Abstract
Effects of chitosan pentamer ((GLcN)5) and chitosan hexamer ((GLcN)6) in vivo and in vitro on gene transcription and protein translation of interleukin-1 (IL-1), tumour necrosis, factor alpha (TNF-α), interleukin-2 and interferon-γ (IFN-γ) were investigated by relatively quantitative reverse transcription-polymerase chain reaction and enzyme-linked immunosorbent assay. The results showed that the expression of IL-1 mRNA, TNF-α mRNA, IFN-γ mRNA as well as the secretion of IL-1, TNF-α and IFN-γ can both be promoted by (GLcN)6 and (GLcN)5. The promotion effects caused by (GLcN)6 were greater than that of (GLcN)5. Gene transcription and protein secretion of cytokines were promoted by (GLcN)5 and (GLcN)6 in vivo and in vitro and thus the immuno-modulating ability was promoted. This may be one of the molecular mechanisms for elucidation of immunity of chitooligosaccharides.
Introduction
Chitooligosaccharides (COS), the hydrolysed form of chitin and chitosan, are water-soluble amino sugars with low degree of polymerisation (DP; Xia, Citation2001). Generally, the DP for COS is from 2 to 20. COS include chitin oligosaccharide and chitosan oligosaccharides. They are homo-oligosaccharides and hetero-oligosaccharides, connected by β-1,4 indican bond between N-acetyl-D-glucosamine (GLcNAc) and D-glucosamine (GLcN). Previous studies showed that COS possessed not only some similar properties to chitin and chitosan, but also special physiological activities or functional properties that differed from chitin and chitosan (Jeon, Shahidi, & Kim, Citation2000). Recent studies of COS are reported to have special functions such as anti-tumour activity (Du, Bai, & Jin, Citation2002; Jeon & Kim, Citation2002; Suzuki et al., Citation1986), immuno-stimulating effect (Jeon & Kim, Citation2001), antimicrobial activity (Kendra, Christian, & Hadwiger, Citation1989; Park, Je, Byun, Moon, & Kim, Citation2004) and radical scavenging activity (Je, Park, & Kim, Citation2004). COS with the DP equal to or greater than six have greater antifungal activity (Kendra & Hadwiger, Citation1984). Immune activities of COS have caused interest among researchers (Jeon & Kim, Citation2001). A low molecular weight chitosan and a COS mixture isolated from this chitosan hydrolysate were shown to have different stimulatory effects on the cell proliferation and IgM secretion of the human hybridoma HB4C5 cells (Wu & Tsai, Citation2004). Oligochitosan consisting of chitohexaose has been reported to have a stimulatory effect on the release of interleukin-1 beta (IL-1β) and tumour necrosis factor alpha (TNF-α) in macrophages (Mϕ) in vitro (Feng, Zhao, & Yu, Citation2004). However, effect of the DP of COS on gene expression and secretion of cytokines was not understood. Herein, the immune mechanism of higher DP chitooligomers (pentamer and hexamer) obtained by enzymatic hydrolysis of chitosan on gene expression of important cytokines such as interleukin-1 (IL-1) and TNF-α in Mϕ as well as interleukin-2 (IL-2) and interferon-γ (IFN-γ) in lymphocytes were studied.
Materials and methods
Materials and reagents
Chitosan pentamer ((GLcN)5) and chitosan hexamer ((GLcN)6) were isolated and purified from enzymatic hydrolysis of chitosan and their structures were identified by liquid chromatography/mass spectrometry (LC/MS) in our laboratory. The purity of (GLcN)5 and (GLcN)6 was higher than 95% according to HPLC analysis. TRIZOL reagent and Taq DNA polymerase were obtained from Bio. Basic, Inc. (Markham, ON, Canada). DEPC was purchased from AMRESCO Inc. (OH, USA) and DNase I was purchased from Worthington Biochemical Co. (NJ, USA). Agarose I was obtained from Command Spanish Inc. (MS, USA). Oligo(dT)15, M-MLV reverse transcriptase and dNTPs were purchased from Promega Co. (WI, USA). DL-2000 DNA Marker was purchased from TaKaRa Bio. Inc (Dalian, China). RPMI-1640 was purchased from Gibco Inc. (NY, USA). Fetal bovine serum (FBS) was obtained from Shanghai Institute of Cell Research (Shanghai, China). Lymphocyte separating medium (specific gravity = 1.088) was purchased from Shanghai Biochemical reagent Co. Ltd. of Chinese Medicine and Drug Co. (Shanghai, China). Other reagents were analytical pure products from China.
Target primers specific to murine IL-1, TNF-α, IL-2, IFN-γ and β-actin genes were generated based on the published sequences in the Genbank database, designed by software Primer 3 and synthesised by Shanghai Bio-asia biologic technique Co. (Shanghai, China). The sequences of primers are indicated in .
Table 1. The primer sequences of murine IL-1, TNF-α, IL-2, IFN-γ and β-actin.
Animals
Institute of Cancer Research (ICR) mice, four to six weeks old, weighing 18–22 g, one-half of each male and female, were obtained from experimental animal central of Nantong Medical College. All procedures were performed according to the Institute Ethical Committee for Experimental Use of Animals.
Preparation of murine macrophages (Mϕ) and splenocytes with chitosan pentamer ((GLcN)5) and chitosan hexamer ((GLcN)6) in vitro
Mice were sacrificed by cervical dislocation and cleansed by 75% alcohol solution. After the intra-peritoneal administration of sucrose solution (0.34 mol/l), ascites were harvested and centrifuged, and then the supernatant was discarded. Cell viability was determined by trypan blue dye exclusion. The final cell suspension was adjusted to 5×106 cells/ml in complete RPMI 1640 culture medium, then the cells were plated in 24 well-plates supplemented with 100 µg/ml (GLcN)5 or (GLcN)6. The control group was added equal volume of medium. The mixtures were incubated at 37°C for 48 h in a ThermoForma water jacketed CO2 incubator (USA) in a humidified atmosphere containing 5% CO2. Supernatants containing IL-1 and TNF-α were collected and kept at –70°C for measuring the production of IL-1 and TNF-α. Total RNA was extracted from spleen Mϕ by TRIZOL reagent and then mRNA expression of IL-1 and TNF-α were measured by reverse transcription-polymerase chain reaction (RT-PCR) methods.
Mice were sacrificed and cleansed by 75% alcohol, and spleens were removed aseptically and pounded to pieces. The pieces of tissues were filtered with a 200-mesh nylon net, added slowly to a centrifuge tube with lymphocytes separating medium and centrifuged. After supernatant medium, subnatant separating medium and precipitation were discarded, the intermedial layer was retained and centrifuged again. The cell precipitation was obtained. The final cell suspension was adjusted to 5×106 cells/ml in complete RPMI 1640 culture medium, then 100 µg/ml of (GLcN)5 or (GLcN)6 was added in 24 well-plates as well as equal volume of medium as control. The mixtures were incubated at 37°C for 48 h in a 95% humidified atmosphere containing 5% CO2. Supernatant containing IL-2 and IFN-γ were collected and kept at –20°C for measuring the production of IL-1 and TNF-α. Total RNA were extracted from spleen splenocytes by TRIZOL reagent and mRNA expression of IL-2 and IFN-γ were performed by RT-PCR.
Preparation of macrophages (Mϕ) and lymphocytes with chitosan pentamer ((GLcN)5) and chitosan hexamer ((GLcN)6) in vivo
Mice were divided randomly into three groups: (GLcN)5 group, (GLcN)6 group and control group. There are eight mice in each group, and they were intraperitoneal (i.p.) administered with (GLcN)5, (GLcN)6 and physiologic saline, respectively, with dose of 10 mg/kg/day for three days. Mice were sacrificed by cervical dislocation at fourth day, and Mϕ were obtained by the pre-described method. The production of IL-1, TNF-α were measured by enzyme-linked immunosorbent assay (ELISA). Total RNA was extracted by TRIZOL reagent and mRNA expression of IL-1 and TNF-α were measured by RT-PCR methods.
RNA preparation and reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted using TRIZOL reagent according to the manufacturer's protocol. Total RNA was quantified by the ratio of 260/280 nm in Biophotometer (Hamburg, German). Then, total RNA was dealt with DNase I without RNase to remove genomic DNA.
To synthesise single strand cDNA, reverse transcription of 2 µg of total mRNA was carried out in a 25 µl reaction volume using PTC-100TM Peltier Thermal Cycler (MA, USA) and reaction mixture was as follows: 1 µl RNA oligo (dT)15 primer (0.5 µg/µl), 1 µl RNA M-MLV reverse transcriptase (200 U/µl), 5 µl deoxynucleotide triphosphate-dNTP mix (2.5 mM of each of dATP, dTTP, dCTP, dGTP), 1×RT buffer (10 mM Tris-HCl pH 8.8, 50 mM KCl, 0.1% v/v Triton X-100) in a final volume of 25 µl. The reaction was performed at 37°C for 1 h, heated at 70°C for 10 min and chilled by ice. Then, reverse transcribed samples – the first train of cDNA were extracted and kept at –20°C.
The PCR was carried out in a 25 µL reaction volume using 1 µl of the previous RT reaction product, 0.4 µM of the 5′ and 3′ primer, 0.2 mM of each dNTP, 2 mM MgCl2 and 1 U Taq DNA polymerase. The primer sequences are indicated in . The amplification reactions were run on PTC-100™ Peltier Thermal Cycler under different conditions for corresponding target gene which are shown in .
Table 2. The PCR parameters for amplification of target DNA.
Electrophoresis and image analysis
PCR products were separated on a 1.5% agarose gel containing 1 mg/ml ethidium bromide (EB). Images of EB-stained bands for amplified target fragment were digitised by SX-300 Image System (Shanghai, China) and quantitatively analysed by the image analysis software-Scion Image. To reduce the error, the level of β-actin was served as the control, and the cDNA ratios of target gene/β-actin were estimated. Collected data were expressed as mean±standard error of arithmetic mean (SEM). Statistical analysis was performed by statistic software SPSS10.0.
Enzyme-linked immunosorbent assay (ELISA)
IL-1, TNF-α, IL-2 and IFN-γ assay kits (Diaclose) were used according to supplier's instruction. Coated ELISA plates were incubated with 50 µl culture supernatants for 2 h at room temperature, and then 50 µl aliquots of biotin-conjugated antibody was added and the plates were incubated at 37°C for another 90 min. The plates were washed thoroughly and 100 µl streptavidin–HRP was added. After incubated further for 30 min at 37°C, the plates were washed thoroughly and 100 µl substrate solution (freshly prepared tetramethylbenzidine with H2O2 solutions) was added. The plates were incubated at 37°C for 20 min in a dark chamber and the optical density (OD) at 450 nm was read. Recombinant murine IL-1, TNF-α, IL-2 and IFN-γ were diluted and used as the standard separately.
Results
Effects of chitosan pentamer ((GLcN)5) and chitosan hexamer ((GLcN)6) on mRNA expression and secretion of interleukin-1 (IL-1) and tumour necrosis factor alpha (TNF-α) in macrophages (Mϕ) in vivo and in vitro
As shown in and , the expression of IL-1 mRNA and TNF-α mRNA in murine Mϕ was up-regulated more significantly by (GLcN)5 and (GLcN)6 group than control and the transcription levels of IL-1 mRNA and TNF-α mRNA induced by (GLcN)6 exceeded that of (GLcN)5. These results indicated that (GLcN)5 and (GLcN)6 both can markedly promote the expression of IL-1 mRNA and TNF-α mRNA in Mϕ and (GLcN)6 has stronger effect than (GlcN)5. As shown in , the secretion of IL-1 and TNF-α increased obviously by the induction of (GLcN)5 and (GlcN)6 group than control in vivo and in vitro, and the production of IL-1 and TNF-α induced by (GLcN)6 exceeded that of (GLcN)5.
Figure 1. Effects of (GLcN)5 and (GLcN)6 on the expression of IL-1 mRNA and TNF-α mRNA in macrophages in vivo and in vitro. A represented the expression of IL-1 mRNA. B represented the expression of TNF-α mRNA. Lanes 1–3 showed intraperitoneal (i.p.) administration with physiological saline, 10 mg/kg of (GLcN)5 and 10 mg/kg of (GlcN)6 for three days, respectively. Lanes 4–6 showed murine macrophage cell treated directly with culture medium, 100 µg/ml of (GLcN)5 and 100 µg/mL of (GLcN)6 for 48 h at 37°C, respectively. Total RNA was isolated from the macrophages and PT-PCR was performed.
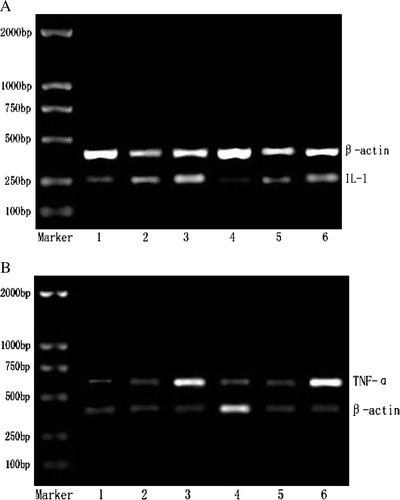
Table 3. Effects of (GLcN)5 and (GLcN)6 on gene transcription levels of IL-1 and TNF-α in macrophages.
Table 4. Effects of (GLcN)5 and (GLcN)6 on secretion of IL-1 and TNF-α in macrophages.
These results showed that (GLcN)5 and (GLcN)6 both can markedly promote the expression of IL-1 mRNA and TNF-α mRNA as well as the secretion of IL-1 and TNF-α, furthermore (GLcN)6 was much more than that of (GLcN)5. The study indicated that (GLcN)5 and (GLcN)6 firstly activated the transcription of IL-1 mRNA and TNF-α mRNA through a certain signal conduction, and then produced a great deal of IL-1 and TNF-α and secreted them from cell.
Effects of chitosan pentamer ((GLcN)5) and chitosan hexamer ((GLcN)6) on mRNA expression and secretion of interleukin-2 (IL-2) and interferon-γ (IFN-γ) in lymphocytes in vivo and in vitro
As shown in A and , (GLcN)5 and (GLcN)6 differed little with control in the expression of IL-2 mRNA in murine lymphocytes both in vivo and in vitro, and (GLcN)6 is different with (GLcN)5 in the expression of IL-2 mRNA. However, as indicated in B and , (GLcN)5 and (GLcN)6 were much stronger than control in the expression level of IFN-γ mRNA in murine lymphocytes both in vivo and in vitro, and the transcription level of (GLcN)6 was much stronger than that of (GLcN)5. The results indicated that (GLcN)5 and (GLcN)6 both couldn't promote the expression of IL-2 mRNA in lymphocytes. However, we could conclude that (GLcN)5 and (GLcN)6 both could markedly promote the expression of IFN-γ mRNA in lymphocytes, and furthermore it revealed that (GLcN)6 has stronger effect than (GLcN)5.
Figure 2. Effects of (GLcN)5 and (GLcN)6 on the expression of IL-2 mRNA and IFN-γ mRNA in lymphocytes in vivo and in vitro. A represented the expression of IL-2 mRNA. B represented the expression of IFN-γ mRNA. Lanes 1–3 showed i.p. administration with physiological saline, 10 mg/kg of (GLcN)5 and 10 mg/kg of (GLcN)6 for three days, respectively. Lanes 4–6 showed murine lymphocytes cell treated directly with culture medium, 100 µg/ml of (GLcN)5 and 100 µg/ml of (GLcN)6 for 48 h at 37°C, respectively. Total RNA was isolated from the lymphocytes and PT-PCR was performed.
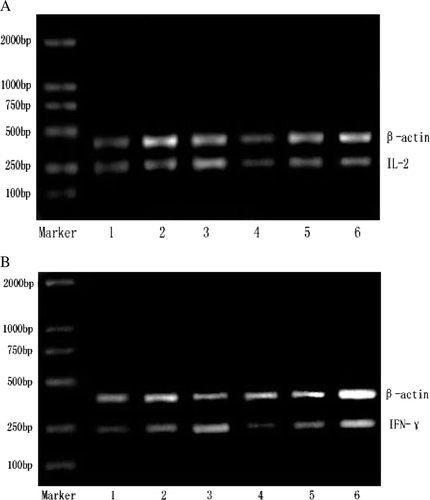
Table 5. Effects of (GLcN)5 and (GLcN)6 on gene transcription levels of IL-2 and IFN-γ in lymphocytes in vivo and in vitro.
As shown in , (GLcN)5 and (GLcN)6 differed from control in production of IL-2 in a dose of 10 mg/kg/day in vivo and in a dose of 100 µg/ml in vitro, although (GLcN)5 and (GLcN)6 could promote the production of IL-2 of spleen lymphocytes at a certain degree. The result was consistent in vivo and in vitro, (GLcN)5 and (GLcN)6 both could not markedly promote the expressive quantity of IL-2 mRNA () as well as the secretion of IL-2, furthermore that of (GLcN)6 was not much different from that of (GLcN)5. The production of IFN-γ was increased more significantly in (GLcN)5 and (GLcN)6 group than control in a dose of 10 mg/kg/day in vivo and in a dose of 100 µg/ml in vitro, and the production of IFN-γ induced by (GLcN)6 exceeded that of (GLcN)5.
Table 6. Effects of (GLcN)5 and (GLcN)6 on secretion of IL-2 and IFN-γ in lymphocytes in vivo and in vitro.
The results showed that (GLcN)5 and (GLcN)6 both can markedly promote the expression of IFN-γ mRNA as well as the secretion of IFN-γ, furthermore (GLcN)6 was much more than that of (GLcN)5. The study confirmed the stimulation of (GLcN)5 and (GLcN)6 was concerned with the production of IFN-γ induced by these two COS which was consistent with Shibata's results (Shibata, Citation1998).
Discussion
In our previous studies, we have found that proliferation of splenocytes by (GLcN)6 was obviously more effective than by (GLcN)5 and the phagocytic percentage and phagocytic indexes, and HC50 in mice by i.p. administration of (GLcN)6 were much more than that of (GLcN)5. These data have demonstrated that (GlcN)6 has stronger immune activity than (GLcN)5.
IL-1, TNF-α, IL-2 and IFN-γ are extracellular signalling cytokines which are important immunostimulatory factors in immune network that restrict the growth of tumour cells and adjust immune system. IL-1 and TNF-α are mostly expressed by monocytes and Mϕ. TNF-α and IL-1 synergise in numerous biological functions, both in vitro and in vivo. IL-1 is important in the host defence against micro-organisms that reside preferentially inside cells (Denis & Ghadirian, Citation1994; Rogers, Tripp, Schreiber, & Unanue, Citation1994). IL-1 stimulates the production of other pro-inflammatory cytokines, except TNF-α which resides upstream from IL-1 in the pro-inflammatory cytokine cascade (Butler, Maini, Feldmann, & Brennan, 1995). TNF-α is one of the most important pro-inflammatory and pro-immune cytokines. It is the most rapidly released cytokine under stress and has the ability to stimulate the production of other pro-inflammatory cytokines, such as IL-1 and IL-6 (Butler et al., Citation1995; Tracey et al., Citation1987). IL-2, originally known as “T-cell growth factor”, has immuno-stimulatory and immuno-suppressive effects on the immune system. IL-2 promotes the proliferation of naive T-cells and their maturation into Type 1 deviated lymphocytess and enhances the cytotoxicity of T-cells and promotes the production of pro-inflammatory cytokines. Furthermore, IL-2 promotes the proliferation of natural killer-cells (NK-cells) and of γ/▵ subsets of T-cells (Robertson & Ritz, Citation1990; Toribio et al., Citation1988). The main producers of IFN-γ are activated T-cells and NK-cells. IFN-γ is the major activator of Mϕ and it stimulates several macrophage functions such as tumour cell cytotoxicity (Le et al., Citation1983; Pace, Russell, Torres, Johnson, & Gray, Citation1983) and antimicrobial activity (Nathan, Murray, Wiebe, & Rubin, Citation1983). IFN-γ stimulates the production of IFN-β, IL-1α, IL-1β, TNF-α, IFN-γ, inducible protein (IP-10) and IL-12 in human and murine systems. Thus, IL-1, TNF-α, IL-2 and IFN-γ were paid attention to in this paper as for immuno-stimulatory activity of (GLcN)5 and (GLcN)6.
There were some reports concerned with gene expression of cytokines of polysaccharides. Ruan, Fu, Zhou, Hu, and Wu (Citation2006) showed the polysaccharide L-2 from Lentinus edode significantly promoted TNF-α production and immunity of the polysaccharide L-2 was associated with TNF-α mRNA expression at the transcriptional leve1. Fujihara et al. (Citation1994) reported that the level of TNF-α mRNA could be measured in 15–30 min when lipopolysaccharide (LPS) acted on macrophage-like cell J774. The level of mRNA could be tardily increased, the maximum appeared at 3–6 h and mildly decreased at 8 h. Shibata, Foster, Metzger, and Myrvik (Citation1997), Shibata et al. (1998) and Shibata et al. (Citation2001) found that chitin can activate Mϕ and make Mϕ secret IL-12, IL-18 and TNF-α, and at the same time chitin can induce spleen cells to produce IFN-γ. The effect of chitin activating Mϕ and the effect of IFN-γ inducing Mϕ to secret cytokines responded and affected each other. From these studies, we can find that the activation of immune cells and gene expression of cytokines affected each other and the immune mechanism of polysaccharides is based on the expression of cytokines in vivo and in vitro.
As for the immune mechanism of COS, there were also some reports based on cytokines in vivo or in vitro. Tokoro et al. (Citation1988) studied the anticancer effect of (GlcNAc)6 and found that (GlcNAc)6 could stimulate Mϕ to produce IL-1 and stimulate spleen cell to produce IL-2. Gorbach et al. (Citation1994) synthesised a kind of COS derivatives and the experiments showed that it could promote Mϕ to produce IL-1 and TNF-α. However, in their studies, the cytokines were measured by cell proliferation assay. Feng, Zhao, and Yu (2004) investigated the release of IL-1β and TNF-α on protein translation level in Mϕ in vitro induced by oligochitosan by ELISA, but in this study COS mixtures were used instead of monomers.
In order to further elucidate the immune mechanism of COS, especially monomers, we used (GLcN)6 monomer and (GLcN)5 monomer which were prepared in the lab as the material and the expression of IL-1, TNF-α, IL-2 and IFN-γ based on transcription level and translation level were investigated in vivo and in vitro. Regarding the dose in vitro, we did preliminary test of ConA stimulating the multiplication of thymus gland cell and concentration dose of 0, 50, 100, 200 and 500 µg/ml were used. The test showed that the transcription levels of IL-1 increase significantly in a dose-dependent manner. We chose 100 µg/ml as the best stimulatory concentration to assess the gene transcription and release of IL-1, TNF-α, IL-2 and IFN-γ in vitro. (GLcN)6 monomer and (GLcN)5 monomer both can promote the gene transcription and protein translation of IL-1, TNF-α and IFN-γ in vivo and in vitro, and the effect of (GLcN)6 was better than that of (GLcN)5. It indicated that (GLcN)6 and (GLcN)5 could promote the immune mediation obviously of mice based on the gene transcription level of IL-1,TNF-α and IFN-γ as well as the protein translation level of these cytokines. After chitin oligosaccharides or chitosan oligosaccharides was treated on mice, Mϕ were activated and adjusted mutually with other components of immune system which hastened the production of reactive medium such as reactive oxygen and NO (Tokoro et al., Citation1989). Chitin oligosaccharides or chitosan oligosaccharides could either directly kill pathogen micro-organism or tumour cells by exerting immune response, or enhance cytotoxic activity and then inhibit tumour cells production by activating T-cells, NK-cells and some other immune cells through some cytokines such as IL-1 and TNF-α (Tsukada et al., Citation1990; Wang & He, Citation2001). TNF-α could exert synergetic effect and adjust the proliferation of Th1 cell systems together with IL-1 and IL-2 in vitro collectively (Zhan & Cheers, Citation1995). These results in our studies indicate that COS induced innate immune responses by up-regulating IL-1, TNF-α and IFN-γ and then played a role on immune functions of lymphocytes. However, it was not understood that they could not induce lymphocytes to secret IL-2. The reason why the effect of (GLcN)6 on cytokines was better than that of (GLcN)5 need to be studied further.
Acknowledgements
We take this opportunity to express our sincere appreciation to Professor Dongshen Qian (College of Basic Medical Sciences, Nantong University) for his generous help with molecular biology experiments. The authors are grateful for financial sponsorship from National High Technology Research and Development (863) Program of China (2008AA10Z322), Shanghai Rising-Star Program (07QB14047), Shanghai-Unilever Research and Development Fund (05SU7094) and National Natural Science Foundation of China (Grant No. 30840067), Shanghai Key Special Project of Chongming Island Ecological Construction (07DZ12043) and Education Committee Foundation of Shanghai (2005DZ28).
References
- Butler , D.M. , Maini , R.N. , Feldmann , M. and Brennan , F.M. 1995 . Modulation of proinflammatory cytokine release in rheumatoid synovial membrane cell cultures . European Cytokine Network , 6 : 225 – 230 .
- Denis , M. and Ghadirian , E. 1994 . Interleukin-1 is involved in mouse resistance to Mycobacterium avium . Infection and Immunity , 62 : 457 – 461 .
- Du , L.G. , Bai , X.F. and Jin , Z.L. 2002 . Study on antitumor effect of chitooligosaccharides . Chinese Marine Drug , 86 : 18 – 21 .
- Feng , J. , Zhao , L.H. and Yu , Q.Q. 2004 . Receptor-mediated stimulatory effect of oligochitosan in macrophages . Biochemical and Biophysical Research Communications , 317 : 414 – 420 .
- Fujihara , M. , Ito , N. , Pace , J.L. , Watanabe , Y. , Russell , S.W. and Suzuki , T. 1994 . Role of endogenous interferon-beta in lipopolysaccharide-triggered activation of the inducible nitric-oxide synthase gene in a mouse macrophage cell line, J774 . Journal of Biological Chemistry , 269 : 12773 – 12778 .
- Gorbach , V.I. , Krasikova , I.N. , Luk'yanov , P.A. , Loenko , Y.N. , Solov'eva , T.F. Ovodov , Y.S. 1994 . New glycolipids (chitooligosaccharide derivatives) possessing immunostimulating and antitumor activities . Carbohydrate Research , 260 : 73 – 82 .
- Je , J.Y. , Park , P.J. and Kim , S.K. 2004 . Free radical scavenging properties of hetero-chitooligosaccharides using an ESR spectroscopy . Food and Chemical Toxicology , 42 : 381 – 387 .
- Jeon , Y.J. and Kim , S.K. 2001 . Potential immuno-stimulating effect of antitumoral fraction of chitosan oligosaccharides . Journal of Chitin and Chitosan , 6 : 163 – 167 .
- Jeon , Y.J. and Kim , S.K. 2002 . Antitumor activity of chitosan oligosaccharides produced in ultrafiltration membrane reactor system . Journal of Microbiology and Biotechnology , 12 : 503 – 507 .
- Jeon , Y.J. , Shahidi , F. and Kim , S.K. 2000 . Preparation of chitin and chitosan chitooligosaccharides and their applications in physiological functional foods . Food Reviews International , 16 : 159 – 176 .
- Kendra , D.F. , Christian , D. and Hadwiger , L.A. 1989 . Chitosan oligomers from Fusarium solani/pea interactions, chitinase/b-glucanase digestion of sporelings and from fungal wall chitin actively inhibit fungal growth and enhance disease resistance . Physiological and Molecular Plant Pathology , 35 : 215 – 230 .
- Kendra , D.F. and Hadwiger , L.A. 1984 . Characterization of the smallest chitosan oligomer that is maximally antifungal to Fusarium solani and elicits posatin formation in Pisum sativum . Experimental Mycology , 8 : 276 – 281 .
- Le , J. , Prensky , W. , Yip , Y.K. , Chang , Z. , Hoffman , T. Stevenson , H.C. 1983 . Activation of human monocyte cytotoxicity by natural and recombinant immune interferon . Journal of Immunology , 131 : 2821 – 2826 .
- Nathan , C.F. , Murray , H.W. , Wiebe , M.E. and Rubin , B.Y. 1983 . Identification of interferon-gamma as the lymphokine that activates human macrophage oxidative metabolism and antimicrobial activity . Journal of Experimental Medicine , 158 : 670 – 689 .
- Pace , J.L. , Russell , S.W. , Torres , B.A. , Johnson , H.M. and Gray , P.W. 1983 . Recombinant mouse gamma interferon induces the priming step in macrophage activation for tumor cell killing . Journal of Immunology , 130 : 2011 – 2013 .
- Park , P.J. , Je , J.Y. , Byun , H.G. , Moon , S.H. and Kim , S.K. 2004 . Antimicrobial activity of hetero-chitosans and their oligosaccharides with different molecular weights . Journal of Microbiology and Biotechnology , 14 : 317 – 323 .
- Robertson , M.J. and Ritz , J. 1990 . Biology and clinical relevance of human natural killer cells . Blood , 76 : 2421 – 2438 .
- Rogers , H.W. , Tripp , C.S. , Schreiber , R.D. and Unanue , E.R. 1994 . Endogenous IL-1 is required for neutrophil recruitment and macrophage activation during murine listeriosis . Journal of Immunology , 53 : 2093 – 2101 .
- Ruan , Z. , Fu , X.F. , Zhou , Q.C. , Hu , X.B. and Wu , M.C. 2006 . Effect of polysaccharide L-2 from lentinus edodes on TNF-α and IL-2 . Food Science , 27 : 223 – 226 .
- Shibata , Y. , Foster , L.A. , Kurimoto , M. , Okamura , H. , Nakamura , R.M. Kawajiri , K. 1998 . Immunoregulatory roles of IL-10 ininnate immunty: IL-10 inhibits macrophage production of IFN-gamma-inducing factors but enhances NK cell production of IFN-gamma . Journal of Immunology , 161 : 4283 – 4288 .
- Shibata , Y. , Foster , L.A. , Metzger , W.J. and Myrvik , Q.N. 1997 . Alveolar macrophage priming by intravenous administration of chitin particels, polymers of N-acetyl-D-glucosamine in mice . Infection and Immunity , 65 : 1734 – 1741 .
- Shibata , Y. , Honda , I. , Justice , J.P. , Scott , M.R.V. , Nakamura , R.M. and Myrvik , Q.N. 2001 . Th1 adjuvant N-acetyl-D-glucosamine polymer up- regulates Thl immunity but down-regulates Th2 immunity against a mycobacterial protein (MPB-59) in interleukin-10-knockout and wild-type mice . Infection and Immunity , 69 : 6123 – 6130 .
- Suzuki , K. , Mikami , T. , Okawa , Y. , Tokoro , A. , Suzuki , S. and Suzuki , M. 1986 . Antitumor effect of hexa-N-acetylchitohexaose and chitohexaose . Carbohydrate Research , 151 : 403 – 408 .
- Tokoro , A. , Kobayashi , M. , Tatewaki , N. , Suzuki , K. , Okawa , Y. Mikami , T. 1989 . Protective effect of N-acetyl-chito-hexaose on Listeria monocytogenes infection in mice . Microbiology and Immnology , 33 : 357 – 367 .
- Tokoro , A. , Suzuki , K. , Matsumoto , T. , Mikami , T. , Suzuki , S. and Suzuki , M. 1988 . Chemotactic response of human neutrophils to N-cetyl chitohexaose in vitro . Microbiology and Immunology , 32 : 387
- Toribio , M.L. , de la Hera , A. , Borst , J. , Marcos , M.A. , Marquez , C. Alonso , J.M. 1988 . Involvement of the interleukin 2 pathway in the rearrangement and expression of both alpha/beta and gamma/delta T cell receptor genes in human T cell precursors . Journal of Experimental Medicine , 168 : 2231 – 2249 .
- Tracey , K.J. , Fong , Y. , Hesse , D.G. , Manogue , K.R. , Lee , A.T. Kuo , G.C. 1987 . Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia . Nature , 330 : 662 – 664 .
- Tsukada , K. , Matsumoto , T. , Aizawa , K. , Tokoro , A. , Naruse , R. Suzuki , S. 1990 . Antimetastatic and growth-inhibitory effects of N-acetylchitohexaose in mice bearing Lewis lung carcinoma . Japanese Journal of Cancer Research , 81 : 259 – 265 .
- Wang , F.Y. and He , Y.S. 2001 . Study on antitumor effect of water-soluble chitosan . Journal of Chinese Biochemical Drug , 22 : 21 – 22 .
- Wu , G.J. and Tsai , G.J. 2004 . Cellulase degradation of shrimp chitosan for the preparation of a water-soluble hydrolysate with immunoactivity . Fisheries Science , 70 : 1113 – 1120 .
- Xia , W.S. 2001 . Advance in enzymatic modification of chitosan . Journal of Wuxi University of Light Industry , 20 : 550 – 554 .
- Zhan , Y. and Cheers , C. 1995 . Differential induction of macrophage-derived cytokines by live and dead intracellular bacteria in vitro . Infection and Immunology , 63 : 720 – 727 .