Abstract
Petasites japonicus (PJ, family Asteraceae) was fermented with lactic acid bacteria (LAB), Bifidobacterium breve (BB) or Lactobacillus acidophilus (LA), and its allergic effect was investigated. LAB-fermented PJs more potently inhibited IgE–antigen-stimulated degranulation of RBL-2H3 cells than non-fermented PJ, with BB-fermented PJ more potent than LA-fermented PJ. Fermented PJ more potently inhibited TNF-α and IL-4 cytokine expression and transcription factor NF-κB activation in IgE-induced RBL-2H3 cells. PJ and BB-fermented PJ also inhibited IgE–antigen-induced passive cutaneous anaphylaxis (PCA) reactions as well as compound 48/80-induced scratching behaviours in mice, with fermented PJ more potent than non-fermented PJ. These findings suggest that PJ may show anti-allergic effects by inhibiting degranulation, and TNF-α and IL-4 expression and its anti-allergic effects can be enhanced by LAB fermentation.
Introduction
Fermentation produces beneficial products for humans. These processes are mostly performed by lactic acid bacteria (LAB), such as Bifidobacterium sp. and Lactobacillus sp., and some moulds, such as Saccharomyces sp. (Nyman, Citation1995; Steinkraus, Citation1983). These microbes transform food components and can convert sugars to alcohol and lactic acid. For example, LAB fermentation of ginseng produces lactic acid and compound K, which is transformed from ginsenoside Rb1, Rb2 and Rc and exhibits potent cytotoxicity against tumour cells and anti-allergic effect (Bae, Kim, Han, Choo, & Kim, Citation2000; Choo, Park, Han, & Kim, Citation2003; Shin, Bae, Kim, Lee, & Kim, Citation2005).
Petasites japonicus (PJ, family Compositae) is frequently used in Korea, China and Japan as a vegetable, herbal medicine and Kimchi ingredient. Its leaves contain the flavonoid glycoside and petasiphenol, which inhibit DNA polymerase lambda activity (Matsubara, Mori, & Mizushina, Citation2004; Mizushina et al., Citation2003), petaslignolide A, which is neuroprotective against oxidative stress induced by kainic acid in the brains of mice (Cui, Kim, & Sok, Citation2005), and furofuran lignan, which exhibits anti-oxidant and anti-seizure activities (Min, Cui, Lee, Sok, & Kim, Citation2005). However, the inhibitory effects of PJ leaves with and without LAB fermentation against allergic reactions have not been studied.
In preliminary experiments, PJ leaves fermented with LAB, Bifidobacterium breve (BB) and Lactobacillus acidophilus (LA), inhibited antigen–IgE complex-induced degranulation of RBL-2H3 cells, with BB-fermented PJ more potent. Here, we investigated the inhibitory effects of BB-fermented PJ leaves on the passive cutaneous anaphylaxis (PCA) reaction and scratching in mice.
Materials and methods
Materials
Betamethasone, 12-O-tetradecanoylphorbol-13-acetate (TPA), lipopolysaccharide (LPS), compound 48/80, egg albumin, p-nitrophenyl-N-acetyl-β-d-glucosaminide, anti-dinitrophenol (DNP)-IgE, DNP-human serum albumin (HSA) and Evans blue were purchased from Sigma Co. (USA). The Griess reagent was purchased from Promega Co. (USA).
Extraction of Petasites japonicus (PJ) and Bifidobacterium breve (BB)-fermented Petasites japonicus (PJ)
The fresh leaves of PJ (0.2 kg), artificially cultured at Yang-Yang, Kangwondo, Korea, were dried in a room temperature, extracted with water 1 L for 2 h in boiling water bath, concentrated under a vacuum and freeze dried. The freeze-dried powder (5 g) was fermented with 1×1011 cells of LAB, such as Bifidobacterium breve KCCM1097 (BB) or Lactobacillus acidophilus KCTC3164 (LA), at 37°C for 48 h. The LAB-fermented PJ were then extracted with 0.5 L of ethyl acetate: yields fermented with and without BB or LA were 1.7%, 2.8% and 2.4%, respectively.
Assay of degranulation–inhibitory activity against RBL-2H3 cells
The inhibitory activity of test agents against the release of β-hexosaminidase from RBL-2H3 cells was evaluated according to Choo et al. (Citation2003). RBL-2H3 cells were grown in Dulbecco's modified Eagle's medium supplemented with 10% foetal bovine serum and L-glutamine. Cells were dispensed into 24-well plates at 5×105 cells per well in medium containing 0.5 µg/mL of mouse monoclonal IgE, the cells were sensitised by incubation overnight at 37°C in 5% CO2. They were then washed with 500 mL of Siraganian buffer (pH 7.2, 119 mM NaCl, 5 mM KCl, 0.4 mM MgCl2, 25 mM PIPES and 40 mM NaOH) and incubated in 160 µL Siraganian buffer containing 5.6 mM glucose, 1 mM CaCl2 and 0.1% BSA for an additional 10 min at 37°C. The cells were exposed to 40 µL of test agents for 20 min, and then treated with 20 µL of antigen (DNP-HSA, 1 µg/mL) for 10 min at 37°C to activate cells and to evoke allergic reactions. The reaction was stopped by cooling in an ice bath for 10 min. The reaction mixture was centrifuged at 2000×g for 10 min, and then 25 µL aliquots of the supernatant were transferred to 96-well plates and incubated with 25 µL of substrate (1 mM p-nitrophenyl-N-acetyl-β-d-glucosaminide) for 1 h at 37°C. The reaction was stopped by adding 200 µL of 0.1 M Na2CO3/NaHCO3. Absorbance was measured by using an enzyme-linked immunosorbent assay (ELISA) reader at 405 nm.
Immunoblot
The inhibitory effects of the test agents against the degranulation of RBL-2H3 cells were measured according to Choo et al. (Citation2003). Assay of IL-4 and TNF-α in RBL-2H3 cells stimulated by IgE–antigen complex was performed by ELISA. RBL-2H3 cells (5×105 cells), previously cultured in DMEM (Sigma Co., USA), were treated with 0.5 µg/mL of mouse monoclonal IgE to sensitise the cells. The cells (1.8 mL) were exposed to 0.2 mL of the test agents (dissolved in 0.5% dimethyl sulfoxide) for 4 h, followed by treatment with 0.2 mL DNP-HSA (1 µg/mL) for 40 min at 37°C. The supernatant (50 µL) was transferred to 96-well ELISA plates, and the IL-4 and TNF- α concentrations were then determined using commercial ELISA kits (Pierce Biotechnology, Inc., Rockford, IL, USA) (Matsuda, Morikawa, Ueda, Managi, & Yoshikawa, 2002).
Collected cells (3×106 cells) for immunoblot assay were lysed on ice for 15 min in a hypotonic buffer containing 10 mM Tris (pH 8.0), 1.5 mM MgCl2, 1 mM dithiothreitol (DTT), 0.1% NP-40, 5 µg/mL pepstatin A and 5 µg/mL aprotinin, and centrifuged at 12,000 g at 4°C for 15 min. The supernatant was used as the cytosolic fraction for the IκBα immunoblot assay. The pelleted nuclei fraction for the NF-κB immunblot assay was resuspended in extraction buffer containing 10 mM Tris (pH 8.0), 50 mM KCl, 300 mM NaCl, 1 mM DTT, 5 µg/mL pepstatin A and 5 µg/mL aprotinin, and then lysed on ice for 30 min. The lysed nuclei fraction was centrifuged at 12,000 g at 4°C for 30 min. Cell lysates (40 µg) were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions, and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Hertfordshire) at 30 V for 2 h. The membranes were blocked with 2% skimmed milk in PBS, containing 0.05% Tween 20, and incubated for 2 h at room temperature. The cytosolic IκBα and nucleic p65 NF-κB were assayed using their corresponding antibodies, according to a previously reported method (Matsuda et al., Citation2002; Shin et al., Citation2005). Immunodetection was carried out using an enhanced chemiluminescence detection kit.
Animals
Male and female ICR mice (20–22 g, 5 weeks old) and male BALB/c mice (18–22 g, 5 weeks old) were supplied from the Charles River Orient Experimental Animal Breeding Center (Seoul, Korea). All the animals were housed in wire cages at 20–22°C, a relative humidity of 50±10%, air ventilation frequency of 15–20 times/h and 12 h illumination (07:00–19:00; intensity, 150–300 Lux), fed standard laboratory chow (Charles River Orient Experimental Animal Breeding Center, Seoul, Korea), and allowed water ad libitum. All the procedures relating to the animals and their care conformed to international guidelines, ‘Principles of Laboratory Animal Care’ (NIH publication no. 85–23, revised 1985).
Passive cutaneous anaphylaxis (PCA) reaction
The IgE-dependent PCA reaction was measured according to the method of Choo et al. (Citation2003). Each group consisted of six male ICR mice that were injected intradermally with 10 µg of anti-DNP IgE at two dorsal skin sites that had been shaved 48 h earlier and outlined with a water-insoluble red marker. Forty-eight hours later, each mouse received a test compound (either orally or intraperitoneally), followed 1 h later by an injection of 200 µL of 3% Evans blue phosphate-buffered saline containing 200 µg of DNP-HSA via the tail vein. Thirty mins after the DNP-HSA injection, the mice were sacrificed and their dorsal skin removed to measure the pigment area. After extraction with 1 mL of 1.0 N KOH and 4 mL of a mixture of acetone and 0.6 N phosphoric acid (13:5), the amount of dye was determined colorimetrically (absorbance at 620 nm).
Scratching behaviour experiments
The behavioural experiments were all performed according to the method of Sugimoto, Umakoshi, Nojiri, and Kamei (Citation1998). Before the experiments, male BALB/c mice were put into acrylic cages (22×22×24 cm) for about 10 min for acclimation, and divided into groups of six. The rostral part of the skin on the back was clipped, and 50 µg/50 µL of compound 48/80 or 300 µg/50 µL of histamine dissolved in saline intradermally injected using a 29-gauge needle. The control mice received a saline injection instead of the scratching agent. Immediately after the intradermal injection, the mice (one animal/cage) were put back into the same cages and their scratching behaviour recorded using an 8-mm video camera (SV-K80, Samsung, Seoul, Korea) under automated conditions. The number of times the injected site was scratched using the hind paws was counted and compared with the scratching of other sites, such as the ears. Each mouse was only used for one experiment. The mice generally scratched several times during 1 s, thus a series of such behaviour over 60 min was counted as one incident of scratching.
Assay of nitric oxide (NO) production in lipopolysaccharide (LPS)-induced RAW 264.7 cells
RAW 264.7 cells were seeded at 5×104 cells per well in flat-bottomed 96-well plates. LPS (1 µg/mL) and test agents were added to the culture medium, and incubated at 37°C for 16 h, briefly centrifuged, and then 150 µL of cell culture supernatant was mixed with 150 µL of Griess reagent and incubated for 10 min at room temperature (light protected). The absorbance was measured using an ELISA reader at 540 nm and compared to a standard calibration curve prepared from sodium nitrite (Shin et al., Citation2005).
Statistics
All the data are expressed as the mean±standard deviation, and statistical significance was analysed using one way ANOVA followed by a Student–Newman–Keuls test.
Results
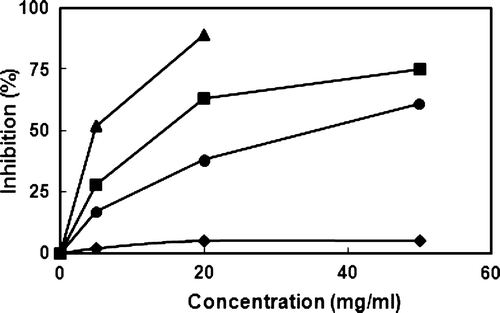
We first determined the inhibitory effect of PJ fermented with BB or LA against the degranulation of histamine-releasing cells, such as mast cells and basophiles. PJ and LAB-fermented PJs were extracted with ethyl acetate and tested against IgE–antigen complex-induced degranulation of RBL-2H3 cells (). LAB-fermented PJs were more potent than non-fermented PJ, and BB-fermented PJ was the most potent, with an IC50 value of 73 µg/mL. Importantly, these agents did not show cytotoxic effects against RBL-2H3 cells at a concentration to 200 µg/ml. We also measured inhibitory effect of PJ and BB-fermented PJ against nitric oxide (NO) production of LPS-stimulated macrophages, which produces NO in chronic allergic diseases (Leung, Citation1995) (). These agents potently inhibited NO production, with IC50 values of 37 µg/ml and 14 µg/ml, respectively. However, the ethyl acetate fraction of BB alone did not significantly inhibit NO production.
Figure 1. Inhibitory effects of PJ and BB-fermented PJ on IgE-antigen complex-induced degranulation of RBL-2H3 cells. RBL-2H3 cells, grown in DMEM supplemented with 10% foetal bovine serum and l-glutamine, were dispensed into 24-well plates at 5×105 cells per well and sensitised using 0.5 µg/mL of mouse monoclonal IgE. The cells were then washed with 500 µL of a Siraganian buffer, exposed to 40 µL of various concentrations of PJ (•), BB-fermented PJ (▪), LA-fermented PJ (▴), azelastine (□), BB alone (□) or LA (□) alone for 20 min, and treated with 20 µL of an antigen (DNP–HSA, 1 µg/mL) for 10 min at 37°C. The degranulation of RBL-2H3 cells was evaluated by measuring β-hexosaminidase activity released from the cells into the medium. Inhibition values indicate the mean±SD (n=3).
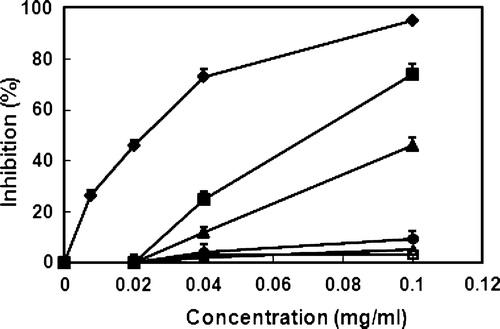
Figure 2. Effects of PJ and BB-fermented PJ on NO synthesis of LPS-stimulated RAW 264.7 cells. Nitric oxide was determined by measuring the amount of nitrite in cell culture supernatant using Griess reagent, according to the manufacturer's protocol. RAW 264.7 (mouse macrophage leukemia) cells were stimulated with lipopolysaccharide (LPS, 1 µg/ml) and the test agents (closed circle, PJ; closed square, fermented PJ; closed triangle, dexamethasone) for 24 h. The cells were briefly centrifuged and 150 µL of cell culture supernatant mixed with 150 µL of Griess reagent. Incubation was conducted at 10 min at room temperature (light protected), and absorbance was measured using an ELISA reader at 540 nm using a sodium nitrate determined calibration curve as a standard.
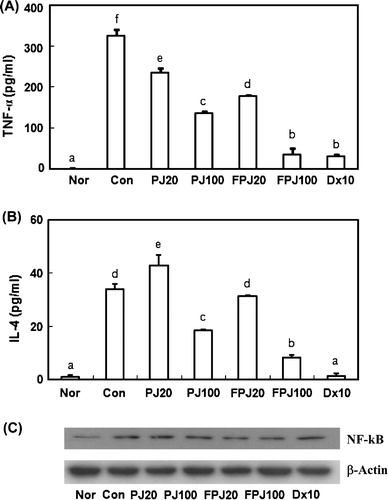
We then measured the ability of PJ and BB-fermented PJ to inhibit the expression of allergic cytokines, TNF-α and IL-4, in RBL-2H3 cells stimulated with IgE–antigen complex using ELISA analysis (). These agents, at 100 µg/ml, inhibited the expression of TNF-α by 55 and 89%, respectively, and IL-4 by 47 and 78%, respectively. However, BB alone did not significantly inhibit the expression of these cytokines (Data not shown). We also investigated the inhibitory effect of these agents on the transcription factor NF-κB. These agents attenuated NF-κB activation in RBL-2H3 cells induced by the IgE–antigen complex, but increased IκB inactivation. However, BB alone did not significantly inhibit NF-κB activation (data not shown). The inhibitory effect of BB-fermented PJ was more potent than that of non-fermented one.
Figure 3. Inhibitory effects of PJ and BB-fermented PJ on TNF-α (A) and IL-4 (B) expression and transcription factor NF-κB activation (C) in RBL-2H3 cells induced by an IgE–antigen complex. RBL-2H3 cells (5×105 cells) were treated with 0.5 µg/ml of mouse monoclonal IgE for 1 h, exposed to 0.2 mL of the test agent (Con, vehicle alone; PJ20, 20 µg/ml of PJ extract; PJ100, 100 µg/ml of PJ extract; FPJ20, 20 µg/ml of BB-fermented PJ; FPJ100, 100 µg/ml of BB-fermented PJ; DX10, 10 µM dexamethasone) for 20 min, and treated with 0.2 mL dinitrophenol–human serum albumin (DNP–HSA, 1 µg/ml) for 1 h (immunoblot) or 4 h (for ELISA) at 37°C, and then immunoblot for NF-κB and ELISA for TNF-α and IL-4 were performed. The normal group (Nor) was treated with the vehicle alone, and the control group (Con) was treated with vehicle and the IgE–antigen complex Values represent the mean±SD for triplicate experiments. a,b,c,d,e,fItems with the same letter were not significantly different (p>0.05).
Next, we measured the inhibitory effects of BB-fermented PJ on the IgE–antigen complex-induced PCA reaction in mice (). While PJ both with and without fermentation reduced the PCA reaction, BB-fermented PJ more potently inhibited it than non-fermented PJ. PJs fermented with and without BB, at 50 mg/kg, inhibited the PCA reaction by 46 and 36%, respectively. However, BB alone did not exhibit anti-PCA effects.
Table 1. Inhibitory effects of PJ and BB-fermented PJ on the PCA reaction in mice.
We also investigated the inhibitory effect of PJ fermented with and without BB on compound 48/80-induced scratching (). Both agents inhibited scratching, with BB-fermented PJ more potent than non-fermented PJ, inhibiting scratching frequency by 44 and 33% at 50 mg/kg, respectively. These agents also inhibited scratching induced by histamine with the same efficacy as seen with compound 48/80. BB alone did not exhibit anti-scratching effects.
Table 2. Inhibitory effects of PJ and BB-fermented PJ on the scratchingr behaviour in mice.
Discussion
Allergic reactions, including atopy, rhinitis, asthma and anaphylaxis, produce inflammatory mediators and cause anaphylaxis, scratching, inflammation, pain and increased vascular permeability (Stevens and Austen, Citation1989; Wuthrich, Citation1989). Anti-histamines, NSAIDs, steroids and immunosuppressants can be used to treat allergic diseases, but the repeated application of these agents causes side effects (Friedman, La Natra, & Stiller, Citation2002; Sakuma et al., Citation2001; Schafer-Korting, Schmid, & Korting, Citation1996; Simons, Citation1992). Therefore, herbal medicines and functional foods have been receiving increased attention as alternative treatments for allergic diseases (Bielory, Citation2004). The anti-allergic effects, particularly the anti-PCA reaction and anti-scratching activities, of PJ leaves have been not studied, although its rhizomes exhibit anti-histaminic actions (Tobinaga et al., Citation1983).
In this study, PJ with and without BB fermentation inhibited the IgE-induced PCA reaction as well as compound 48/80-induced scratching. BB-fermented PJ more potently inhibited degranulation of RBL-2H3 cells than non-fermented PJ. BB alone showed only a weak anti-allergic effect. These results suggest that PJ and BB-fermented PJ may inhibit the PCA reaction by inhibiting mast cell degranulation.
Mast cells and basophiles produce histamine, as well as proinflammatory cytokines, TNF-α and IL-6, and IgE-producing cytokine, IL-4 (Stevens and Austen, Citation1989), which is important in allergic reactions. Mast cells are a principal source of TNF-α in human dermis, and degradation of mast cells in the dermal endothelium is abrogated by inhibition of TNF-α (Kempuraj et al., Citation2003; Stevens and Austen, Citation1989). IL-6 produced from mast cells, when locally accumulated, is associated with the PCA reaction (Ito, Miyazaki, Ono, & Sakurai, Citation1998). IL-4 induces IgE production in B lymphocytes (Stevens & Austen, Citation1989). Inhibition of cytokine expression in mast cells may reduce allergic symptoms. PJ and BB-fermented PJ inhibited degranulation and expression of TNF-α and IL-4 in IgE-stimulated RBL-2H3 cells, as well as NO production in RAW 264.7 macrophage cells, as do other natural anti-allergic agents, such as quercetin (Ito et al., Citation1998; Jiang et al., Citation2006). NF-κB activation is an important signalling pathway in the immune response (Furumoto, Nunomura, Terada, Rivera, & Ra, Citation2004; Zhao, Oskeritzian, Pozez, & Schwartz, Citation2005), and NF-κB activation is essential for the expression of proinflammatory cytokines, such as TNF-α and IL-6. PJ and BB-fermented PJ inhibited IgE-induced activation of NF-κB in RBL-2H3 cells. These agents inhibited degranulation and IL-4/TNF-α expression with the same potency, suggesting that the anti-allergic effects of PJ and BB-fermented PJ may be due to the inhibition of degranulation and cytokine biosynthesis via the inhibition of NF-κB activation. Based on these findings, BB-fermented PJ may be useful for improving the PCA reaction and scratching, which are representative IgE-mediated skin allergic diseases.
Acknowledgements
This work was supported by a Grant from the Korean Rural Development Administration (2006).
References
- Bae , E.A. , Kim , N.Y. , Han , M.J. , Choo , M.K. and Kim , D.H. 2000 . Transformation of ginsenosides to compound K by lactic acid bacteria of human intestine . Journal of Microbiology and Biotechnology , 13 : 9 – 14 .
- Bielory , L. 2004 . Complementary and alternative interventions in asthma, allergy, and immunology . Annuals of Allergy and Asthma Immunology , 93 ( 2 Suppl. 1 ) : S45 – S54 .
- Choo , M.K. , Park , E.K. , Han , M.J. and Kim , D.H. 2003 . Antiallergic activity of ginseng and its ginsenoside . Planta Medica , 69 : 518 – 522 .
- Cui , H.S. , Kim , M.R. and Sok , D.E. 2005 . Protection by petaslignolide A, a major neuroprotective compound in the butanol extract of Petasites japonicus leaves, against oxidative damage in the brains of mice challenged with kainic acid . Journal of Agricultural Food Chemistry , 53 : 8526 – 8532 .
- Friedman , E.S. , La Natra , N. and Stiller , M.J. 2002 . NSAIDs in dermatologic therapy: Review and preview . Journal of Cutaneous Medicine and Surgery , 6 : 449 – 459 .
- Furumoto , Y. , Nunomura , S. , Terada , T. , Rivera , J. and Ra , C. 2004 . The FcepsilonRIbeta immunoreceptor tyrosine-based activation motif exerts inhibitory control on MAPK and IkappaB kinase phosphorylation and mast cell cytokine production . Journal of Biological Chemistry , 279 : 49177 – 49187 .
- Ito , H. , Miyazaki , T. , Ono , M. and Sakurai , H. 1998 . Antiallergic activities of rabdosiin and its related compounds: Chemical and biochemical evolutions . Bioorganic and Medicinal Chemistry , 6 : 1051 – 1056 .
- Jiang , J.S. , Shih , C.M. , Wang , S.H. , Chen , T.T. , Lin , C.N. and Ko , W.C. 2006 . Mechanisms of suppression of nitric oxide production by 3-O-methylquercetin in RAW 264.7 cells . Journal of Ethnopharmacology , 2103 : 281 – 287 .
- Kempuraj , D. , Huang , M. , Kandere-Grzybowska , K. , Basu , S. , Boucher , W. Letourneau , R. 2003 . Azelastine inhibits secretion of IL-6, TNF-alpha and IL-8 as well as NF-kappaB activation and intracellular calcium ion levels in normal human mast cells . International Archives of Allergy and Immunology , 132 : 231 – 239 .
- Leung , D.Y. 1995 . Atopic dermatitis: The skin as a window into the pathogenesis of chronic allergic diseases . International Archives of Allergy Immunology , 96 : 302 – 318 .
- Matsubara , K. , Mori , M. and Mizushina , Y. 2004 . Petasiphenol which inhibits DNA polymerase lambda activity is an inhibitor of in vitro angiogenesis . Oncology Report , 11 : 447 – 451 .
- Matsuda , H. , Morikawa , T. , Ueda , K. , Managi , H. and Yoshikawa , M. 2002 . Structural requirement of flavonoids for inhibition of antigen-induced degranulation, TNF-a and IL-4 production from RBL-2H3 cells . Bioorganic and Medicinal Chemistry , 10 : 2123 – 2128 .
- Min , B.S. , Cui , H.S. , Lee , H.K. , Sok , D.E. and Kim , M.R. 2005 . A new furofuran lignan with antioxidant and antiseizure activities from the leaves of Petasites japonicus . Archives of Pharmacal Research , 28 : 1023 – 1026 .
- Mizushina , T. , Ishidoh , T. , Kamisuki , S. , Nakazawa , S. , Takemura , M. Sugawara , F. 2003 . Flavonoid glycoside: A new inhibitor of eukaryotic DNA polymerase alpha and a new carrier for inhibitor-affinity chromatography . Biochemical and Biophysical Research Communication , 301 : 480 – 487 .
- Nyman , M. 1995 . Effects of processing on dietary fibre in vegetables . European Journal of Clinical Nutrition , 49 ( Suppl. 3 ) : S215 – S218 .
- Sakuma , S. , Higashi , Y. , Sato , N. , Sasakawa , T. , Sengoku , T. Ohkubo , Y. 2001 . Tacrolimus suppressed the production of cytokines involved in atopic dermatitis by direct stimulation of human PBMC system (Comparison with steroids) . International Immunopharmacology , 1 : 1219 – 1226 .
- Schafer-Korting , M. , Schmid , M.H. and Korting , H.C. 1996 . Topical glucocorticoids with improved risk-benefit ratio . Drug Safety , 14 : 375 – 385 .
- Shin , Y.W. , Bae , E.A. , Kim , S.S. , Lee , Y.C. and Kim , D.H. 2005 . Effect of ginsenoside Rb1 and compound K in chronic oxazolone-induced mouse dermatitis . International Immunopharmacology , 5 : 1183 – 1191 .
- Simons , F.E.R. 1992 . The antiallergic effects of antihistamines (H1-receptor antagonists) . Journal of Allergy and Clinical Immunology , 90 : 705 – 715 .
- Steinkraus , K.H. 1983 . Lactic acid fermentation in the production of foods from vegetables, cereals and legumes . Antonie Van Leeuwenhoek , 49 : 337 – 348 .
- Stevens , R.L. and Austen , K.F. 1989 . Recent advances in the cellular and molecular biology of mast cells . Immunology Today , 10 : 381 – 386 .
- Sugimoto , Y. , Umakoshi , K. , Nojiri , N. and Kamei , C. 1998 . Effect of histamine H1 receptor antagonists on compound 48/80-induced scratching behavior in mice . European Journal of Pharmacology , 351 : 1 – 5 .
- Tobinaga , S. , Takeuchi , N. , Kasama , T. , Yamashita , J. , Aida , Y. and Kaneko , Y. 1983 . Anti-histaminic and anti-allergic principles of Petasites japonicus Maxim . Chemical and Pharmaceutical Bulletin , 31 : 745 – 758 .
- Wuthrich , B. 1989 . Epidemiology of the allergic diseases: Are they really on the increase? . International Archives of Allergy and Applied Immunology , 90 ( Suppl. 1 ) : 3 – 10 .
- Zhao , W. , Oskeritzian , C.A. , Pozez , A.L. and Schwartz , L.B. 2005 . Cytokine production by skin-derived mast cells: Endogenous proteases are responsible for degradation of cytokines . Journal of Immunology , 175 : 2635 – 2642 .