obtained from sino-American Biotechnology Co (Beijing, China). All other chemicals were of analytical reagent grade. The instruments used were a magnetic stirrer (HJ-3, Shanghai, China), a high-speed centrifuge (CR 21G), a thermo advance ISE (MA, USA), a UV-vis scanning spectrophotometer (NS-6, Japan), an electron box (303AS, Shanghai, China), an ELISA plate reader (F039300, Austria). Polystyrene 96-well microtiter plates were purchased from Costar (Cambridge, MA) and ELISA plate washer from Nunc Maxisorp (Roskilde, Denmark). ELX800 Universal Microplate Reader (BIO-TEK Instrument INC, Winooski, USA) was used to measure the OD of the microplates.
Buffer and solutions
Phosphate-buffered saline (PBS) containing 8.0 g/l NaCl, 0.2 g/l KH2PO4, 2.9 g/l Na2HPO4·12H2O, 0.2 g/l KCl, pH 7.4. Coating buffer containing 1.5 g/l Na2CO3, 2.9 g/l NaHCO3, pH 9.6. Blocking solution was 1% OVA in PBS. Washing solution was prepared with PBS containing 0.05% Tween-20 (PBST). TMB solution contained 3.3 µl of 30% H2O2, and 400 µl of 0.6 TMB in DMSO. Stopping solution was 2 M H2SO4.
All buffers were filtered through 0.22 µm pore size filter before use.
Synthesis and verification of haptens to doxycyclines (DOXs)
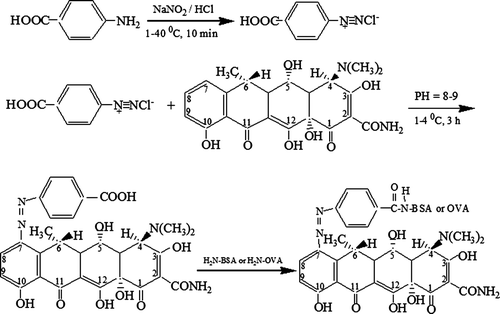
The synthetic routes for the two haptens used in this study are shown in .
4-aminobenzoic acid (27.4 mg) was dissolved in 1 mol/L HCl (4 ml) and cooled under an ice bath. Afterwards, sodium nitrite (69 mg) in water (0.5 ml) was added drop wise to maintain the temperature below 4°C and the resulting diazonium salt solution was stirred for 10 min. The reaction mixture was slowly added to a solution of DOX (88 mg) in three distilled water (3 ml). The pH of the mixture was set at 8–9 by adding solid sodium carbonate. The mixture was stirred for 3 h at 4°C, and then, 6.8 µl of tributylamine and 3.8 µl of isobutyl chloroformate were added. The mixture was centrifuged and the supernatant was collected. About 680 mg of BSA (or 440 mg of OVA) dissolved in 5 ml water, was slowly added into the supernatant, and the mixture obtained was reacted for overnight. The mixture was then centrifuged under 10,000 rpm for 20 min and the supernatant was endly collected and dialysed against 0.01 mol/l of PBS for three days. The PBS was replaced with fresh solution every 8 hours. The conjugates prepared by this method were designated as DOX–PABA–BSA and DOX–PABA–OVA, respectively. Then DOX–PABA–BSA (or DOX–PABA–OVA) was lyophilised and stored at −20°C. UV-vis spectral data were used to confirm the structures of the final conjugates. The hapten densities (the number of hapten molecules per molecule of protein) of the conjugates were estimated directly by the mole absorbance ε:Hapten density=(εconjugation −εprotein)/εhapten.
Immunisation schedule and antiserum preparation
Routinely, 500 µg of the conjugate (DOX–PABA–BSA) was dissolved in 500 µl of PBS and emulsified with FCA (1:1, v/v). It was then injected intradermally at multiple sites on the back of each female New Zealand white rabbit. Animals were boosted at 21-day intervals with the same immunogen (500 µg of the conjugate emulsified with FIA). Booster injections were given at 2-week intervals. On the eighth day after each booster, 5 ml blood samples were taken from an ear vein to check the titre of the antiserum using a non-competitive ELISA protocol. When the schedule was finished, blood were obtained from the rabbit's heart, and allowed to coagulate overnight at 4°C. Then, the antisera were separated by centrifugation, and sodium azide was added as a preservative at a final concentration of 0.02% (Min, Hye-Sung, Duck, & Yong, Citation2003). Antiserum was then aliquoted and stored at −70°C.
Sample pretreatment
Muscle samples and liver samples were obtained from pigs that had not been exposed to DOX. A homogenised sample of muscle, or liver was accurately weighed. For recovery studies, DOX standard solution (1µg/ml, prepared in 0.01M PBS) was added into homogenised tissue samples to produce spiked concentrations of 50, 100, 150, 200, 300 and 600 µg/kg. The sample pretreatment was accomplished by sample homogenisation, extraction and drying. Each pork or liver sample was homogenised in ethyl acetate (2 ml/g weight). The homogenates were vortexed for 3 min and then centrifuged at 2000 rpm for 10 min. The resultant supernatant (400 µl) was evaporated by dryness in 60°C water bath under a gentle flow of nitrogen. The residue was re-suspended in 1 ml of phosphate buffer (pH 7.4) and mixed thoroughly before analysis.
Indirect competitive enzyme-linked immunosorbent assay (ic-ELISA) procedure
The coating antigen (DOX–PABA–OVA) was diluted with coating buffer (0.05 M carbonate bicarbonate buffer, pH 9.6). Microtitre plates were incubated overnight at 4°C with 100 µl per well of the hapten-OVA conjugate. After the plates were washed with PBS-T (PBS with 0.05% Tween-20), the surface of the wells was blocked by adding a solution of OVA (1.0% in PBS, 100 µl per well) and incubating for one hour at 37°C. After the washing step, 50 µl per well of antiserum was diluted in PBS-T and 50 µl per well of serial dilutions of the analyte in 10% methanol-PBS-T were dispensed into the wells and incubated for 1 h at 37°C. Following another washing step (three times), 100 µl per well of GAR/HRP (diluted 1:20,000 in PBS-T) was added to each well and incubated at 37°C for 45 min. The plates were washed three times, and then 100 µl of substrate solution was added in each well. After incubation at 37°C for 15 min, the colour development was stopped by adding 50 µl per well of H2SO4. Finally, the OD of each well was measured at 450 nm. The titre dilution of the antiserum was considered suitable in case that the OD450 nm value was around 1.0. Competition curves were obtained by plotting absorbance against the logarithm of the analyte concentrations. The concentration of analyte giving 50% inhibition (IC50) value was expressed as sensitivity of ELISA.
Screening of sera and coating conjugate
Optimum concentrations of sera and coating conjugates were chosen to produce absorbance values ranging 0.8–1.2 units in the absence of analytes (Pastor-Navarro et al., 2007). The titre of the antiserum was determined by checkerboard titration assay using a non-competitive indirect ELISA. The binding of each antiserum at serial dilutions (1/1000–1/128,000) to microtitre plates coated with different concentrations of hapten conjugate (0.25–16.0 µg/ml) was measured. The optimum serum and coating dilutions were predetermined by the checkerboard titration results.
Enzyme-linked immunosorbent assay (ELISA) optimisation
The screening of the variables to set up competitive ELISA procedures was performed in conjugate and antibody-coated formats, following the protocol described by Tijssen (Li, Shi, & Wang, Citation2008).
The optimisation was performed for the most sensitive assay (DOX–PABA–OVA) using DOX as competitor analyte. The influence of several experimental parameters (concentration of coating, dilutions of antisera, coating time and reacting time) on assay characteristics was examined in order to improve the sensitivity of the immunoassay. Criteria used to evaluate the assay performances were the IC50 value, the absorbance of the wells without DOX (B0).
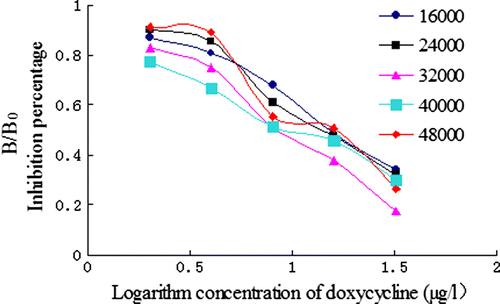
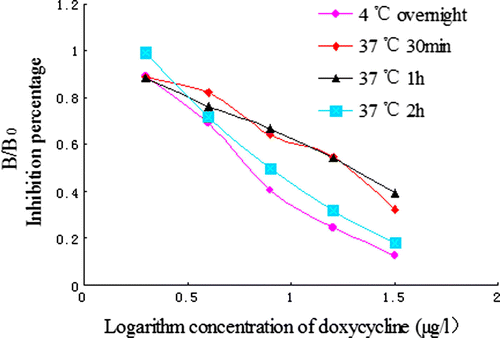
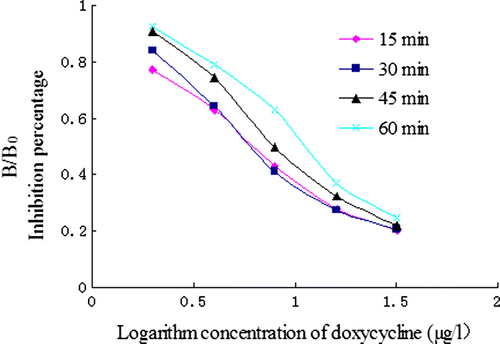
The effect of various concentration of coating antigen was evaluated using different concentrations of coating: 0.25, 0.5, 1, 2 and 4 µg/ml. To estimate the influence of the dilutions of the antisera, a series of dilution was made: 1:16,000, 24,000, 32,000, 40,000 and 48,000. The effect of coating time (4°C for overnight, 37°C for 30 min, 37°C for 1 h and 37°C for 2 h) was studied. Finally, the influence of antigen–antibody reacting time (15 min, 30 min, 45 min and 60 min) was investigated.
A set of standard concentrations of DOX (1.25, 2.5, 5, 10, 20, 40, 80 and 160 µg/l) were prepared in assay buffer and added (50 µl/well) to the appropriate wells of each microtitre plate. After the optimisation process, the best results were obtained by employing ELISA Optimisation. After incubation and development, the average OD of zero standard wells containing all components except the analyte was normalised to 100%. The IC50 was calculated as the concentration of analyte that caused a 50% reduction in binding of the enzyme-labelled analyte, which was repeated three times.
Evaluation of the optimised indirect competitive enzyme-linked immunosorbent assay (ic-ELISA)
Cross-reactivity (CR)
The specificity of the ic-ELISA procedures was determined against the standard solution of the DOX and other structurally related TC, including OTC, TC, CTC, MNC, MTC, DMC and several antibiotics. Each compound was prepared in PBS-T containing 5% methanol and tested in the concentration ranging 0–160 µg/l. The CR values were calculated as follows: CR (%)=(IC50 (DOX)/IC50 (analogue))×100. Here, CR (%) of DOX was defined as 100%.
Sensitivity and precision
According to the results of the section of ELISA optimisation, the optimised ELISA conditions were determined, and then under the best parameters, ic-ELISA in serial concentrations of DOX standard was repeated five times (n=5) in order to work out the mathematical simulation equation and the linear detection range. The limits of detection (LOD) were calculated by taking the mean value of 20 blank samples plus three times standard deviations of the mean (mean±3SD). The precision (intra-assay variability and inter-assay variability) and the sensitivity were also evaluated.
Accuracy (analysis of spiked samples)
The accuracy was evaluated by spiked sample experiments. To study the recovery, liver samples and muscle samples were spiked with different concentrations of DOX and analysed in a blind fashion by the ELISA protocol. For the recovery test, the final concentrations of DOX for each of the above samples were prepared. The recoveries of DOX were calculated on the basis of the DOX standard curve constructed by ic-ELISA.
Matrix effects
The DOX dissolved in PBS, the liver extraction and muscle extraction containing DOX were set as follows: 0, 2.5, 5, 10, 20, 40, 80 and 160 µg/l. The calibration curve was developed according to the ic-ELISA performance.
Results and discussion
Identification of artificial antigens
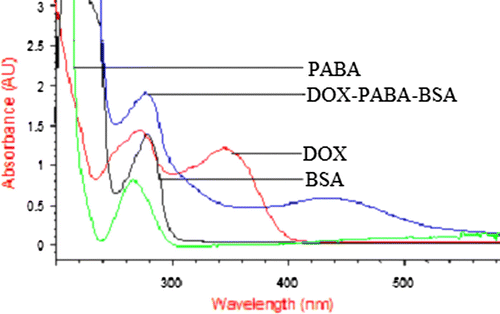
The protocol for the preparation of the two hapten carrier (DOX–PABA–BSA and DOX–PABA–OVA) is shown in . The UV-VIS spectra graphs of DOX–PABA–BSA are shown in . There were several obvious changes between the spectra of the conjugates and its carrier protein. The BSA and PABA showed the maximum absorbance wavelength at 280 nm and 266 nm, respectively. For DOX, the maximum absorbance wavelength ranged between 271 and 346 nm. The spectrum of DOX–PABA–BSA conjugate showed the maximum absorbance wavelength at 278 nm and 435 nm. This red shift could be the result of combination of the carrier protein and hapten. The same change can be seen in the DOX–PABA–OVA spectrum. These results indicated that the synthesis of the artificial antigens was successful. The molar ratio of hapten to protein for DOX–PABA–BSA and DOX–PABA–OVA was 10.5:1 and 6.3:1, respectively. The synthesis of haptens is the key step in a procedure of research on rapid immunoassay for antibiotics residue. Some haptens of TC have been reported (Pastor-Navarro et al., 2007). Here, we designed and synthesised a new combination of immunising/coating hapten with the spacer-arm to DOX. This method was different from those reported and its success was effectively proved by the following results.
Optimal concentration of coating antigen and antiserum titre
The antisera titre was determined by non-competitive ELISA and the value was 1:32,000 with optimal concentration of coating antigen set at 0.5 µg/ml. So optimal concentration of the coating antigen DOX–PABA–OVA (0.5 µg/ml) and antiserum titre (1:32,000) were selected for further development.
Enzyme-linked immunosorbent assay (ELISA) optimisation
In the ELISA method optimisation, two main parameters must be satisfied: B0 and IC50. The effect of the concentration of coating antigen and the dilutions of antibody on ic-ELISA performance were investigated. According to the data from and , a concentration of 0.5 µg/ml of coating antigen and a dilutions of antisera set at 1:32,000 were found to be optimal for antigen/antibody reaction with a higher B0 value (the absorbance of the sample without DOX) and lower IC50.
For the coating time optimisation, 4°C overnight gave a lower IC50 value than 37°C for 2 h, while B0 values were similar ( and ). Thus, the coating at 4°C for overnight was selected. Compared to the reaction time (15, 30, 45 or 60 min), an incubation time of 15 min showed a low IC50 value. The rapid equilibrium of antigen/antibody reaction implied that the polyclonal antibody developed here may have a high constant association and dissociation rate (Chen et al., Citation2008). Considering the rapidity of the manipulation, the incubation time of the antigen and the antibody were chosen at 15 min. With this saving time, the ELISA method developed here provided a rapid determination of DOX compared to the conventional ELISA.
Through studies of several factors, the main parameters of ELISA procedure were determined. The concentration of coating antigen (DOX–PABA–OVA) was 0.5 µg/ml, and the dilution of antibody was set at 1:32,000. The coating time was made at 4°C for overnight. The incubation time of antigen and antibody complex was set in 15 min.
Cross-reactivity (CR) studies
The CR not only determines the specificity of the antibody, but also the reliability of the assay. To determine the specificity of the optimised ic-ELISA, a set of several antibiotics were tested forCR. shows the results of CR tests. The interference observed was negligible. The highest interference was obtained for OTC which showed a CR of 10.71%. The high level of CR shown with OTC might be due to the similar structure of these two compounds except the only difference of the function group of hydrogen replaced by hydrogen hydroxyl. Thus, the developed immunoassay could be considered as a generic assay for the most commonly used DOX. A weak CR was also observed by structurally related compounds, such as MC (5.17%), TC (4.10%), CTC (1.89%) and DMC (0.92%). The low cross-reactivities of the antibody toward these compounds are understandable because their aromatic structures are very similar to DOX. However, for the other three antibiotics (MNC, DR and DXR), the cross-reactivity rates were below 0.1%. No CR was observed with any of the other compounds tested. Therefore, it can be concluded that the ic-ELISA developed for DOX is highly specific.
Table 1. Cross-reactivity (CR) data of the DOX assay for different tetracyclines and related compounds.
Sensitivity and precision
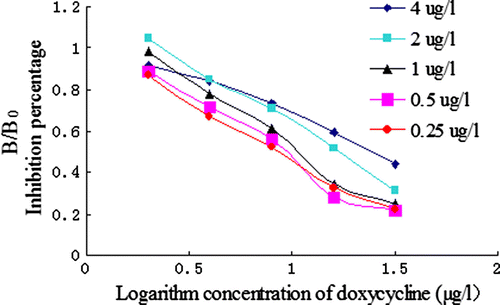
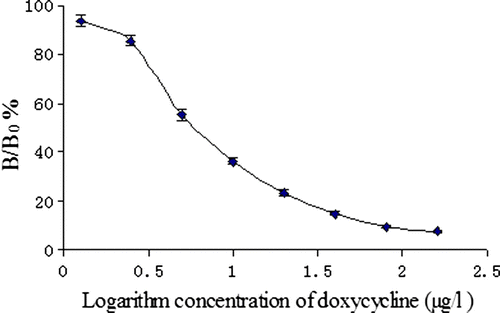
After the optimisation process, the best results were obtained by employing optimised ELISA. Based on these results, the standard curves of ic-ELISA for DOX were constructed as shown in . Under the optimised conditions mentioned above, the ic-ELISA procedures were conducted in quintuplicate with a set of standard concentration of DOX at different times (within 3 days). We used inhibition ratio (B/B0) as the longitudinal coordinates (y), logarithm of concentration of DOX (ng/ml) as the lateral coordinates (x). Positive correlation was obtained between B/B0 value and the standard concentrations of DOX ranged from 0 to 160 µg/l. After optimising the concentration of immunoreagents by checkerboard titration, the ic-ELISA for DOX gave an IC50 value of 8.74 µg/l using DOX–PABA–OVA as the coating antigen. In this optimised ic-ELISA, IC50 value was 8.74 µg/l and the limit of detection was 1.96 µg/l. The ELISA has a dynamic range between 1.96 and 160 µg/L. Compared with the TC polyclonal antibody as described by Pastor-Navarro et al. (2007), the DOX polyclonal antibody developed here has greater affinity and sensitivity.
The variability of intra-assay and inter-assay of the ELISA curve for DOX was used to show the precision of this protocol. The intra-assay variability was given by the average of five replicated wells in one microplate. The inter-assay variability was given by the average of three replicated microplates at different times. As shown in , the intra-assay average variation coefficients in the liver samples and the muscle samples were 5.75 and 7.53%, respectively. The inter-assay average variation coefficient was 5.92% with the liver samples and 7.21% for muscle sample. These results demonstrated that it was feasible to determine DOX using the ic-ELISA.
Table 2. Precision of DOX measurement from different samples by ic-ELISA (n=5).
Accuracy (analysis of spiked samples)
Liver and muscle samples spiked with different concentrations of DOX were simultaneously analysed by ic-ELISA as described above. The results are shown in . The recoveries of DOX in liver ranged from 80.19 to 89.41%. In muscle samples, the recoveries ranged from 83.98 to 94.75%.
Table 3. Recovery test of DOX added to different samples by ic-ELISA (n=5).
Matrix effects
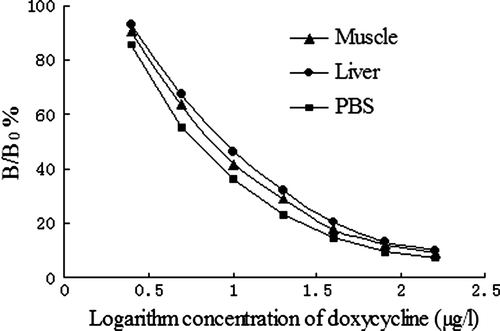
Previous studies have reported that various impurities in grain extracts have caused matrix effects in the antigen–antibody reaction (Pastor-Navarro et al., 2007). According to the calibration curves, with PBS, the IC50 was 8.74 µg/l. For the muscle samples and the liver samples from swine, the IC50 were 9.45 µg/l and 10.22 µg/l, respectively. These results indicated that the matrix has no significant effect on the specificity and sensitivity of the ic-ELISA (see ).
Conclusions
In this study, a rapid ELISA for DOX was developed. The polyclonal antibody against DOX was generated after immunising rabbit with DOX conjugated to BSA using a shorter aliphatic spacer-arm of PABA. The IC50 was 8.74 µg/l, and a weak CR was also observed to the other structurally related compounds. The detection limit was 1.96 µg/l. With the ELISA method developed in this study, a good recovery was obtained in spiked liver and muscle samples from swine. Selective haptens for DOX was synthesised and purified for the first time and further used to produce sensitive and generic polyclonal antibodies. The DOX haptens used in this study indicated that the selecting of carrier protein could be very significant. Persisting immunisations in rabbit using a pure immunogen (DOX–PABA–BSA) can produce more sensitive antibodies for DOX. Therefore, the optimised ELISA may become a new convenient, rapid and accurate analytical tool for monitoring DOX residues in animal food productions.
Acknowledgements
The author thanks the National Science & Technology Pillar Program (2006BAK02A09) of the People's Republic of China for the financial support, which enabled this work to be carried out.
References
- Aga , D.S. , O'Connor , S. , Ensley , S. , Payero , J.O. , Snow , D. and Tarkalson , D. 2005 . Determination of the persistence of tetracycline antibiotics and their degradates in manure-amended soil using enzyme-linked immunosorbent assay and liquid chromatography-mass spectrometry . Journal of Agricultural and Food Chemistry , 53 : 7165 – 7171 .
- Asensio , L. , Gonzalez , I. , Garca , T. and Martin , R. 2008 . Determination of food authenticity by enzyme-linked immunosorbent assay (ELISA) . Food Control , 19 : 1 – 8 .
- Axisa , B. , Naylor , A.R. , Bell , P.R.F. and Thompson , M.M. 2000 . Simple and reliable method of doxycycline determination in human plasma and biological tissues . Journal of Chromatography B , 744 : 359 – 365 .
- Chen , Y.Q. , Wang , Z.Q. , Wang , Z.H. , Tang , S.S. , Zhu , Y. and Xiao , X.L. 2008 . Rapid enzyme-linked immunosorbent assay and colloidal gold immunoassay for kanamycin and tobramycin in swine tissues . Journal of Agricultural and Food Chemistry , 56 : 2944 – 2952 .
- Chinese Agriculture Ministry . 2002 . Maximum residue limits (MRL) for veterinary chemicals in animal tissues . (Bulletin No, 235). Agriculture Ministry of P.R. China .
- Choma , I. , Grenda , D. , Malinowska , I. and Suprynowicz , Z. 1999 . Determination of flumequine and doxycycline in milk by a simple thin-layer chromatographic method . Journal of Chromatography B , 734 : 7 – 14 .
- Croubels , S. , Vermeersch , H. , De Backer , P. , Santos , M.D.F. , Remon , J.P. and Van Peteghem , C. 1998 . Liquid chromatographic separation of doxycycline and 4-epidoxycycline in a tissue depletion study of doxycycline in turkeys . Journal of Chromatography B , 708 : 145 – 152 .
- EMEA/MRL/803/01-FINAL . 2001 . http://www.emea.eu.int/pdfs/vet/mrls/080301en.pdf
- Gil , E.C. , Van Schepdael , A. , Roets , E. and Hoogmartens , J. 2000 . Analysis of doxycycline by capillary electrophoresis: Method development and validation . Journal of Chromatography A , 895 : 43 – 49 .
- Hamscher , G. , Sczesny , S. , Hoper , H. and Nau , H. 2002 . Determination of persistent tetracycline residues in soil fertilized with liquid manure by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry . Analytical Chemistry , 74 : 1509 – 1518 .
- Jiang , C.Q. and Zhang , N. 2004 . Enzyme-amplified lanthanide luminescence based on complexation reaction-a new technique for the determination of doxycycline . Journal of Pharmaceutical and Biomedical Analysis , 35 ( 5 ) : 1301 – 1306 .
- Karlicek , R. and Solich , P. 1994 . Flow injection spectrophotometric determination of tetracycline antibiotics . Analytica Chimica Acta , 285 : 9 – 12 .
- Kay , P. , Blackwell , P.A. and Boxall , A.B.A. 2004 . Fate of veterinary antibiotics in a macroporous tile drained clay soil . Environmental Toxicology and Chemistry , 23 ( 5 ) : 1136 – 1144 .
- Li , B. , Shi , H.Y. and Wang , M.H. 2008 . An enzyme-linked immunosorbent assay for the detection of bifenthrin . Chinese Journal of Analytical Chemistry , 36 ( 1 ) : 34 – 38 .
- Lindsey , M.E. , Meyer , M. and Thurman , E.M. 2001 . Analysis of trace levels of sulfonamide and tetracycline antimicrobials in groundwater and surface water using solid-phase extraction and liquid chromatography/mass spectrometry . Analytical Chemistry , 73 : 4640 – 4646 .
- Min , J.K. , Hye-Sung , L. , Duck , H.C. and Yong , T.L. 2003 . Synthesis of haptens of organophosphorus pesticides and development of enzyme-linked immunosorbent assays for parathion-methyl . Analytica Chimica Acta , 493 : 47 – 62 .
- Nadine , A. and Joseph , G. 2001 . Sensitive detection of tetracyclines using europium-sensitized fluorescence with EDTA as co-ligand and cetyltrimethylammonium chloride as surfactant . Analyst , 126 : 694 – 697 .
- Pastor-Navarro , N. , Morais , S. , Maquieira , A. and Puchades , R. 2007 . Synthesis of haptens and development of a sensitive immunoassay for tetracycline residues application to honey samples . Analytica Chimica Acta , 594 : 211 – 218 .
- Santos , M.D. , Vermeersch , H. , Remon , J.P. , Schelkens , M. , De Backer , P. Ducatelle , R. 1996 . Validation of a high-performance liquid chromatographic method for the determination of doxycycline in turkey plasma . Journal of Chromatography B , 682 : 301 – 308 .
- Syropoulos , A.B. and Calokerinos , A.C. 1991 . Continuous-flow chemiluminometric determination of some tetracyclines. Analytica Chimica Acta , 255 : 403 – 411 .