Abstract
Food processing induces protein modifications by Maillard reactions. This generates advanced glycation end products (AGEs) that are known to affect human health. Therefore, it is of interest to monitor AGEs in food products. Currently Maillard products are detected by measuring fluorescence. However, several AGEs are non-fluorescent, while non-AGE components can exhibit autofluorescence. Therefore, specific AGE immunodetection was investigated. Immunofluorescence of AGEs as well as autofluorescence were determined in cookie extracts. Autofluorescence increases with baking time and sugar level, where AGE immunofluorescence increases with baking time until 20 minutes. Replacing sucrose by fructose confirmed the higher reactivity of fructose in AGE formation. The pattern of autofluorescence correlates well with the acrylamide and antioxidant activity. However, the immunodection of AGEs did not show such a correlation. At higher baking times the autofluorescence probably results from the generation of non-proteineious compounds. The immunofluorescence reduction likely results from the transient character of AGE epitopes.
Introduction
Industrial processing or cooking of food is known to promote the formation of flavours, aromas and colours that are characteristic of baked, roasted and boiled foods. The generation of such features is directly correlated to the generation of Maillard products (Ames, Citation1998). Maillard products are formed after a series of complex reactions that can be divided into three steps (Machiels & Istasse, Citation2002).
In the first step, protein glycation is initiated by a condensation reaction between the carbonyl group of a reducing sugar and an unprotonated amine group of a protein (preferably lysine, arginine and asparagine) to form a Schiff base. This is subsequently stabilised after rearrangement into Amadori products or Heyns products according to the type of sugar involved (aldoses or ketoses). The resulting early glycated products are submitted to the second stage during which additional complex rearrangements take place (such as oxidation, enolisation, dehydration, condensation and fragmentation), leading to highly reactive intermediates of which the identity has been established, as well as poorly characterised species (Kanska & Boratynski, Citation2002). The final stage is defined by further reactions (cross-linkages and polymerisation) of the resulting reactive intermediates with free amino groups, leading to the emergence of highly coloured, water insoluble polymeric compounds (melanoidins) and a variety of advanced glycation end products (AGEs; Miller & Gerrard, Citation2005).
The food industry is more and more interested in controlling Maillard reaction products by modulating the glycation process (Maillard reaction) to upgrade the functional properties of proteins such as their emulsifying, foaming and gelling capacity as well as their solubility (Kim, Choi, Shin, & Moon, Citation2003; Oliver, Melton, & Stanley, Citation2006). In addition, Maillard products can exhibit antioxidant, antimutagenic or anticarcinogenic activities that are beneficial for human health (Friedman, Citation2005; Sun, Hayakawa, Puangmanee, & Izumori, Citation2006). On the other hand, more and more studies point at the implication of AGEs in causing a variety of dietary or age-related diseases like diabetes, atherosclerosis, Alzheimer's disease (Peppa, Uribarri, & Vlassara, Citation2003), chronic diseases associated with underlying inflammation (Uribarri et al., Citation2005) as well as cancer (Van Heijst, Niessen, Hoekman, & Schalkwijk, Citation2005). Other detrimental effects of the Maillard reaction such as its potential contribution to increase protein allergenicity and the production of toxic and carcinogenic compounds such as the low-molecular weight products, keto-aldehydes, glyoxal, methylglyoxal, 3-deoxyglucosone, heterocyclic amines and acrylamide renders their presence in food products undesirable (Chung & Champagne, Citation1999; Gruber, Becker, & Hofmann, Citation2005).
This increasing role of AGEs in human health issues strengthens the importance to monitor their presence in food products. Such monitoring activities as set out in European Recommendation 2007/331/EC (European Commission, Citation2007) can support the development of new industrial processes that decrease or inhibit the formation of AGEs or acrylamide.
Fluorescence spectroscopy has been reported as a common and reliable technique to measure the emergence of Maillard products during food processing such as baking (Birlouez-Aragon, Locquet, De St. Louvent, Jouan-Rimbaud Bouveresse, & Stahl, Citation2005; Leclere & Birlouez-Aragon, Citation2001). However, fluorescence spectroscopy does not allow to make any distinction between early, intermediate and late Maillard products (Ahmed, Thorpe, & Baynes, Citation1986; Dyer, Blackledge, Thorpe, & Baynes, Citation1991). Furthermore, whereas several structures of AGEs elucidated so far exhibit fluorescence properties (e.g. pentosidine, crossline, glucosepane) many others do not (e.g. N-carboxymethyl lysine (CML), N-carboxyethyl lysine (CEL), pyrraline, 3-deoxyglucosone-imidazolone, methylglyoxal-imidazolone, argpyrimidine).
Therefore, immunological detection using antibodies specific for AGEs might be more suitable to reveal AGE formation in food products. However, immunodetection of AGEs in food products so far have been rare (Goldberg et al., Citation2004; Tauer, Hasenkopf, Kislinger, Frey, & Pischetsrieder, Citation1999). Most studies utilising antibodies specific for AGEs have been performed in the biomedical area, where the immunological detection of AGEs in the serum of diabetic patients has been proposed as a diagnostic tool, a potential disease-related marker (Takeuchi et al., Citation2001). Since AGEs emerging from food processing are absorbed as free adducts after digestion they are likely to constitute a major source of intracellular and plasma AGEs (Faist & Erbersdobler, Citation2001; Foerster & Henle, Citation2003). It can therefore be expected that the antibodies used for the detection of intracellular AGEs in biomedical studies (Horiuchi, Araki, & Morino, Citation1991; Krajcovicova-Kudlackova, Sebekova, Schinzel, & Klvanova, Citation2002) might also be able to detect food AGEs.
In this study such antibodies have been employed for the immunodetection of AGEs in cookies. Monitoring of AGE formation in cookies prepared according to different recipes and with applying different baking times allowed us to establish multiresponse models. This type of modelling can be a powerful tool to improve our understanding of the evolution of AGEs during food processing.
In addition, this cookie material was previously analysed to evaluate the progression of the antioxidant capacity of the formed Maillard products, as well as the level of acrylamide (Summa, Wenzl, Brohee, DeLaCalle, & Anklam, 2006), which had demonstrated the existence of a correlation between the formation of both. The autofluorescence as well as the immunoflourescence at 580 nm was determined in the cookie extracts and the results were compared to the acrylamide content and the antioxidant activities as determined by Summa et al. (2006).
Materials and methods
Cookies
The cookie samples used in this study were previously analysed for the determination of acrylamide content and antioxidant activity (Summa et al., Citation2006). The composition of the cookies is summarised in . Ground cookies stored at –20°C were used for protein extraction.
Table 1. Cookie recipes.
Extraction
Extraction of proteins from cookies was performed as follows: 250 mg of ground cookies were weighted into 15 ml Falcon tubes to which 4.75 ml extraction buffer (containing 0.05% Tween 20, 1% SDS, 5% β-mercaptoethanol and 50 mM Tris-HCl at pH 7.4) was added. Extraction was carried out overnight at room temperature under gentle agitation. Subsequently, the tubes were centrifuged for 20 minutes at 3000 g at room temperature and the supernatant that included extracted proteins was collected.
Protein quantitation
Proteins from cookie extracts were quantified with the 2-D Quant kit (GE Healthcare, Uppsala, Sweden) based on trichloroacetic acid/acetone precipitation according to the manufacturer's instructions.
Maillard product levels determined by autofluorescence detection
Cookie extracts from the different recipes were spotted in triplicate onto a nitrocellulose membrane (Biorad-laboratories, Hercules, CA, USA) in equal quantities (1.5 µg protein). After drying, the membranes were saturated at room temperature for 1 hour with diluted Sea Block Blocking Buffer (Pierce Biotechnology, Inc., Rockford, IL, USA) composed of non-mammalian protein (steelhead salmon serum) to reduce the risk of non-specific interactions. The membranes were rinsed three times for 10 minutes in Tris buffered saline (TBS) supplemented with 0.05% Tween 20 before being inserted into a 96 well-plate device for fluorescence measurements (Perkin Elmer, Waltham, MA, USA). Autofluorescence was measured using a multilabel plate reader Victor (Perkin Elmer) employing an excitation and emission wavelength of 530 nm and 580 nm, respectively.
Advanced glycation end products’ (AGEs) determination by immunochemical dot blot analysis
After determination of the autofluorescence emerging from the Maillard products, AGEs were detected on the same membranes by utilising an anti-AGE antibody. The membranes were rinsed in TBS/0.05% Tween 20 and incubated for 1 hour with 0.5 µg ml–1 of polyclonal rabbit anti-AGE antibodies (AbCAM, Cambridge, UK) prepared in TBS/0.05% Tween 20. The membranes were subsequently washed three times for 10 minutes in TBS/0.05% Tween 20 before being incubated 1 hour with a 1500 times diluted fluorescent anti-rabbit antibody – Alexa fluor 555 (Molecular probes, Eugene, OR, USA). Three washes of 10 minutes preceded the insertion of the membranes in the 96 well-plate device. The immunofluorescence was subsequently measured with the multilabel plate reader Victor employing the same excitation and emission wavelengths as mentioned above (530 nm/580 nm).
Statistical data analysis
Fluorescence intensity data are expressed as means±1 SD resulting from three readings of sample triplicates. Presence of outliers in the fluorescence data population was determined with Grubb's test and the normality of the distribution was tested according to Kolmogorov–Smirnov, which allowed the use of ANOVA and Student's t-tests. ANOVA tests (single and two factors) were performed to study the variations triggered by the different independent multilevel variables: baking time (5, 10, 15 and 20 minutes) and sugar content (0, 9, 16 and 28%) as quantitative factors, and the type of sugar (fructose, sucrose) as a qualitative factor. Multiple comparisons were investigated with two-factor ANOVA for repeated measurements. When the F-value was found to be significant, post hoc multiple comparisons were performed by Student's t-test. Statistical analysis was confirmed using response surface modelling and an experimental fractional factorial design created with the software Modde version 8 (Umetrics Academy, Umeå, Sweden) was used to generate 3D contour plot models. The data sets containing autofluorescence and the background corrected immunofluorescence values were used for modelling.
Results and discussion
Protein quantitation
Protein quantitation was performed after the extraction of proteins from the cookie material. This was required since equal amounts of protein were to be analysed for a comparison of all samples by means of autofluorescence and AGE immunofluorescence.
The determination of the protein content in the sample extracts also allowed analysing the effects that different baking conditions and different saccharide contents exerted on the extractability of proteins. shows the protein concentration in all cookie extracts, and points out that the extractable protein concentration decreased with increasing baking time. This significant decrease (p<0.001) most likely resulted from a combination of protein degradation and lower extraction efficiency due to polymerisation and the creation of intermolecular resistant structures all triggered by the heat treatment. Our data are in agreement with previous studies reporting a decrease of peanut proteins that can be extracted after submission to heat treatments (Chassaigne, Brohee, Norgaard, & van Hengel, Citation2007; Poms, Capelletti, & Anklam, Citation2004). Furthermore, our findings are in agreement with a study on wheat protein in dough reporting a decrease of protein content during heat treatment that was found to be related to a decrease in solubility and the formation of disulfide bond interactions (Rumbo, Chirdo, Fossati, & Anon, Citation2001).
Table 2. Protein concentration of cookie extracts.
In addition to this, the level of sugar was found to significantly (p<0.001) affect the protein extraction whereby higher sugar concentrations in the dough, resulted in a lower efficiency of protein extraction from the cookies. This reduction is potentially due to a saccharide-mediated polymerisation that negatively affects solubility. The presence of saccharides has been reported to protect the protein structure from heat denaturation but on the other hand saccharides bind to the proteins leading to protein/sugar polymers (Divair, Takeuchi, & Cunha, Citation2005).
Maillard products determined by fluorescence detection at 580 nm
In contrast to Maillard products formed during the ‘early stage’, AGEs have rarely been quantified in food unless in protein–carbohydrates models (Kislinger et al., Citation2003). The few assessements of AGEs performed on food were based on immunotechniques with a monoclonal antibody specific for a single AGE, CML, which is claimed to be a marker of the late stage of glycation (Goldberg et al., Citation2004). In this study, we used a polyclonal antibody capable of binding different AGEs (including CML and imidazolone) to follow the evolution of AGE production within a cookie matrix.
Before proceeding to the detection of AGEs by immunofluorescence at excitation/emission wavelengths of 530/580 nm, the natural autofluorescence of Maillard products was investigated in cookie extracts of all four recipes. Although fluorescence of Maillard products is commonly detected at an emission wavelength of 420 nm (Leclere & Birlouez-Aragon, Citation2001; Matiacevich, Santagapita, & Buera, Citation2005), a significant fluorescence could also be observed at 580 nm. Since this autofluorescence from cookie extracts at 580 nm interferes with an AGE specific signal, it is required to be substracted from the immunofluorescence signal measured at the same wavelength.
Analysis by Student paired t-tests demonstrated that the immunofluorescence was always significantly higher than the autofluorescence for all different recipes and baking times, with a single exception (protein extract of the cookie sample that contained 37% sucrose and was baked for 30 minutes). We therefore conclude that food AGEs can indeed be detected by utilising the polyclonal antibodies employed in this study.
Influence of baking time on autofluorescence and immunodetection of advanced glycation end products (AGEs)
Both the autofluorescence and the immunofluorescence were determined by analysing equal amounts of protein from all cookie extracts. shows the mean of the autofluorescence and ΔAGE fluorescence values as obtained after analysis of the samples of recipe 1. The values for ΔAGE fluorescence represent the difference of intensities between immunofluorescence and autofluorescence measured at 580 nm.
Table 3. Autofluorescence and ΔAGE fluorescence (immunofluorescence minus autofluorescence) intensity in cookie extracts of recipe 1.
The autofluorescence clearly increases with increasing the baking time from 5 to 20 minutes. This increase was found to be highly significant (p<0.0001). This effect of baking time on the development of Maillard products as detected by autofluorescence is in line with the increase in acrylamide content in the same cookies as reported by Summa et al. (2006) and is also in accordance with previous studies on the determinant factors influencing acrylamide formation (Bråthen & Knutsen, Citation2005). The kinetics of the Maillard reaction are known to depend on the duration of baking (Charissou, Ait-Ameur, & Birlouez-Aragon, 2007) and are correlated to the variation of water activity, which decreases during baking to reach optimal conditions for the Maillard reaction (Hurrell & Carpenter, Citation1977). This was confirmed by Summa et al. who showed that the increase of acrylamide content was correlated to the decrease of moisture during baking (Summa et al., Citation2006).
When the detection was performed with the specific anti-AGE antibody, the total AGE fluorescence (immunofluorescence not corrected for autofluorescence) presented overall the same evolution as the autofluorescence of Maillard products with intensification during baking (data not shown). The immunofluorescence signal (as corrected by subtraction of the autofluorescence resulting in ▵AGE fluorescence values) is reported in . This table shows that for recipe 1, ▵AGE fluorescence increased gradually from 5 to 20 minutes. We therefore conclude that employing the method developed in this study has revealed an increase of both the autofluorescence signal (at 580 nm) as well as the immunological detection of AGEs. This confirms the suitability of the method, since our results are in full agreement with the well-known fact that baking increases the formation of Maillard products (Charissou et al., Citation2007).
Influence of baking time and sucrose content
The samples of recipe 2 were used to study the effect of two different parameters on the formation of Maillard products. Both the autofluorescence and the ▵AGE fluorescence were determined for the samples of recipe 2 that varied in baking time (four different baking times) and sucrose content (five different concentrations).
The effect of the variation of those two factors on the evolution of autofluorescence and ▵AGE can be summarised and modelled with the use of a factorial design. A full factorial design set up for the autofluorescence model fitted the experiments by 84.2% (R 2) while the fractional factorial design established for the ▵AGE immunofluorescence model reached 78.4% (R 2). A and B show the resulting response surface models for autofluorescence and the ▵AGE immunofluorescence, respectively. The first model is in complete agreement with a previous study that claimed that Maillard products accumulate with longer baking times and higher sugar contents (Ameur, Mathieu, Lalanne, Trystram, & Birlouez-Aragon, 2007). In contrast, model 1B that is based on the ▵AGE values is clearly distinct. This model reveals that detection of AGE reactive epitopes has a non-linear relation with both baking time and sucrose content. For autofluorescence, the highest values are associated with the highest baking time in combination with the highest sucrose percentage, while this is clearly not the case for the ▵AGE values where the highest values are associated with high baking times or with high sucrose levels, but not a combination of both (). In other words, the strong interaction that exists between baking time and sucrose in both models is associated with an amplification of the autofluorescence during baking while ▵AGE detection is negatively affected when both sucrose content and baking time increase (). This decrease of ▵AGE fluorescence observed after prolonged baking at higher sucrose concentrations might imply that the antibody cannot access the specific AGE epitopes due to the high polymerisation of the glycated proteins. Another explanation could lie in a transient nature of the AGE epitopes recognised, due to further Maillard reactions that might transform immunoreactive AGEs into other products, e.g. melanoidins. This evolution also suggests that the continuous increase of autofluorescence with increased baking times should not all be attributed to AGEs, but might be caused by an increase of other chromophores derived from, for instance, the caramelisation of saccharides. Indeed, at the baking temperature applied (180°C), caramel aroma and brown-coloured products are likely to be formed from the thermal degradation of saccharides (occurring with melting temperatures of around 102 and 132°C for fructose, 146–165°C for glucose and 185–190°C for sucrose) (Hurtta, Pitkanen, & Knuutinen, Citation2004). Caramelisation induces the emergence of aldehydes and dicarbonyl groups, precursors of compounds that strongly absorb in UV leading to an overestimation of the Maillard reaction. Subsequent condensation and polymerisation of those compounds into high molecular mass components contribute to the increase in browning (Buera, Chirife, Resnik, & Wetzler, Citation1987; Kanska & Boratynski, Citation2002). Since caramelisation takes place when sugars are heated alone and/or during baking of food with a high sugar content, this is likely to explain the high autofluorescence observed for the sample containing 37% of sucrose.
Figure 1. 3D contour plot models obtained by fractional factorial design and based on analytical data obtained for samples of recipe 2. The models represent the correlation between the amount of sucrose, baking time and (A) autofluorescence response or (B) ΔAGE fluorescence.
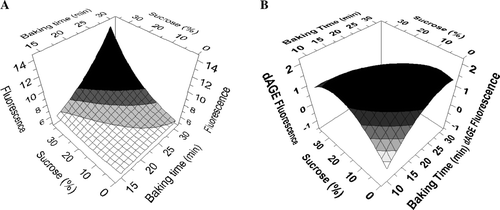
In the absence of carbohydrates like sucrose, other ingredients possessing carbonyl groups, such as oxidised lipids, are known to be able to react with amino groups to produce AGEs and take over the Maillard reaction (Hidalgo, Alaiz, & Zamora, 1999; Zamora & Hidalgo, Citation2005). B shows that in the samples without sucrose, the ▵AGE values show the strongest increase during baking. This observation supports data reported by Goldberg and co-workers on the evaluation of the AGEs in food products in which fatty food products were shown to be richer in AGEs than carbohydrate-rich foods (Goldberg et al., Citation2004). Moreover, it has been shown that proteins treated with oxidised lipids lead to more fluorescence than protein modified by carbohydrates (Hidalgo et al., Citation1999) in contrast to the effect on browning.
Effect of the type of sugar
Besides baking time and sugar percentage, the Maillard reaction and therefore the production of AGEs, is influenced by the type of sugar included in the recipe. The set of cookies produced and investigated by Summa et al. (Citation2006) included cookies containing either fructose or sucrose as ingredients. Sucrose is commonly used in food products like cookies, while also fructose constitutes a main component of the human diet and is more and more used as a sweetener in food products (Hanover & White, Citation1993). We therefore made an evaluation of the effect that changing sucrose for fructose has on the development of autofluorescence and ΔAGE signals by analysing samples from recipes 3 and 4 that differ only in this ingredient.
The variation of the two factors, baking time and the percentage of saccharide, in recipes 3 and 4, was modelled with the use of fractional factorial design to evaluate their effect on the evolution of autofluorescence and ▵AGE. The fit with the experimental data for the autofluorescence and the ΔAGE fluorescence systems was good (88.4 and 92.4%, respectively) and showed a satisfactory efficiency (Geff) exceeding 70% (72.67 and 73.7%, respectively for Models 1 and 2). The resulting models that are based on the data set of both recipes are depicted in . Increasing baking time appears to induce more autofluorescence when fructose was included in the dough (recipe 4) compared to sucrose (recipe 3) (A and B). But, whereas high autofluorescence correlated with long baking times, ▵AGE values show a decline at the highest baking time (C and D). Clearly, the values for ΔAGE fluorescence do not point at a linear relationship between ΔAGE fluorescence and baking time. Instead, they show that the highest value is reached around baking times of 10–15 minutes.
Figure 2. Response surface diagrams obtained by factorial design and based on analytical data obtained for samples of recipes 3 and 4. The first two models represent the correlation between the autofluorescence response, baking time and (A) the amount of fructose or (B) the amount of sucrose. The last two models represent the correlation between ΔAGE fluorescence, baking time and (C) the amount of fructose or (D) the amount of sucrose.
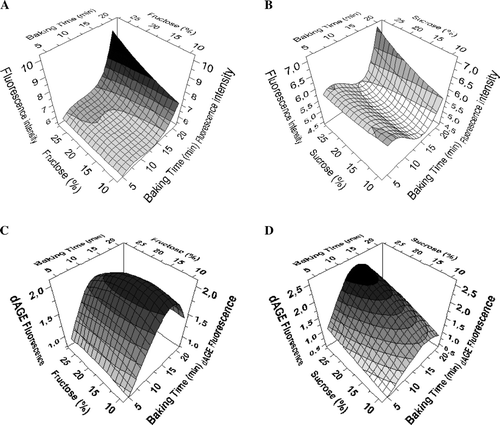
Fructose as a reducing sugar is more reactive than sucrose, which needs to be decomposed into fructose and glucose to interact with reactive amino groups. The small inflexion in the fluorescence at 10 minutes for sucrose, which is apparent in B, seems to indicate that the fluorescence decreases prior to the start of sucrose decomposition, which is required to sustain the progression of the Maillard reaction. Furthermore, the molar ratio sugar to protein is higher for fructose at any percentage used compared to sucrose, which contributes to the difference in the rate of the Maillard reaction. The type of sugar has already been associated with the production of fluorescence and Maillard product formation (Ameur et al., Citation2007; Pomeranz, Johnson, & Shellenberg, Citation1962). Indeed, having fructose instead of sucrose in the recipe has a clear effect on the autofluorescence exhibited at 20 min baking time. In contrast to this, ΔAGE levels are not drastically affected when sucrose is replaced by fructose unless around 15 minutes of baking. This difference might be explained by the fact that fructose is involved earlier in the caramelisation process than sucrose. Caramelisation has been reported to represent up to 40% of total UV-absorbance and 10–36% of brown colour development for fructose (Ajandouze & Puigserver, Citation1999; Ajandouze, Tchiakpel, Dalle Ore, Benajiba, & Puigserver, Citation2001), and is likely to be an important factor in the increase in autofluorescence, especially in the fructose containing samples.
Interestingly, a comparison of C and D reveals that while increasing the sucrose percentage always results in increased ΔAGE levels, this is not the case for fructose where maximum ΔAGE levels were detected in samples containing sugar levels between 15 and 20%.
Since in contrast to fructose, sucrose is not a reducing sugar, sucrose containing samples were expected to exhibit lower ΔAGE levels. This indeed holds true for samples containing lower quantities of sugar.
Correlation to acrylamide and antioxidant activity
The Maillard reaction is associated with the development of compounds with ambivalent activities: carcinogenic and antioxidant (Bressa, Tesson, Dalla Rosa, Sensidoni, & Tubaro, Citation1996; Van Nguyen, Citation2006). This has been demonstrated by Summa et al. (2006) who highlighted a correlation between the acrylamide content and the antioxidant activity in the same cookies that were used in the present study.
Therefore, it was of interest to investigate potential correlations of AGE detection with either the antioxidant activity and/or the acrylamide data reported by Summa et al. (2006). For this purpose, the acrylamide and antioxidant activity was plotted against autofluorescence, immunofluorescence and ΔAGE signals. This revealed a correlation between acrylamide and antioxidant activity with both autofluorescence as well as immunofluorescence. This correlation was especially apparent when the reducing sugar fructose was used in the recipe. visualises this correlation and depicts the immunofluorescence versus acrylamide content (A), and the relation between immunofluorescence and antioxidant activity (B) based on the data obtained after analysis of samples of recipe 4 with different percentages of fructose.
Figure 3. Correlations between the immunofluorescence measured (=the sum of autofluorescence and ΔAGE fluorescence) with (A) the acrylamide content and (B) the antioxidant activity for recipe 4 (fructose). Curves relate to data obtained for samples with a fructose content of 0, 9, 16 or 28%.
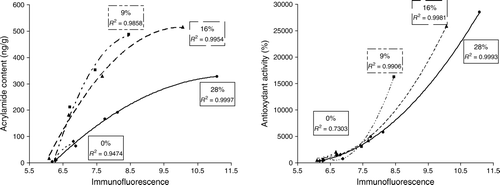
With increasing baking time, the relationship between immunofluorescence and acrylamide content reaches a plateau (parabolic curve). In contrast to this, the relationship between immunofluorescence and antioxidant activity shows an increase (hyperbole). This is in agreement with the observations made by Morales and Jimenez-Perez (2001) linking the formation of fluorescent substance to the production of compounds possessing antioxidant capacity (Morales & Jimenez-Perez, Citation2001; Yen & Chung, Citation1999). In all cases, the shapes of the curves change with increasing percentage of saccharides being either fructose or sucrose ().
No such correlation could be detected between ΔAGE levels and acrylamide content, or between ΔAGE levels and antioxidant activity. In fact, the correlations observed from immunofluorescence with acrylamide content and antioxidant activity (as shown above) are based on the major contribution of autofluorescence to the immunofluorescence signal. The absence of any apparent correlation between antioxidant activity and ΔAGE levels suggests that the autofluorescence at 580 nm is mainly derived from molecules with fluorescent properties other than the AGEs that were immunologically detected.
Conclusions
Fluorescence detection is a commonly used technique to detect Maillard products but it is not specific for AGE detection (Bellmunt, Portero, Pamplona, Muntaner, & Prat, Citation1995) and does not allow to discriminate between different stages or the progression of protein modification resulting from Maillard reactions. This paper reports the detection of AGEs in food matrices with AGE-specific antibodies. The exposure to heating was studied which revealed that increasing the period of heating resulted in an increase and subsequent decrease of the immunologically reactive AGE epitopes. This suggests that first, the heating generates the epitopes, while after prolonged heating they are destroyed and/or become unaccessible for the antibodies. Potentially, heat-mediated transformation of (immunoreactive) AGEs into other products that might exhibit fluorescent properties contribute to the observed increase of autofluorescence. The variations in the detection of Maillard products by autofluorescence and immunological detection of AGEs also point at the potential participation of two other pathways that are not directly related to AGE formation, such as caramelisation and lipid oxidation that might both contribute to the fluorescence intensity by the generation of fluorescent compounds.
The presence of AGEs in food products raises concern since only one-third of absorbed dietary AGEs (from browned foods) are excreted, while the rest is presumably incorporated into body tissues and is suspected to be responsible for food and age-related diseases (Koschinsky et al., Citation1997; Levi & Werman, Citation1998; Suarez, Rajaram, Oronsky, & Gawinowiczj, Citation1989). Therefore, it is clear that a better understanding and monitoring of AGE production during food production is required. The transient nature of the immunoreactive AGE epitopes as described in this study could provide an important tool to determine the extent of the Maillard reaction on proteins present in food products. In contrast to acrylamide formation and antioxidant activity that both show a steady increase with increasing heat exposure, this new method based on immunofluorescence detection provides a deeper insight into the evolution of the advanced stages of AGE formation during the baking process.
Acknowledgements
The authors would like to thank Dr Franz Ulberth for helpful discussions and valuable comments on the manuscript.
References
- Ahmed , M.U. , Thorpe , S.R. and Baynes , J.W. 1986 . Identification of N epsilon-carboxymethyllysine as a degradation product of fructoselysine in glycated protein . Journal of Biological Chemistry , 261 : 4889 – 4894 .
- Ajandouze , E.H. and Puigserver , A. 1999 . Nonenzymatic browning reaction of essential amino acids: Effect of pH on caramelization and Maillard reaction kinetics . Journal of Agricultural and Food Chemistry , 47 : 1786 – 1793 .
- Ajandouze , H. , Tchiakpel , S. , Dalle Ore , F. , Benajiba , A. and Puigserver , A. 2001 . Effects of pH on caramelization and Maillard reaction kinetics in fructose-lysine model systems . Journal of Food Science , 66 : 926 – 931 .
- Ames , J.M. 1998 . Applications of the Maillard reaction in the food industry . Food Chemistry , 62 : 431 – 439 .
- Ameur , L.A. , Mathieu , O. , Lalanne , V. , Trystram , G. and Birlouez-Aragon , I. 2007 . Comparison of the effects of sucrose and hexose on furfural formation and browning in cookies baked at different temperatures . Food Chemistry , 101 : 1407 – 1416 .
- Bellmunt , M. , Portero , M. , Pamplona , R. , Muntaner , M. and Prat , J. 1995 . Age-related fluorescence in rat lung collagen . Lung , 173 : 177 – 185 .
- Birlouez-Aragon , I. , Locquet , N. , De St. Louvent , E. , Jouan-Rimbaud Bouveresse , D. and Stahl , P. 2005 . Evaluation of the Maillard reaction in infant formulas by means of front-face fluorescence . Annals of the New York Academy of Sciences , 1043 : 308 – 318 .
- Bråthen , E. and Knutsen , S.H. 2005 . Effect of temperature and time on the formation of acrylamide in starch-based and cereal model systems, flat breads and bread . Food Chemistry , 92 : 693 – 700 .
- Bressa , F. , Tesson , N. , Dalla Rosa , M. , Sensidoni , A. and Tubaro , F. 1996 . Antioxidant effect of Maillard reaction products: Application to a butter cookie of a competition kinetics analysis . Journal of Agricultural and Food Chemistry , 44 : 692 – 695 .
- Buera , P. , Chirife , J. , Resnik , S.L. and Wetzler , G. 1987 . Non enzymatic browning in liquid model systems of high water activity, kinetics of color changes due to MAILLARD's reaction between different single sugars and glycine and comparison with caramelization browning . Journal of Food Science , 52 : 1063 – 1067 .
- Charissou , A. , Ait-Ameur , L. and Birlouez-Aragon , I. 2007 . Kinetics of formation of three indicators of the Maillard reaction in model cookies: Influence of baking temperature and type of sugar . Journal of Agricultural and Food Chemistry , 55 : 4532 – 4539 .
- Chassaigne , H. , Brohee , M. , Norgaard , J.V. and van Hengel , A.J. 2007 . Investigation on sequential extraction of peanut allergens for subsequent analysis by ELISA and 2D gel electrophoresis . Food Chemistry , 105 : 1671 – 1681 .
- Chung , S. and Champagne , E. 1999 . Allergenicity of Maillard reaction products from peanut proteins . Journal of Agricultural and Food Chemistry , 47 : 5227 – 5231 .
- Divair , C. , Takeuchi , K.P. and Cunha , R.L. 2005 . Effect of sucrose addition and heat treatment on egg albumen protein gelation . Journal of Food Science , 70 : 230 – 238 .
- Dyer , D.G. , Blackledge , J.A. , Thorpe , S.R. and Baynes , J.W. 1991 . Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo . Journal of Biological Chemistry , 266 : 11654 – 11660 .
- European Commission . 2007 . Recommendation of 3 May 2007 on the monitoring of acrylamide levels in food . Official Journal of European Union , L123 , 33 40 .
- Faist , V. and Erbersdobler , H. 2001 . Metabolic transit and in vivo effects of melanoidins and precursor compounds deriving from the Maillard reaction . Annals of Nutrition and Metabolism , 45 : 1 – 12 .
- Foerster , A. and Henle , T. 2003 . Glycation in food and metabolic transit of dietary AGEs (advanced glycation end-products): Studies on the urinary excretion of pyrraline . Biochemical Society Transactions , 31 : 1383 – 1385 .
- Friedman , M. 2005 . “ Biological effects of Maillard browning products that may affect acrylamide safety in food ” . In Chemistry and safety if acrylamide in food , Edited by: Friedman , M. and Mottram , D. 135 – 156 . Albany : Springer .
- Goldberg , T. , Cai , W. , Peppa , M. , Dardaine , V. , Baliga , B. Uribarri , J. 2004 . Advanced glycoxidation end products in commonly consumed foods . Journal of the American Dietetic Association , 104 : 1287 – 1291 .
- Gruber , P. , Becker , W.M. and Hofmann , T. 2005 . Influence of the maillard reaction on the allergenicity of rAra h 2, a recombinant major allergen from peanut (Arachis hypogaea), its major epitopes, and peanut agglutinin . Journal of Agricultural and Food Chemistry , 53 : 2289 – 2296 .
- Hanover , L.M. and White , J.S. 1993 . Manufacturing, composition, and applications of fructose . American Journal of Clinical Nutrition , 58 : 724 – 732 .
- Hidalgo , F.J. , Alaiz , M. and Zamora , R. 1999 . Effect of pH and temperature on comparative nonenzymatic browning of proteins produced by oxidized lipids and carbohydrates . Journal of Agricultural and Food Chemistry , 47 : 742 – 747 .
- Horiuchi , S. , Araki , N. and Morino , Y. 1991 . Immunochemical approach to characterize advanced glycation end products of the Maillard reaction. Evidence for the presence of a common structure . Journal of Biological Chemistry , 266 : 7329 – 7332 .
- Hurrell , R.F. and Carpenter , K.J. 1977 . Mechanisms of heat damage in proteins. 8. The role of sucrose in the susceptibility of protein foods to heat damage . British Journal of Nutrition , 38 : 285 – 297 .
- Hurtta , M. , Pitkanen , I. and Knuutinen , J. 2004 . Melting behaviour of D-sucrose, D-glucose and D-fructose . Carbohydrate Research , 339 : 2267 – 2273 .
- Kanska , U. and Boratynski , J. 2002 . Thermal glycation of proteins by D-glucose and D-fructose . Archivum Immunologiae et Therapiae Experimentalis (Warszawa) , 50 : 61 – 66 .
- Kim , H.J. , Choi , S.J. , Shin , W.S. and Moon , T.W. 2003 . Emulsifying properties of bovine serum albumin-galactomannan conjugates . Journal of Agricultural and Food Chemistry , 51 : 1049 – 1056 .
- Kislinger , T. , Humeny , A. , Peich , C.C. , Zhang , X. , Niwa , T. Pischetsrieder , M. 2003 . Relative quantification of N(epsilon)-(Carboxymethyl)lysine, imidazolone A, and the Amadori product in glycated lysozyme by MALDI-TOF mass spectrometry . Journal of Agricultural and Food Chemistry , 51 : 51 – 57 .
- Koschinsky , T. , He , C.J. , Mitsuhashi , T. , Bucala , R. , Liu , C. Buenting , C. 1997 . Orally absorbed reactive glycation products (glycotoxins): An environmental risk factor in diabetic nephropathy . Proceedings of the National Academy of Sciences (USA) , 94 : 6474 – 6479 .
- Krajcovicova-Kudlackova , M. , Sebekova , K. , Schinzel , R. and Klvanova , J. 2002 . Advanced glycation end products and nutrition . Physiological Research , 51 : 313 – 316 .
- Leclere , J. and Birlouez-Aragon , I. 2001 . The fluorescence of advanced Maillard products is a good indicator of lysine damage during the Maillard reaction . Journal of Agricultural and Food Chemistry , 49 : 4682 – 4687 .
- Levi , B. and Werman , M.J. 1998 . Long-term fructose consumption accelerates glycation and several age-related variables in male rats . Journal of Nutrition , 128 : 1442 – 1449 .
- Machiels , D. and Istasse , L. 2002 . La réaction de Maillard: Importance et applications en chimie des aliments . Annales de Médecine Vétérinaire , 146 : 347 – 352 .
- Matiacevich , S.B. , Santagapita , P.R. and Buera , M.P. 2005 . Fluorescence from the Maillard reaction and its potential applications in food science . Critical Reviews in Food Science and Nutrition , 45 : 483 – 495 .
- Miller , A.G. and Gerrard , J.A. 2005 . The Maillard reaction and food protein crosslinking . Progress in Food Biopolymer Research , 1 : 69 – 86 .
- Morales , F.J. and Jimenez-Perez , S. 2001 . Free radical scavenging capacity of Maillard reaction products as related to colour and fluorescence . Food Chemistry , 72 : 119 – 125 .
- Oliver , C.M. , Melton , L.D. and Stanley , R.A. 2006 . Creating proteins with novel functionality via the Maillard reaction: A review . Critical Reviews in Food Science and Nutrition , 46 : 337 – 350 .
- Peppa , M. , Uribarri , J. and Vlassara , H. 2003 . Glucose, advanced glycation end products, and diabetes complications. What is new and what works . Clinical Diabetes , 21 : 186 – 187 .
- Pomeranz , Y. , Johnson , J.A. and Shellenberg , J.A. 1962 . Effect of various sugars on browning . Journal of Food Science , 27 : 350 – 354 .
- Poms , R.E. , Capelletti , C. and Anklam , E. 2004 . Effect of roasting history and buffer composition on peanut protein extraction efficiency . Molecular Nutrition & Food Research , 48 : 459 – 464 .
- Rumbo , M. , Chirdo , F.G. , Fossati , C.A. and Anon , M.C. 2001 . Analysis of the effects of heat treatment on gliadin immunochemical quantification using a panel of anti-prolamin antibodies . Journal of Agricultural and Food Chemistry , 49 : 5719 – 5726 .
- Suarez , G. , Rajaram , R. , Oronsky , A.L. and Gawinowiczj , M.A. 1989 . Nonenzymatic glycation of bovine serum albumin by fructose (fructation). Comparison with the Maillard reaction initiated by glucose . The Journal of Biological Chemistry , 264 : 3674 – 3679 .
- Summa , C. , Wenzl , T. , Brohee , M. , DeLaCalle , B. and Anklam , E. 2006 . Investigation of the correlation of the acrylamide content and the antioxidant activity of model cookies . Journal of Agricultural and Food Chemistry , 54 : 853 – 859 .
- Sun , Y. , Hayakawa , S. , Puangmanee , S. and Izumori , K. 2006 . Chemical properties and antioxidative activity of glycated α-lactalbumin with a rare sugar, d-allose, by Maillard reaction . Food Chemistry , 95 : 509 – 517 .
- Takeuchi , M. , Yanase , Y. , Matsuura , N. , Yamagishi , S. , Kameda , Y. Bucala , R. 2001 . Immunological detection of a novel advanced glycation end-product . Molecular Medicine , 7 : 783 – 791 .
- Tauer , A. , Hasenkopf , K. , Kislinger , T. , Frey , I. and Pischetsrieder , M. 1999 . Determination of Nε-carboxymethyllysine in heated milk products by immunochemical methods . European Food Research and Technology , 209 : 72 – 76 .
- Uribarri , J. , Cai , W. , Sandu , O. , Peppa , M. , Goldberg , T. and Vlassara , H. 2005 . Diet-derived advanced glycation end products are major contributors to the body's AGE pool and induce inflammation in healthy subjects . Annals of the New York Academy of Sciences , 1043 : 461 – 466 .
- Van Heijst , J.W.J. , Niessen , H.W.M. , Hoekman , K. and Schalkwijk , C.G. 2005 . Advanced glycation end products in human cancer tissues: Detection of N-(Carboxymethyl)lysine and argpyrimidine . Annals of the New York Academy of Sciences , 1043 : 725 – 733 .
- Van Nguyen , C. 2006 . Toxicity of the AGEs generated from the Maillard reaction: On the relationship of food-AGEs and biological-AGEs . Molecular Nutrition & Food Research , 50 : 1140 – 1149 .
- Yen , G.C. and Chung , D.Y. 1999 . Antioxidant effects of extracts from Cassia tora L. Prepared under different degrees of roasting on the oxidative damage to biomolecules . Journal of Agricultural and Food Chemistry , 47 : 1326 – 1332 .
- Zamora , R. and Hidalgo , F.J. 2005 . Coordinate contribution of lipid oxidation and Maillard reaction to the nonenzymatic food browning . Critical Reviews in Food Science and Nutrition , 45 : 49 – 59 .