Abstract
An indirectly competitive enzyme-linked immunosorbent assay (ic-ELISA) was developed for the quantitative detection of fenvalerate. The hapten FVa (α-carboxy-3-phenoxyphenyl-2-(4-chloro-phenyl)-3-methyl-butyrate) and FVb (N-2-(carboxybutyl)carbamoyl-3-phenoxyphenyl-2-(4-chlorophenyl)-3-methylbutyrate) for the fenvalerate were prepared starting from 2-(4-chlorophenyl)-3-methyl-butyric acid and α-cyano-3-phenoxyhenzyl-benzyl alcohol. The hapten FVa was conjugated to ovalbumin (OVA) to form the coating antigen (FVa–OVA); the hapten FVb was conjugated to bovine serum albumin (BSA) to form the immunogen (FVb–BSA). The rabbits were immunised by conjugate of FVb–BSA, and titres of anti-fenvalerate serum (1.28×104) was determined by non-competitive indirect ELISA. After optimisation of the ic-ELISA, such as pH values, ionic strengths, concentrations of methanol, pH 7.5, phosphate-buffered saline of 0.3 M Na+ and 30% methanol were determined to optimum assay condition. The medium inhibitory concentration (IC50) for fenvalerate was (1.19±0.09) mg/L, and limit of detection (LOD) was (0.017±0.002) mg/L. Pyrethroids, such as fenpropathrin, cyhalothrin, permethrin, cypermethrin, deltamethrin and bifenthrin did not cross-react significantly in this assay.
Introduction
Fenvalerate is one of the most widely used pyrethroids in China. It is predominantly used to control various insects and mites that infest cotton, fruit plants, greenstuff and other crops (Wang, Citation2004). Since the 1990s, fenvalerate was one of the two pesticides which were most familiar detected from tea (Chen, Xia, Wang, & Xue, Citation1987).
So far, the fenvalerate was always detected through gas chromatography (GC) (Lin, Gong, & Xie, Citation2008) and HPLC (Shen et al., Citation2006). Multistep sample cleanup procedures are used in convnentional fenvalerate residue analysis. These methods are very complex, and not especially fit for fast detection. Immunoassay method is a simple, cost-effective, highly sensitive and selective tool for detecting trace amounts of pesticides. It developed very fast during these years (Lu & Wang, Citation2007). Here, we opted to synthesise the haptens and immunogen for fenvalerate. The development of the immunoassay resulting from the above haptens is described in this study.
Materials and methods
Reagents
The raw material for hapten synthesis and pyrethroid standards was obtained from Redsun Co., Ltd. (Nanjing, China). 4-aminobutyric acid (98%) was purchased from China National Medicines Co., Ltd. N-hydroxysuccinimide (NHS), anti-rabbit immunoglobulin, raised in goats and conjugated to horseradish peroxidase (GAR/HRP), O-phenylenediamine (OPD), bovine serum albumin (BSA) and ovalbumin (OVA) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). All the other chemicals were of analytical grade and purchased in China market.
Instrument
HT-5 beater (Ronghua apparatus); R-200 Rotavapor (Büchi, Swiss). NMR spectra were acquired in CDCl3 on an Avance DRX-500 spectrometer (Bruker, Germany) using solvent signal and tetramethylsilane (TMS) as internal standards. Mass spectra were recorded on an LC-MSQDECA (Finnigan, USA). UV-vis spectra were recorded on a DU-800 spectrophotometer (Beckman, USA). Enzyme-linked immunosorbent assays (ELISAs) were carried out using 96-well microtitre plates and the absorbance was read with the Vmax reader from Infinite M200 (Tecan, Switzerland).
Hapten synthesis
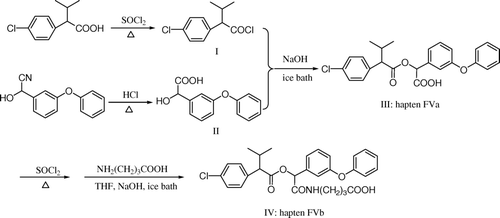
Synthesis of 2-(4-chlorophenyl)-3-methyl-butylacyl chloride (I)
2-(4-chlorophenyl)-3-methyl-butyric acid (4.25 g) was added in 15 ml redistilled SOCl2. The reaction mixture was stirred at 75°C for 2 h. The redundant SOCl2 was removed after reaction to get yellow oil (I) ().
Synthesis of α-hydroxyl-3-phenoxyhenzyl-benzyl acid (II)
α-cyano-3-phenoxyhenzyl-benzyl alcohol (4.5 g) was added in 20 ml concentrated chlorhydric acid. The reaction mixture was stirred at 80°C for 2 h. The mixture was extracted with ethyl ether, followed by concentration to dry. The giblets were dissolved in saturated sodium bicarbonate solution, which was extracted with ethyl ether later. The ethyl ether phase was thrown away, and the water phase was acidified to pH 2.0 with concentrated hydrochloric acid. The extraction was conducted triplicate to get white crystal (II).
Synthesis of α-carboxy-3-phenoxyphenyl-2-(4-chlorophenyl)-3-methylbutyrate (FVa)
The intermediate products I and II were added to 20 ml THF. The mixture was stirred in an ice bath for 2 h. Then the mixture was alkalified to pH 9 with 1 mol/L sodium hydrate solution, followed by stirring for 2 h. The resultant liquor was chromatographed on a silica gel column eluted with petroleum ether/ethyl acetate to afford the hapten FVa, which was yellow oil.
Synthesis of N-2-(carboxybutyl) carbamoyl-3-phenoxyphenyl-2-(4-chlorophenyl)-3-methylbutyrate (FVb)
FVa (10 mmol) and redistilled SOCl2 (10 ml) was added in redistilled toluene (15 ml). The mixture was stirred at 80°C for 4 h. Then the mixture was evaporated at reduced pressure to remove redundant SOCl2 and toluene to afford yellow oil, followed by dissolution in 25 ml THF and cooling in an ice bath. Aminobutyric acid (1.03 g) was added to 5 ml sodium hydrate solution (2 mol/L), followed by stirring in an ice bath for 10 minutes. Then 5 ml THF solution was added in the mixture for an interval of 10 minutes every time, and 2 ml sodium hydrate solution (2 mol/L) was added together in the last four times. The mixture was stirred for another 2 h in an ice bath. The resultant liquor was chromatographed on a silica gel column eluted with petroleum ether/ethyl acetate/acetic acid to afford the hapten FVb, which was light yellow oil.
Synthesis and identification of the coating antigen and immunogen
Hapten FVa was conjugated to OVA for the coating antigens (FVa–OVA) by the mixed anhydride method. Hapten FVb was conjugated to BSA for the immunogen (FVb–BSA) by the carbodiimide method (Li, Shi, & Wang, Citation2008). The coating antigen and immunogen were purified by dialysis in phosphate-buffered saline ((PBS), 0.01 mol/L, pH 7.4) for 72 h at 4°C, and then stored at −20°C. UV-vis spectral data were used to confirm the structures of the final conjugates. The hapten densities (the number of hapten molecules per molecule of protein) of the conjugates were estimated directly by the mole absorbance ε (Yu et al., Citation2006):
Preparation and purification of the antibody
Two New Zealand white rabbits were immunised by the conjugate FVb–BSA for raising polyclonal antibodies according to the conventional protocol (Zhu, Mao, Shi, & Cheng, Citation2005). Test ear-bleeds were taken 8 days after each boost to monitor the antisera titre using a non-competitive ELISA protocol. On the eighth day after five times boost, antisera were obtained from the rabbits’ hearts, from which the antibodies were separated by the method of salting out (with caprylic acid–ammonium sulfate) and stored at −20°C after being freeze dried (Wengatz, Stoutamire, Gee, & Hammock, Citation1998).
Enzyme-linked immunosorbent assay (ELISA)
Heterologous assays
Because the haptens for immunogen and coating antigen are the same, the titres detected by homologous assays are higher than it detected by heterologous assays (Mak et al., Citation2005). But it is reverse in analysis of chemicals. The heterologous assays were selected in this experiment. The best concentrations of antibody and coating antigen were choosed by the ic-ELISA when the OD490nm=1.0.
Assay optimisation
Several parameters can influence the binding of the antibody to hapten, including pH and ionic strength of the assay buffer during the competition. Organic solvents are often added to the assay buffer to improve the solubility of the analyte or to decrease non-specific binding. The effects of these were determined (Shan et al., Citation2000).
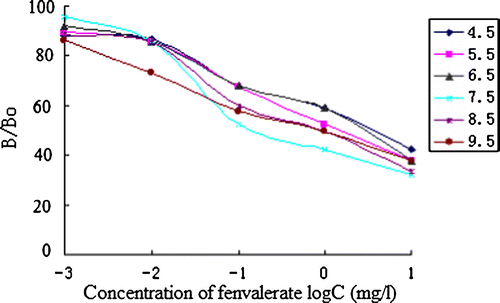
pH effect
To evaluate the effect of the pH of the medium on assay performance, the PBS buffers with pH values between 4.5 and 9.5 with an increment of 1.0 pH unit were prepared. Analyte was prepared as described above in 30% methanol in PBS. The standard inhibition curve was run in three well replicates at each pH.
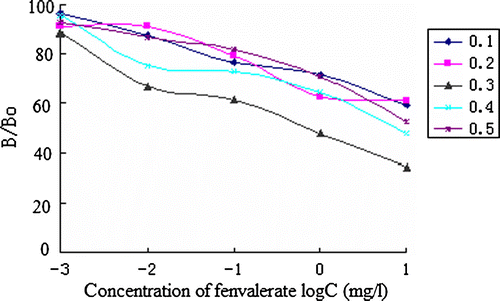
Ionic strength
The effect of PBS buffer with increasing content of NaCl (0.1 − 0.5 M) was evaluated. As above, the antibody was diluted in buffers of various ionic strengths, and the assays were conducted as above.
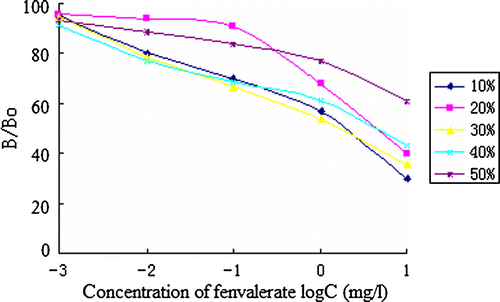
Solvent effect
Methanol was chose as the solvent basis on the previous investigation. Standard curves were measured in PBS containing 10%, 20%, 30%, 40% and 50% (v/v) methanol to determine the effects of solvent. The assays were conducted as above in triplicates.
Standard curve and cross-reactivities
Standard curve
Different concentrations of fenvalerate standard solution were prepared. With optimised assays, a standard curve for fenvalerate was obtained by ic-ELISA versus concentration of fenvalerate and inhibition rate. The IC50 and IC10 were determined in this assay (Watanabe, Shan, Stoutamire, Gee, & Hammock, Citation2001).
Cross-reactivities
The optimised assays were applied to cross-reactivity studies using the standard solution of the fenvalerate and other structurally related pyrethroids, including cyhalothrin, deltamethrin, permethrin, cypermethrin, bifenthrin and fenpropathrin. The specificity of the ELISA procedures was determined and calculated as follows: (Shan et al., Citation2004).
The recovery rate of fenvalerate in distilled water
The fenvalerate was added in distilled water as five concentrations (0.1, 0.5, 1, 5 mg/l). The samples were analysed by ic-ELISA to determine the recoveries and RSD. The experiments were conducted in triplicate.
Results and discussion
Verification of hapten
The final product (FVb) of the reaction was characterised by MS and 1H-NMR. MS: m/z 546 [M + Na]+. 1H-NMR (CDCl3): ▵ 7.0~7.41(m, 13H, Ar–H), 6.96 (s, 1H, −NH), 5.90 (s, 1H, −CH − O), 3.46~3.49 (m, 1H, CHCO), 3.16~3.23(m, 2H, CH2N), 2.25~2.28 (m, 1H, CH), 2.05~2.09 (m, 2H, CH2COO), 1.65~1.73 (m, 2H, CH2), 0.98 (s, 3H, CH3), 1.07 (s, 3H, CH3).
Identification of conjugates
In the UV-vis spectra obtained from continuous wavelength scanning, there are obvious differences between the spectra of the conjugate and that of the corresponding carrier protein, especially at around 280 nm. The conjugate of uptaking peak shape is the result of lapping between the pristine carrier protein and hapten that have been coupled. The molar ratio of haptens to protein (as assessed by this spectrophotometric method) were 10:1 and 35:1 for FVa–OVA and FVb–BSA, respectively.
Antiserum characterisation and titre
Non-competitive ELISA was used to characterise the reactivity of the antiserum to heterologous hapten conjugate (FVa–OVA). The result showed that rabbit antiserum displayed a high level. When the FVa–OVA is 10 µg/ml, titres of the antiserum and freeze-dried antibodies (FVb-Ab) were determined as 1.28×104 and 1×105 (OD490nm=1.0).
Assay optimisation
The best concentrations of antibody and coating antigen used in ic-ELISA procedure were determined by the two-dimensional titration method for high sensitivity. The result turned out to be 4 µg/ml and 16 µg/ml to FVa–OVA/FVb-Ab. The result of the assay optimisation is shown in Figures . The pH, ionic strength and methanol concentration were determined as 6.5, 0.3 mol/L and 30% for the IC50 is lower.
Standard curve and cross-reactivities
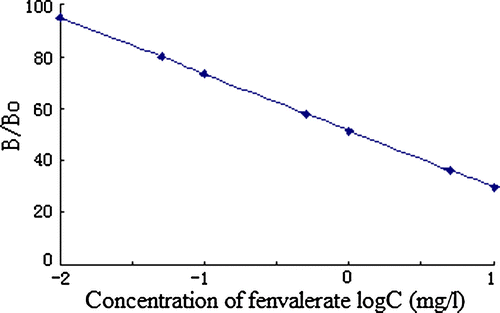
With optimised assays, a standard curve for fenvalerate was obtained by OD (490 nm) versus concentration of fenvalerate and plotting inhibition rate versus logC (): Y = 0.2174logC + 0.4834, R 2=0.9949 (working range was assigned to concentration 0.01~10 mg/L for fenvalerate). In the assay, the IC50 value was (1.19±0.09) mg/L, and the low detection limit (LDL) was (0.017±0.002) mg/L. The antibody was relatively selective for fenvalerate. Most of the compouds tested (), including a group of pyrethroids showed no significant cross-reactivity, except 2-(4-chlorophenyl)-3-methyl-butyric acid (CR = 35.21%).
Table 1. Cross-reactitives of the other pyrethroids.
Recovery rate
The fenvalerate was added in distilled water and the recoveries and RSD was determined by the ic-ELISA ().
Table 2. Spiked recovery of fenvalerate in distilled water.
Discussion
The design and synthesis of the hapten is the key point of the immunoassay (Lee, Mcadam, & Skerritt, Citation1998). The coating-hapten FVa and immuno-hapten FVb were prepared staring from 2-(4-chlorophenyl)-3-methyl-butyric acid and α-cyano-3-phenoxyhenzyl-benzyl alcohol after several chemical reactions. FVb is perfect to be a hapten for fenvalerate in structure. Hapten FVa was conjugated to OVA to form the coating antigen, and the hapten FVb was conjugated to BSA to form the immuno-antigen. The New Zealand rabbits were immunised to produce antibody. The ic-ELISA was set up under the optimised assay conditions and it indicated IC50 of (1.19±0.09) mg/L and the LDL of (0.017±0.002) mg/L. The assay had little cross-reactivity to other pyrethroids, which made it useful for the selective detection of fenvalerate.
Acknowledgements
This work was supported by the National “863” High-Tech Research Program of China (No. 2006AA108447).
References
- Chen , Z.M. , Xia , H.L. , Wang , Y.H. and Xue , Y.Z. 1987 . Present situation and countermeasures of pesticide residues in tea . China Tea , 6 : 6 – 8 .
- Lee , N.J. , Mcadam , D.P. and Skerritt , J.H. 1998 . Development of immunoassays for type II synthetic pyrethroids.1. Hapten design and application to heterologous and homologous assays . Journal of Agricultural and Food Chemistry , 46 : 520 – 534 .
- Li , B. , Shi , H.Y. and Wang , M.H. 2008 . An enzyme-linked immunosorbent assay for the detection of bifenthrin . Chinese Journal of Analytical Chemistry , 36 : 34 – 38 .
- Lin , Z.A. , Gong , Q.Y. and Xie , Z.H. 2008 . Determination of pyrethroid multi-residues in vegetables by high-performance liquid chromatography . Journal of Fuzhou University – Natural Science Edition , 36 : 122 – 125 .
- Lu , X.Q. and Wang , M.H. 2007 . Development of hapten synthesis and ELISA for the pyrethroid pesticides . Agrochemical , 10 : 653 – 655 .
- Mak , S.K. , Shan , G.M. , Lee , H.J. , Watanabe , T. , Stoutamire , D.W. Gee , S.J. 2005 . Development of a class selective immunoassay for the type II pyrethroid insecticides . Analytica Chimica Acta , 534 : 109 – 120 .
- Shan , G.M. , Huang , H.Z. , Stoutamire , D.W. , Gee , S.J. , Leng , G. and Hammock , B.D. 2004 . A sensitive class specific immunoassay for the detection of pyrethriod metabolites in human urine . Chemical Research in Toxicology , 17 : 218 – 225 .
- Shan , G.M. , Leeman , W.R. , Stoutamire , D.W. , Gee , S.J. , Chang , D.P. and Hammock , B.D. 2000 . Enzyme-linked immunosorbent assay for the pyrethroid permethrin . Journal of Agricultural and Food Chemistry , 48 : 4032 – 4040 .
- Shen , C.Y. , Shen , W.J. , Jiang , Y. , Xu , J.Z. , Zhao , Z.Y. Chen , H.L. 2006 . Determination of pyrethroid pesticide residues by gas chromatography-negative chemical ionization mass spectrometry and its application . Chinese Journal of Analytical Chemistry , 34 ( Suppl. 1 ) : 36 – 40 .
- Wang , M.H. 2004 . Pyrethroids chemistry , Beijing : China Agricultural Press .
- Watanabe , T. , Shan , G.M. , Stoutamire , D.W. , Gee , S.J. and Hammock , B.D. 2001 . Development of a class-specific immunoassay for the type I pyrethroid insecticides . Analytica Chimica Acta , 444 : 119 – 129 .
- Wengatz , I. , Stoutamire , D.W. , Gee , S.J. and Hammock , B.D. 1998 . Development of an enzyme-linked immunosorbent assay for detection of the pyrethroid insecticide fenpropathrin . Journal of Agricultural and Food Chemistry , 46 : 2211 – 2221 .
- Yu , R.Z. , He , M. , Shi , H.C. , Liu , J. , Guo , J.H. Wang , N. 2006 . Conjugation and immunogenic evaluation of complete immunogen for the small molecular environmental pollutants 2,4-D . Chinese Journal of Environmental Science , 27 : 146 – 150 .
- Zhu , G.N. , Mao , L.J. , Shi , H.Y. and Cheng , J.L. 2005 . Synthesis and identification of artificial antigen against quinclorac . Scientia Agricultura Sinica , 38 : 86 – 90 .