Abstract
A novel synthesis method of methyl-3-quinoxaline-2-carboxylic acid (MQCA) and the preparation of its hapten were described. MQCA was synthesised involving in two steps with a high purity. For improving the sensitivity of detection, five different haptens were synthesised and corresponding immunogens and coating antigens were prepared. After comparing the sensitivity of immunoassay with different pairs of antibody and coating antigen, a specific immunoassay was obtained using an antibody raised against hapten (four-atom spacer arm) – BSA and a suitable coating antigen with a heterologous spacer arm group. The 50% inhibitory concentration of MQCA by indirect competitive enzyme-linked immunosorbent assay with optimised antibody and coating antigen was 3.84 ng/ml and the limit of detection was 0.25 ng/ml.
Introduction
Olaquindox (OLA) is an antibacterial agent that has been used as a medicinal feed additive for growth promotion in animal production (Hurtaud-Pessel, Pirotais, Blot, & Sanders, Citation2006). OLA is a quinoxaline-N-dioxide drug and can be rapidly metabolised (Joint FAO/WHO Expert Committee on Food Additives, Citation1991). MQCA is the last major remaining detectable metabolite of OLA in animals. Therefore, it was designated as the biomarker substance for this drug (Joint FAO/WHO Expert Committee on Food Additives, Citation1995). Since 1 January 1999, OLA has been banned in the EU, due to health concerns over possible carcinogenic, mutagenic and photoallergenic effects of the drug and its metabolites (European Commission, Citation1998). In China, OLA is approved for use in porcine feed, the maximum residue limit (MRL) of its metabolite (MQCA) in porcine liver is 50 µg/kg and in porcine muscle is 4 µg/kg (Joint FAO/WHO Expert Committee on Food Additives, Citation1990).
To avoid the misuse and enforce the ban of OLA, tissues of food-producing animals must be guaranteed free from such residues in the EU. Many published methods just described the analysis of OLA or its desoxy metabolites (MQCA is the main metabolite) in tissue (Nagata & Saeki, Citation1987; Speirenburg, Van Lenthe, De Graaf, & Jager, Citation1988), such as high performance liquid chromatography (HPLC) with UV detection (Wu et al., Citation2007), liquid chromatographic method (Huang et al., Citation2005), liquid chromatography-electrospray and tandem mass spectrometry (Hutchison, Young, & Kennedy, Citation2005), which are either lengthy manual procedures or sophisticated, expensive instruments. These chromatographic methods cannot be used to handle large amounts of samples for the routine analytical purpose. The enzyme-linked immunosorbent assay (ELISA) method, which is rapid, convenient and sensitive, was used to detect OLA (Situ & Elliott, Citation2005). However, up to now, there has been no report about the detection of MQCA using immunoassay.
The molecular weight of MQCA is small and the structure is illustrated in . To obtain antibodies to small molecule with high sensitivity, suitable haptens need to be synthesised and conjugated to proteins to induce the immune response of the host animal. Haptens must have a spacer arm (linker) for their covalent attachment to carrier proteins. It is generally accepted that the length of the hapten spacer arm is an important factor in the production of antibodies with high affinity to the analyte and that ELISA sensitivity improves with increasing spacer length (Harrison, Goodrow, Gee, & Hammock, Citation1991; Marco, Gee, & Hammock, Citation1995; Szurdoki, Bekheit, Marco, Goodrow, & Hammock, Citation1995). A long spacer arm is thought to avoid the shielding effect of the carrier. However, a spacer arm that is too long may fold and shield the hapten. Thus, a medium size spacer arm with three or four to six atoms has often been quoted as an optimal range (Marco et al., Citation1995; Szurdoki et al., Citation1995). Heterology is commonly used to eliminate problems associated with the strong affinity of antibodies for the immunogen (Kim et al., Citation2003a, Citationb; Liu et al., Citation2007).
Although the target molecules have a carboxylic group that is available for coupling haptens to protein, a direct conjugation with a functional group of the target molecule and amino groups of protein should be avoided because an important antigenic determinant of the molecule disappears that could lead to less specific antibodies.
Hapten synthesis is a key step for developing an immunoassay-based screening method for MQCA. In this paper, MQCA was synthesised by a novel method and the purity of MQCA was ≥99%. Based on the considerations mentioned above, an appropriate hapten permitting easy hapten recognition was designed. After the selection of coating antigen and antibodies, the indirect competitive ELISA (ic-ELISA) method was established and the half maximal inhibitory concentration (IC50) and limit of detection (LOD) were measured.
Experimental
Reagents and materials
Unless otherwise stated, all reagents and solvents were of analytical regent grade and from Sigma (St. Louis, USA).
Mequindox was kindly gifted by Wuxi Zhongsheng Forage Co., Ltd. (Jiangsu Province, China); Tri-n-butylamine, isobutylchloroformate were obtained from Feixiang Chemical Plant (Shanghai, China); Silica gel for column chromatography (100–200 meshes) was purchased from Aldrich (Milwaukee, USA). Analytical (silica gel F254) and preparative TLC plates (silica gel, 1 mm) were obtained from Merck (Darmstadt, Germany); 3-aminobenzoic acid, glycine, gamma aminobutyric acid, 6-aminocaproic acid (BR) were all obtained from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China); bovine serum albumin (BSA) and ovalbumin (OVA) were purchased from Boao Biotechnology Company (Shanghai, China); goat anti-rabbit immunoglobulin conjugated to horseradish peroxidase (IgG-HRP) and Tween-20 were obtained from Shanghai KangChen Bio-tech. Tetramethylbenzidine (TMB) was from Fluka (Switzerland).
Apparatus
NMR spectra were obtained with AVANCE DMX 500 spectrometer (Bruker, Berlin, Germany), operating at 400 MHz; polystyrene microtitre plates were purchased from Nunc (Roskilde, Denmark); Multiska Mks microplates reader was obtained from Thermo Labsystem (Helsinki, Finland); ultraviolet spectra were recorded on a UV-2000 scanner (Ruili Company, Beijing, China); the HPLC/MS spectrometer used for analysing was a Waters Platform ZMD 4000 (Waters Company, Milford, MA, USA).
Preparation of methyl-3-quinoxaline-2-carboxylic acid (MQCA)
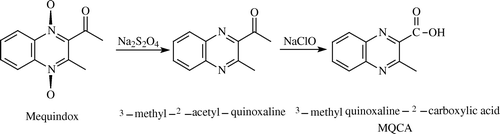
The main procedure scheme of preparing MQCA is shown in .
Aliquots of 6 g mequindox were dissolved in a mixed solution of 160 ml of tetrahydrofuran, 30 ml of chloroform and 30 ml of water. The 6 g of sodium hydrosulfite was added and reaction mixture was stirred at 70°C for 25 min, the organic phase was analysed using thin-layer chromatography (ethyl acetate: petroleum ether, 1:1). If there was not only one spot, another 6 g of sodium hydrosulfite was added and reaction mixture was stirred at 70°C for 25 min, the 6 g of sodium hydrosulfite was not added and the reaction was stopped until there was only one point. The organic phase was removed, evaporated and washed with ice-water. This compound was dried under vacuum in a drying oven overnight. The product was identified as 3-methyl-2-acetyl-quinoxaline (desoxy mequindox) by HPLC-MS and NMR analysis and the purity was over 99% with a yield of 79% (4.7 g). 1H NHR (CDCl3): δ 2.87 (s, 3H, COCH3), δ 3.00 (s, 3H, CH3), δ 7.77–7.88 (m, 2H, H6, H7,), δ 8.06 (d, 1H, H5, J=7.6), δ 8.14 (d, 1H, d, H8, J=7.2); HPLC-MS molecular ion peak (m/z = 186) was the base peak.
Solution of 3-methyl-2-acetyl-quinoxaline (0.4 g) in chloroform (2 ml) was added to a stirred solution of polyethyleneglycol (PEG) 2000 (0.4 g) in water (60 ml). After churning up the resulting mixture, 4 ml of sodium hypochlorite solution was added at regular intervals. The reaction was monitored with starch potassium iodide paper, and continued until the colour of starch potassium iodide paper remained blue. Chloroform was removed by rotary evaporation. The solution was cooled with ice-water, while dropping sodium bisulfite to remove Cl−, and then, acidified with HCl to pH 1–2. Stored at 4°C, the solution was centrifuged, the white deposit was collected and dried under vacuum. The end product was MQCA. 1H NHR (CDCl3): δ 3.20 (s, 3H, CH3), δ 7.84–7.95 (m, 2H, H6, H7), δ 8.14 (m, 2H, H5, H8); HPLC-MS molecular ion peak (m/z = 188) was the base peak.
Synthesis of hapten
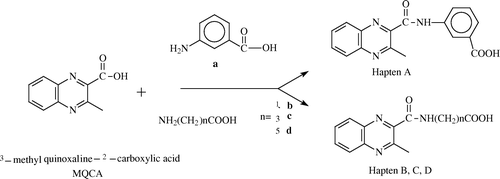
The synthetic route for Haptens A–D is illustrated in .
Preparation of Hapten A
Solution A
MQCA (188 mg) was dissolved in 6 ml of N, N-Dimethylformamide (DMF), and Tri-n-butylamine (120 µL) was added. The carboxylic acid on the MQCA was then activated with isobutylchloroformate (75 µL), stirring for 30 min at room temperature.
Solution B
3-Aminobenzoic acid (137 mg) was dissolved in a stirred solution of 9 ml of carbonate buffer (0.05 mol/L, pH 9.6).
Solution A was added dropwise to B, the reaction was stirred at 4°C for 1 h, and a pale yellow deposit appeared. The mixture was centrifuged at 4500 rpm to remove the liquid and the deposit was dried in vacuum.
Hapten A (MQCA–Aminobenzoic acid): 1H NHR (DMSO): δ 2.89 (s, 3H, CH3), δ 7.19 (s, H, NH), δ 7.52 (t, 1H, H16, J=7.8), δ 7.75–8.19 (m, 6H, H5, H6, H7, H8, H15, H17), δ 8.51 (s, 1H, H19), δ 11.02 (s, 1H, COOH); HPLC-MS molecular ion peak (m/z = 307) was the base peak.
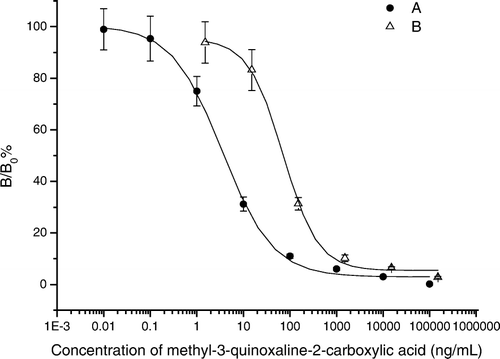
Figure 3. Standard curves of ic-ELISA for MQCA. Conditions: A: coating antigen (Hapten A–OVA, 25 ng/well), antibody (raised using Hapten C–BSA, 1:40,000); B: coating antigen (Hapten E–OVA, 50 ng/well), antibody (raised using Hapten E–BSA, 1:24,000); IgG-HRP (1:3000); each point represents the mean of 16 determinations; vertical bars indicate ±standard deviation about the mean.
Preparation of Hapten B–D
Solution A
A solution of 188 mg (1 mmol) MQCA in 7 ml of tetrahydrofuran was activated with 200 µL of Tri-n-butylamine and 600 µL of isobutylchloroformate in ice-salt bath at about −10°C.
Solution B–D
Glycine, gamma aminobutyric acid and 6-aminocaproic acid, of 2 mmol, respectively, was dissolved in a stirred solution of 4 ml of carbonate buffer (0.05 mol/L, pH 9.6).
Solution A was added dropwise to solution B–D, and the reaction was conducted in an ice-salt bath at about −10°C while being stirred. After 2 h, the mixture was extracted with 4 ml of chloroform, and the solvent was removed under reduced pressure. The crude mixture was purified by column chromatography (silica gel, chloroform: ethyl acetate: acetic acid, 13:6:1).
Hapten B (MQCA–Glycine): 1H NHR (CDCl3): δ 3.13 (s, 3H, CH3), δ 3.59 (s, 2H, NHCH2), δ 7.86 (br-s, 1H, NH), δ 7.72–7.80 (m, 2H, H6, H7), δ 8.05 (m, 2H, H5, H8); HPLC-MS molecular ion peak (m/z = 245) was the base peak.
Hapten C (MQCA–Aminobutyric acid): 1H NHR (CDCl3): δ 2.14 (m, 2H, CH2CH2CH2), δ 2.51 (m, 2H, CH2COOH), δ 3.14 (s, 3H, CH3), δ 3.70 (m, 2H, NHCH2), δ 7.51 (br-s, 1H, NH), δ 7.77–7.93 (m, 2H, H6, H7), δ 8.14 (m, 2H, H5, H8); HPLC-MS molecular ion peak (m/z = 274) was the base peak.
Hapten D (MQCA–Aminocaproic acid): 1H NHR (CDCl3): δ 1.49 (m, 2H, CH2CH2CH2CH2CH2), δ 1.72 (m, 4H, CH2CH2CH2CH2CH2), δ 2.49 (m, 2H, CH2COOH), δ 3.13 (s, 3H, CH3), δ 3.52 (m, 2H, NHCH2), δ 7.19 (br-s, 1H, NH), δ 7.74–7.84 (m, 2H, H6, H7), δ 8.08 (d, 2H, H5, H8, J=8.0); HPLC-MS molecular ion peak (m/z = 301) was the base peak.
Hapten E was MQCA itself.
Preparation of hapten–protein conjugates
MQCA-spacer (0.1 mmol) reacted with 11.5 mg N-Hydroxysuccinimide (NHS) in the presence of 20.6 mg N,N'-dicyclohexylcarbodiimide (DCC) in 3 ml DMF. After 4 h incubation at 4°C, the mixture was added dropwise to another stirred solution of protein (BSA 0.002 mmol to prepare the immunoconjugate or OVA 0.05 mmol for the coating antigen previously dissolved in 6 ml of carbonate buffer (0.05 mol/L, pH 9.6) and incubated overnight. Then, the solution was centrifuged to remove the deposit and dialysed against double-distilled water for the next 2 days, changing the water four times. Finally, it was freeze-dried and preserved.
Coupling ratio of hapten–protein conjugates
A spectrophotometric method was adopted to determine the coupling ratio of hapten–protein (Zhou & Hu, Citation2003). First, the content of protein in the conjugate was tested by the approach of Bradford and Lowry (Zhou & Hu, Citation2003). The coupling ratios could be calculated with Equation (Equation1).
Immunisation of rabbits
MQCA–Aminobenzoic acid–BSA, MQCA–Glycine–BSA, MQCA–Aminobutyric acid–BSA, MQCA–Aminocaproic acid–BSA and MQCA–BSA were used as immunogens. Two New Zealand white rabbits were used for each immunogen for raising antibodies. For the initial immunisation, 1 mg immunogen was emulsified with Freund's complete adjuvant (1:1 v/v) and injected at multiple sites (24–30 sites) subcutaneously on the rabbits’ back. After 2–3 weeks, further injections of 1 mg of the immunogen emulsified with Freund's incomplete adjuvant were given (1:1, v:v). The booster injections were given at 4-week intervals. Blood from rabbits was collected after 10 days of each booster injection for titre monitoring by indirect ELISA. The antiserum was purified by ammonium sulfate precipitation and stored at −20°C.
Determination of antibody titre
The antibody titres were determined by an ELISA chequerboard titration using indirect competitive assay format. The microtitre plates were coated with 100 µL/well of a coating antigen (0.2–2 µg/mL) in carbonate–bicarbonate buffer (0.05 mol/L, pH 9.6) by overnight incubation at 4°C. The plates were washed five times with PBS containing 0.05% Tween-20 (PBST) after coating, and blocked with 2% gelatine in carbonate–bicarbonate buffer (0.05 mol/L, pH 9.6, 200 µL/well). After overnight incubation, the plates were washed again. The antiserum to different haptens were added to the wells (100 µL/well), respectively, with eight different dilutions (1:500–1:20,000 with 0.1% gelatin in PBST) and incubated for 30 min at room temperature. Plates were washed as described above and goat anti-rabbit IgG-HRP with the dilution of 1:3000 was added (100 µL/well) and incubated for 30 min at room temperature. The plates were washed again. 100 µL/well of TMB solution was added and incubated for about 15–25 min at 37°C before the reaction was stopped by adding 2 M H2SO4 (100 µL/well). The absorbance was read at 450 nm.
Indirect competitive enzyme-linked immunosorbent assay (ELISA)
The protocol for ic-ELISA was similar to that of the determination of antibody titre. Firstly, the concentration of antiserum and coating antigen were optimised by chequerboard titration as presented above. The ability of MQCA to compete with coating antigens (Hapten A–E conjugated to OVA) was investigated by adding a serial dilutions of the analyte (from 10−2 ng/ml to 105 ng/ml, in PBS, 50 µL/well), and different antibodies (50 µL/well). The mixture was incubated for 30 min and the plates were then processed as described above.
Results and discussion
Preparation of methyl-3-quinoxaline-2-carboxylic acid (MQCA)
Two reaction steps were adopted to synthesise MQCA. The results indicated that the MQCA was prepared successfully with a high purity (>99%). Due to the high toxicity of the metabolite of mequindox (MQCA) towards humans, it was banned as feed additive. However, for the pursuit of commercial interests, the mequindox was also commonly used. In this study, mequindox was adopted as the starting material. In comparison with other intermediates used in the traditional synthetic approach (Haworth & Robinson, Citation1948), this raw material was cheap and convenient to obtain. Most importantly, there were few impurities during the synthetic process and MQCA could be purified using some simple disposal process.
Hapten synthesis
In order to simplify the synthetic process for this hapten, we developed a new method which requires two steps. This method involves the activation of the carboxylic acid of MQCA with isobutylchloroformate, followed by the reaction with a free amino group on the carrier protein.
Conventionally, the synthesis of amide was as follows: carboxylic acid was activated with SOCl2 or PCl5 to acyl chloride, followed by reaction with amido compound. But we discovered that the acyl chloride group was so active that it can easily react with other substance in the presence of OH− and could decompose rapidly. An improved mixed anhydride method, different kinds of haptens of MQCA were synthesised with good purities and desirable yields.
Hapten design
The initial and critical step in the development of efficient immunoassays for small molecular compound lies in the selection of appropriate haptens. In general, immunising haptens’ determinant groups must not be masked or lost within the protein tertiary structure (Marco et al., Citation1995; Szurdoki et al., Citation1995). Some researchers indicated that the optimum spacer arm is medium-sized (three to six atoms).
In our research, the analyte (MQCA) was a small molecular with the molar weight of 188 which has carboxylic acid on its quinoxaline ring. Although it could be directly conjugated to BSA with its carboxylic acid, from the views described above, we think it is better to synthesise immunising hapten having a spacer arm at the MQCA to achieve highly specific antigen. In order to select a suitable immunogen and to find out whether it is necessary to link a spacer to MQCA, five different haptens of MQCA (Hapten A–E) were used and 10 antisera against these haptens (five haptens coupled to BSA, two rabbits per immuning hapten) were screened from the second boost against coating antigen (five haptens coupled to OVA). All the antisera produced against hapten–protein conjugates presented the stable and high titre at the forth boost, the antiserum raised from MQCA–Aminobutyric acid–BSA had the highest titre (614,400).
Coupling ratio of hapten–protein conjugates
All the haptens were converted to the succinimide esters, which are active esters for coupling haptens to carrier protein. The measuring of hapten–protein ratios were described above and the criterion was fairly uniform. All the results are shown in . The effect of spacer arm of the hapten on the affinity of hapten to protein was not so obvious, but all the haptens were conjugated with proteins at suitable molar ratios.
Table 1. Coupling ratio of hapten–protein conjugates.
Determination of antibody titre
As previous described, different small haptens were linked to protein, so there is a possibility that the haptens may be masked in spatial configuration by protein. Therefore, the antibody may be not definitely sensitive even with a high titre value because of the analyte recognition (Kim et al., Citation2003a, b), Hapten-C–OVA was used as coating antigen to test other five different antibodies titre.
Determination of sensitivity by competitive indirect enzyme-linked immunosorbent assay (ELISA)
For evaluating the effect of different coating antigens, MQCA–Aminobenzoic acid–OVA and MQCA–BSA were used for coating the microplates, respectively. presents the results of competition experiments with different coating antigens and finally MQCA–Aminobenzoic acid–OVA was selected as the most desirable coating antigen because hapten heterology is commonly used to eliminate problems associated with the strong affinity of the antibodies to the spacer arm that leads to no or poor inhibition by the target compound (Harrison et al., Citation1991; Kim et al., Citation2003a, b; Liu et al., Citation2007; Szurdoki et al., Citation1995). From IC50 values of antibodies against Hapten A–E protein conjugates for the determination of MQCA by ELISA method, we found that the covalent linkage of the hapten to a large immunogenic carrier protein by a spacer arm was required to generate high-specific antibody against small haptenic molecule. The Hapten C–BSA was considered to be the most appropriate immunogen. The sensitivity of ELISA has been accessed by several studies based on haptens with different spacer arm length. Abad and Montoya (Abad, Primo, & Montoya, Citation1997) found haptens with three to six atoms spacer arm could improve the sensitivity compared to those with shorter arm. But Moreno, Abad, and Montoya (Citation2001) discovered some opposite results. The former (Shan et al., Citation2000) developed an acceptable assay using haptens with zero spacer arm length. The site of spacer arm and the effect of different length can account for the different results. As for MQCA, we considered if it was directly linked to protein, the quinoxaline ring, which has a conjugated double-ring structure, may be too close to avoid the masking effect by protein's structure. In , we compared the typical inhibition curves obtained under the same condition with different coating antigen/antibody couples. The IC50 of the ic-ELISA based on hapten A–OVA/antibody raised by hapten C–BSA was obvious lower than that based on hapten E–OVA/antibody raised by hapten E–OVA which indicated that the sensitivity of immunoassay on MQCA was highly enhanced by spacer arm.
Table 2. IC50 values of ic-ELISA for determination of MQCA with different conditions.
Conclusion
The aims of this work were to synthesise a most appropriate hapten and develop a specific sensitive and reliable ic-ELISA method for the determination of MQCA. In this text, the commonly used feed additive mequindox was used as starting material for hapten synthesis. The sensitivity of immunoassay was evaluated based on different haptens. The IC50 indicated that it is necessary to link a heterologous structure spacer to MQCA. We also found that the four-atom length spacer was most appropriate, and this could be explained by the masking effect of the protein's structure. In this study we developed new method to synthesise hapten, MQCA–Aminobutyric acid, and gained antibody with sufficient sensitivity to provide an effective screening method for animal-derived food samples.
References
- Abad , A. , Primo , J. and Montoya , A. 1997 . Development of an enzyme-linked immunosorbent assay to carbaryl. 1. Antibody production from several haptens and characterization in different immunoassay formats . Journal of Agricultural and Food Chemistry , 45 : 1486 – 1494 .
- European Commission . 1998 . Commission Regulation (EC) No. 2788/98 of the Commission of December, 22, 1998, concerning additives in feedingstuffs . Official Journal of the European Communities, L347 , 3 – 32 .
- Harrison , R.O. , Goodrow , M.H. , Gee , S.J. and Hammock , B.D. 1991 . “ Hapten synthesis for pesticide immunoassay development ” . In Immunoassays for trace chemical analysis: monitoring toxic chemicals in humans, food, and the environment, ACS symposium series 451 , Edited by: Vanderlaan , M. , Stanker , L.H. , Watkin , B.E. and Robberts , D.W. 14 – 27 . Washington, DC : American Chemical Society .
- Haworth , R.D. and Robinson , S. 1948 . Synthetic antimalarials. Part XXVII. Some derivatives of phthalazine, quinoxaline, and isoquinoline . Journal of the Chemical Society , 174 : 777 – 782 .
- Huang , L. , Xiao , A. , Fan , S. , Yin , J. , Chen , P. Liu , D. 2005 . Development of liquid chromatographic methods for determination of quincetone and its main metabolites in edible tissues of swine and chicken . Journal of the Association of Official Analytical Chemist International , 88 : 472 – 478 .
- Hurtaud-Pessel , D. , Pirotais , Y. , Blot , J. , & Sanders , P. 2006, May . An LC/MS-MS method for the determination of QCA and MQCA, the metabolites of Carbadox and Olaquindox in porcine liver and muscle . Fifth International Symposium on Hormone and Veterinary Drug Residue Analysis , Antwerp , Belgium .
- Hutchison , M.J. , Young , P.Y. and Kennedy , D.G. 2005 . Confirmation of carbadox and olaquindox metabolites in porcine liver using liquid chromatography-electrospray, tandem mass spectrometry . Journal of Chromatography B , 816 : 15 – 20 .
- Joint FAO/WHO Expert Committee on Food Additives . 1990 . Evaluation of certain veterinary drug residues in food . Technical Series , 799 , 37 – 41 .
- Joint FAO/WHO Expert Committee on Food Additives . 1991 . Toxicological evaluation of certain veterinary drug residues in food . Additives Series , 27 , 694 – 705 .
- Joint FAO/WHO Expert Committee on Food Additives . 1995 . Evaluation of certain veterinary drug residues in food . Technical Series , 851 , 19 – 21 .
- Kim , Y.J. , Cho , Y.A. , Lee , H.S. and Lee , Y.T. 2003a . Investigation of effect of hapten heterology on immunoassay sensitivity and development of an enzyme-linked immunosorbent assay for the organophosphorus insecticide fenthion . Analytica Chimica Acta , 494 : 29 – 40 .
- Kim , Y.J. , Cho , Y.A. , Lee , H. , Lee , Y.T. , Gee , S.J. and Hammock , B.D. 2003b . Synthesis of haptens for immunoassay of organophosphrus pesticides and effect of heterology in hapten spacer arm length on immunoassay sensitivity . Analytica Chimica Acta , 475 : 85 – 96 .
- Liu , Y.H. , Jin , M.J. , Gui , W.J. , Cheng , J.L. , Guo , Y.R. and Zhu , G.N. 2007 . Hapten design and indirect competitive immunoassay for parathion determination: Correlation with molecular modeling and principal component analysis . Analytica Chimica Acta , 591 : 173 – 182 .
- Marco , M.P. , Gee , S.J. and Hammock , B.D. 1995 . Immunochemical techniques for environmental analysis II. Antibody production and immunoassay development . Trends in Analytical Chemistry , 14 : 415 – 425 .
- Moreno , M.J. , Abad , A. and Montoya , A. 2001 . Production of monoclonal antibodies to the N-methylcarbamate pesticide propoxur . Journal of Agricultural and Food Chemistry , 49 : 72 – 78 .
- Nagata , T. and Saeki , M. 1987 . Determination of olaquindox residues in swine tissues by liquid chromatography . Journal of the Association of Official Analytical Chemist International , 70 : 706 – 707 .
- Shan , G. , Whitney , R.L. , Donald , W.S. , Gee , S.J. , Chang , D.P.Y. and Hammock , B.D. 2000 . Enzyme-linked immunosorbent assay for the pyrethroid permethrin . Journal of Agricultural and Food Chemistry , 48 : 4032 – 4044 .
- Situ , C. and Elliott , C.T. 2005 . Stimultaneous and rapid detection of five banned antibiotic growth promoters by immunoassay . Analytica Chimica Acta , 529 : 89 – 96 .
- Speirenburg , T.J. , Van Lenthe , H. , De Graaf , G. and Jager , L.P. 1988 . Liquid chromatographic of olaquindox in medicated feeds and in contents of porcine gastrointestinal tract . Journal of the Association of Official Analytical Chemists , 71 : 1106 – 1109 .
- Szurdoki , F. , Bekheit , H.K.M. , Marco , M.P. , Goodrow , M.H. and Hammock , B.D. 1995 . “ Important factors in hapten design and enzyme-linked immunosorbent assay development ” . In New Frontiers in Agricultural Immunoassays , Edited by: Kurtz , D.A. , Skerritt , J.H. and Stanker , L. 39 – 63 . Washington, DC : AOAC .
- Wu , Y. , Yu , H. , Wang , Y. , Huang , L. , Tao , Y. Chen , D. 2007 . Development of high-performance liquid chromatography method for the simultaneous quantification of quinoxaline-2-carboxylic acid and methyl-3-quinoxaline-2-carboxylic acid in animal tissues . Journal of Chromatography A , 1146 : 1 – 7 .
- Zhou , X.W. and Hu , X.Q. 2003 . Instrumental analysis and experimental technology of biochemistry , 271 – 272 . Beijing, , China : Chemistry Industry Press .