Abstract
A class selective enzyme-linked immunosorbent assay (ELISA) was developed for the detection of benzodiazepines, such as nitrazepam, clonazepam, 7-aminonitrazepam, 7-amidoclonzepam, chlordiazepoxide, delorazepam and nordazepam. Class selective artificial antigens were prepared by haptens of 7-aminonitrazepam and 7-aminoclonazepam conjugating to the carrier protein bovine serum albumin (BSA). Class selective polyclonal antibodies (PcAbs) were generated by immunising male New Zealand white rabbits with the class selective immunogens. Characterisation studies of the PcAbs showed that PcAbs raised by 7-aminonitrazepam–BSA had high affinity and specificity to the six relative benzodiazepines, while PcAbs raised by 7-amidoclonazepam–BSA had high affinity and specificity to five. So, PcAbs raised by 7-aminonitrazepam–BSA were selected to establish an indirect competitive ELISA for multi-residue determination. The IC50s of the optimised immunoassay were 2.50 µg/l, 3.13 µg/l, 1.92 µg/l, 2.78 µg/l, 3.13 µg/l and 4.55 µg/l for nitrazepam, clonazepam, 7-aminonitrazepam, 7-aminoclonazepam, delorazepam and nordazepam, respectively. Assay performance was validated using spiked pig urine by adding nirazepam. Recoveries were between 75 and 103%, and the coefficient variation was below 14%. This assay can be applied to detect multi-residues of benzodiazepines in urine.
Introduction
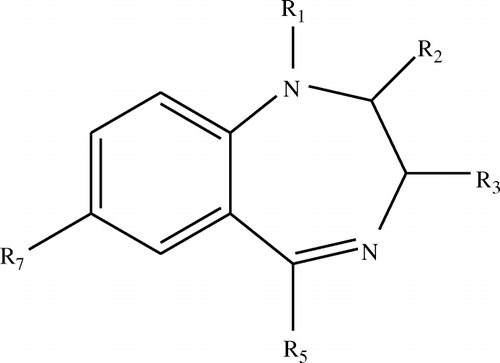
Benzodiazepines (, ) are a large class of drugs, widely prescribed as anxiolytic, hypnotic, anticonvulsant, myorelaxant and amnesic agents (Ashton, Citation1994; Bailey, Ward, & Musa, Citation1994; Hollister, Muller-Oerlinghausen, Rickerls, & Shader, Citation1993). They are relatively safe; overdoses (in the absence of other drugs) rarely result in death. However, when used chronically, benzodiazepines can be addictive (Miller, Gold, & Subst, Citation1991). The addition of benzodiazepines in animal feeds at low levels was shown to induce growth promotion and feed efficiency, and such uses became widespread in animal production. From 2002, benzodiazepines were prohibited in the process of breeding animals by explicit order in China. Because it is effective and relatively safe, benzodiazepines are still abused (Chen et al., Citation2009; Peng et al., Citation2009; Yuan, Xu, & Peng, Citation2008).
Table 1. Chemical structures of the benzodiazepines studied.
Current analysis of benzodiazepines is performed by various methods. Several chromatographic techniques have been developed for testing blood and urine for benzodiazepines, including flow injection analysis (Gambart, Cardenas, Gallego, & Valcarcel, Citation1998), high performance liquid chromatography (Lehmann & Boulieu, Citation1995; Wolff, Garretty, & Hay, Citation1997; Xu et al., Citation2006a,b,c,d), gas chromatography (Pirnay, Ricordel, Libong, & Bouchonnet, Citation2002; Rasanen, Ojanpera, & Vuori, Citation2000; Xu et al., 2006a,Citationb,Citationc,Citationd), thin layer chromatography (Jain, Citation1993), Fluorimetry (Valentour, Monforte, Lorenzo, & Sunshine, Citation1975) and Micellar electrokinetic capillary chromatography (Kuang et al., Citation2009; Li et al., Citation2009a, Citationb; Peng et al., Citation2008a,Citationb; Schafroth, Thormann, & Allemann, Citation1994; Tomita, Okuyama, Sato, & Ishizu, Citation1993; Wernly & Thormann, Citation1992; Xie, Liu, & Xu, Citation2009). Although sensitive and accurate, all these chromatographic methods are time consuming, laborious and expensive; expert technicians and extensive clean-up procedures are also required. They are unsuitable for the analysis of large numbers of samples. With the advantages of sensitivity, specificity, rapidity, simplicity and cost-effectiveness, immunochemical methods, especially enzyme-linked immunosorbent assay (ELISA), is becoming more and more useful for screening benzodiazepines in toxicological and clinical samples. Many immunoassay methods for benzodiazepines have been developed in the past two decades (Barrett et al., Citation1999; Borrey et al., Citation2002; Elian, Citation2003; Huang & Moody, Citation1995; Kemp, Sneed, Kupiec, & Spiehler, Citation2002; Li et al., Citation2008; Meatherall & Fraser, Citation1998; Miller, Wylie, & Oliver, Citation2006; Peng et al., Citation2008a,Citationb; Wang, Peng, Chen, & Xu, Citation2008). Various authors (Elian, Citation2003; Huang & Moody, Citation1995) have tested commercial immunoassays for their specificity against benzodiazepines, benzodiazepine metabolites, clonazepam and 7-aminoclonazepam, however, these assay either did not detect all of the compounds or had low cross-reactivities (CRs) with the compounds (Liu & Xu, Citation2007; Wang, Xu, & Peng, Citation2006; Xu et al., 2006a,b,c,d). Thus, the detection of mixtures of benzodiazepines is not possible with these assays. Class selective immunoassay for benzodiazepines is desirable.
In this study an attempt has been made to develop a class selective immunoassay for benzodiazepines. Two haptens were synthesised using a similar hapten design strategy, and antibodies raised from their bovine serum albumin (BSA) conjugates were screened through an indirect competitive ELISA method. Class selective immunoassay for benzodiazepines has also been developed.
Materials and methods
Reagents
Diazepam, nitrazepam, clonazepam, chlordiazepoxide, delorazepam, nordazepam, oxazepam and promethazine hydrochloride were purchased from Nhwa Pharma Corporation (Jiangsu, China). Triazolam was obtained from Institute of Forensic Science, Ministry of Public Security (Shanghai, China). BSA and ovalbumin (OVA) were purchased from Sigma Chemical Co. (MO, USA). Goat anti-rabbit immunoglobulin conjugated to horseradish-peroxidase (HRP) and 3,3′,5,5′-tetramethylbenzidine (TMB) were purchased from Sigma (MO, USA). Tween-20 was purchased from Zhejiang Longyou Chemical Company. Water used in all experiments was purified with a NANO pure system (Barnstead, USA). All other chemicals and organic solvents used in the hapten synthesis, immunisation and ELISA were commercially available and were of reagent grade or better.
Apparatus
NMR spectra were obtained with an AVANCE DMX 500 spectrometer (Bruker, Berlin, Germany), operating at 400 MHz; polystyrene 96-microwell plates were purchased from Nunc Rockilde (Denmark). The vacutainer blood collection set was acquired from Becton Dickinson (Meylan Cedex, France). Washing steps were carried out using a SLT 96PW microplate washer (SLT Labinstruments GmbH, Salzburg, Austria). Absorbances were read with a Multiskan Plus MK3 microplate reader (Labsystems, Helsinki, Finland) in single wavelength mode at 450 nm. Derivations were identified using WATERS Platform ZMD 4000 LC-MS instrument (Waters Company, USA). The conditions were as follows: lichrospher C18 2.1×250 mm column; water as mobile phase; 0.3 ml/min of flow rate; elution with a linear gradient concentrations from 60 to 100% of methanol for 25 min, which isolated each component completely. Mass spectrum conditions: m/z rates ranged from 200 to 800; capillary pressures of EIS− and EIS+ were 3.88 KV and 3.87 KV, respectively, and degas temperature 300°C; through testing multichannel cation and anion contemporarily with different pressures of piezoelectric diffuser, it was found that the result was the best and could get comparatively high molecule-ion peak when respective capillary pressures of EIS− and EIS+ were 30 V and 24 V.
Hapten synthesis and verification
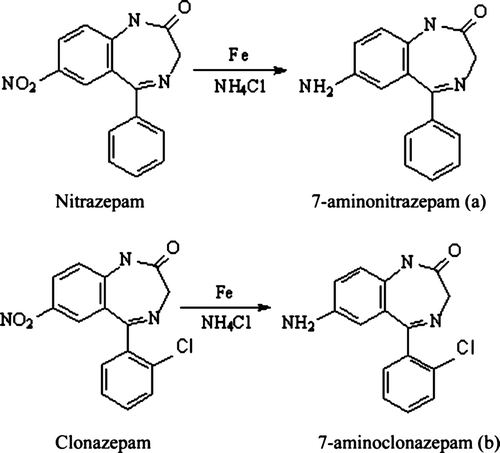
The chemical structures of nitrazepam, clonazepam and their derivatives used for ELISA development are shown in . 7-aminonitrazepam (hapten a) and 7-aminoclonazepam (hapten b) were prepared by a reduction process. Briefly, 1.68 g reduced iron powder and 200 mg ammonium chloride were suspended in 5 ml water and heated for 15 min, 2.81 g nitrazepam (3.15 g clonazepam) in 100 ml methanol was added to the mixture. After refluxing for 5 hours, reduced iron powder was removed by filtration; the methanol was evaporated in vacuo. The precipitate was collected and recrystallised with methanol–hexane to give 2.24 g (2.77 g) of fawn crystalloid.
Preparation of hapten–protein conjugates
Haptens a and b were covalently attached to carrier proteins BSA and OVA according to the diazotisation method described by Dixon (Citation1984). The conjugates formed were purified by dialysis against 2 l phosphate-buffered saline (PBS, pH 7.4), which was changed 3 times a day, for three days. The a-BSA and b-BSA were used as immunogens, a-OVA and b-OVA were used as coating antigens.
Preparation of polyclonal antibodies (PcAbs)
Two New Zealand white rabbits were immunised with hapten a-BSA (rabbits #2 and #7), and another two were immunised with hapten b-BSA (rabbits # 8 and #12). One month after an initial immunisation with 1 mg of the immunogen protein emulsified with Freund's complete adjuvant (1:1, v:v), further injections of 1 mg of the immunogen emulsified with Freund's incomplete adjuvant were given (1:1, v:v). The booster injections were given at 4-week intervals. Blood from rabbits were collected after 10 day of each booster injection for titre monitoring by indirect ELISA. Antibodies were purified by ammonium sulphate precipitation, and further by protein-A-Agarose affinity chromatography column. Antibodies with maximum activity were stored at –80°C. (Immunisation experiment was implemented in Jiangsu Institute of Nuclear Medicine that has the Jiangsu Laboratory Animal Management Committee permission.)
Competitive indirect enzyme-linked immunosorbent assay (ELISA) procedure
Microplates were coated overnight at 4°C with the appropriate coating antigen (100 µl per well in 0.05M carbonate–bicarbonate buffer, pH 9.6), then washed three times over a 3-min cycle with PBS containing 0.05% Tween-20 (PBST), all subsequent washing steps were performed in the same manner with PBST. The plates were blocked with 200 µl of 0.1% gelatin in coating buffer by incubation overnight at 4°C, and then shaken dry. Following a washing step, benzodiazepines standards in 5% methanol–PBS or samples (50 µL) and 50 µl antiserum diluted in antibody diluent (0.1% gelatin in PBST) were added to the well. After 30 min incubation at room temperature, the plates were washed. Goat anti-rabbit IgG-HRP conjugate at a dilution of 1:3000 in the antibody diluent (100 µl per well) was added and incubated for another 30 min at room temperature. The plates were washed again, and 100 µl per well of substrate solution (4 µl of 30% H2O2, 5 ml of 0.06% TMB in glycol per 25 ml of disodium hydrogen phosphate–citric acid buffer, pH 5.0) was added. After incubation for 20 min at room temperature, colour development was stopped with 2M H2SO4 (100 µl per well). The absorbance was measured at 450 nm.
The concentrations of standard analyte ranged from 0.05 to 50 µg/l. Standard curves were obtained by plotting absorbance against the logarithm of the analyte concentration.
Cross-reactivity (CR)
CR was studied by comparing the analyte concentration resulting in half-maximun inhibition (IC50) with the concentration of potential cross-reactants similarly producing 50% inhibition. The CRs (%) were calculated as:
Sample pre-treatment protocol
To 1 ml of pig urine, 4 ml NaOH (0.1M) were added and mixed (vortex) for 2–5 min, then defatted by addition of 5 ml hexane. The mixture was centrifuged for 5 min at 3000 rpm and 15°C. The supernatant was evaporated to dryness under a mild stream of nitrogen at a temperature of 50°C and residues were dissolved in 1 ml PBS. Measurements were carried out in triplicate.
Recovery
To 1 ml of blank urine samples, 5 ng, 10 ng and 20 ng nitrazepam were added, respectively. Urine samples were extracted following the sample pre-treatment protocol. The recoveries from urine samples were estimated.
Result and discussion
Hapten synthesis
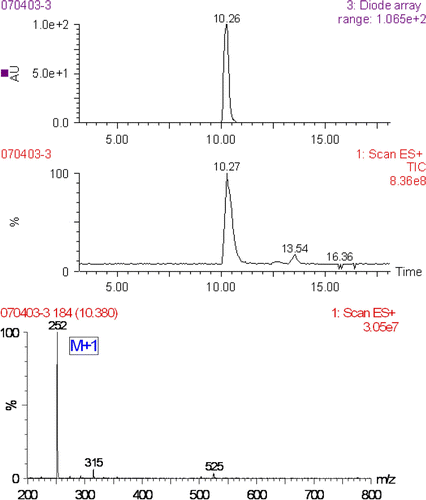
The 7-aminonitrazepam was a fawn crystalloid material. In the TLC system, it showed one spot with R f value of 0.35, while the nitrazepam showed R f value of 0.72, 1H NMR and HPLC-MS further supported the structure of target molecules, 1H NHR (CDCl3): δ 3.85 (m, 2H, NH 2 ), δ 4.52 (s, 2H, 2H3), δ 6.36 (m, 1H, H8), δ 6.83 (m, 1H, H6), δ 7.32–7.50 (m, 4H, H3’, H4’, H5’, H9), δ 7.93–7.96 (m, 2H, H2', H6’), δ 8.31 (m, 1H, H1); showed the HPLC-MS figures of 7-aminonitrazepam: a prominent fragment ions seemed to be generic to 7-aminonitrazepam, namely m/z 252.
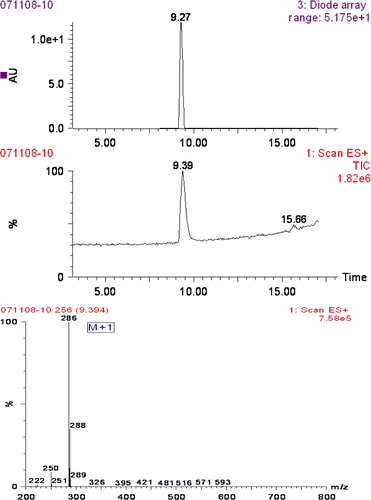
The product of 7-aminoclonazepam was an orange crystalloid material. In the TLC system, it showed one spot with R f value of 0.33, while the clonazepam showed R f value of 0.68, 1H NHR (CDCl3): δ 4.05 (m, 2H, NH 2 ), δ 4.42 (s, 2H, 2H3), δ 6.46 (m, 1H, H8), δ 6.80 (m, 1H, H6), δ 7.44–7.51 (m, 3H, H4', H5', H9), δ 7.78 (m, 1H, H6'), δ 7.91 (m, 1H, H2’), δ 8.11 (m, 1H, H1); the LC-MS figure of 7-amidoclonzepam was shown in , the major fragment ions at m/z 286 was thought to be a result from MH+ for 7-amidoclonzepam.
In general, the above results validated the successful synthesis of 7-aminonitrazepam and 7-aminoclonazepam.
Calculation coupling rates of conjugate
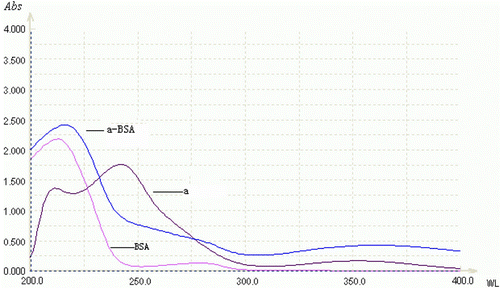
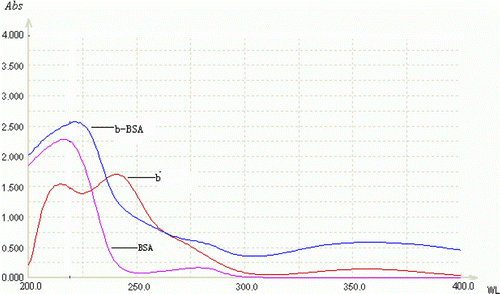
7-aminonitrazepam (7-aminoclonazepam) and BSA had their own ultraviolet absorption curves, so the immunogen which was prepared by coupling 7-aminonitrazepam (7-aminoclonazepam) with BSA would show the ultraviolet absorption characteristics of hapten and protein. and show the UV scanning of 7-aminonitrazepam–BSA (7-aminoclonazepam–BSA), 7-aminonitrazepam (7-aminoclonazepam) and BSA. The absorption of 7-aminonitrazepam (7-aminoclonazepam) reached peaks at 230 nm and 330 nm, and there was also an obvious prominence of conjugates at 230 nm and 330 nm, which indicated that the immunogen had the characteristic of 7-aminonitrazepam (7-aminoclonazepam). So it can be concluded that the coupling of immunogen was successful. The protein concentration was measured by the Lowry method. It was estimated that the molar ratio of 7-aminonitrazepam and BSA of 7-aminonitrazepam – BSA was about 12 by measurement of the difference of UV absorption at 230 nm as a molecular extinction coefficient of 7-aminonitrazepam; for 7-aminoclonazepam, it was 15.
Titre of antibodies
The titre of the sera from each animal was screened by measuring the binding of serial dilutions to microtitre plates coated with several concentrations of a-OVA and b-OVA. According to the method, the serum titre of rabbits #2, #7, # 8 and #12 were 15,3600, 38,400, 15,3600 and 38,400, respectively. Therefore, the serum of #2 and #8 were selected for the further determination.
Optimised working concentration
Working concentrations of antibody–antigen or antibody–enzyme tracer were optimised by checkerboard assays. Optimised working concentration of antibody–antigen were 3200–15,3600 and 25,600–15,3600 for 2# and 8#, respectively.
Enzyme-linked immunosorbent assay (ELISA) procedure
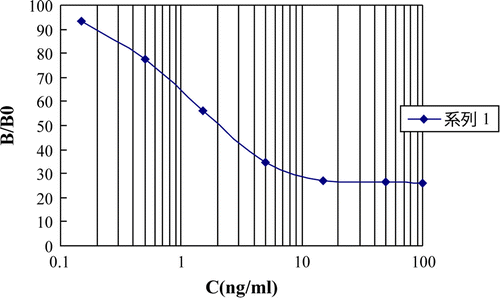
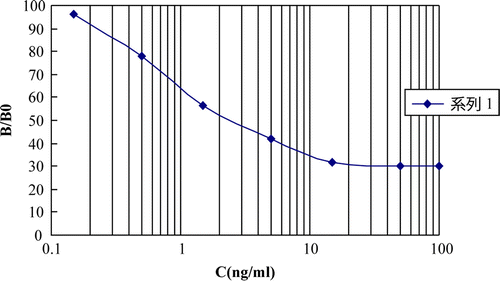
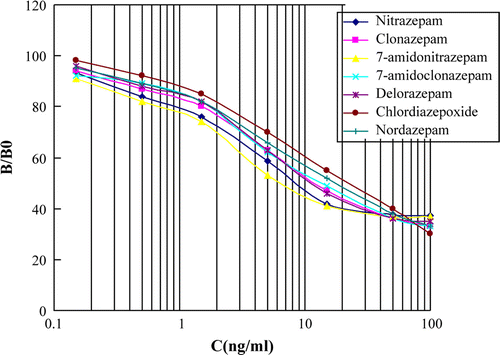
Two ELISA plates were coated with the optimised concentrations of hapten a-OVA and hapten b-OVA, respectively. Inhibitions rates were determined by indirect competitive ELISA. Two typical standard ELISA curves were showed in and . The IC50 (the concentration of chemicals corresponding to 50% inhibition), detection of limit (LOD, IC10) and linear range for nitrazepam and clonazepam are given in .
Table 2. Precision to two benzodiazepines using indirect competitive ELISA format.
Cross-reactivity (CR)
demonstrates the CR of the ELISA system with different benzodiazepines. The hapten a-BSA system had high CR with six benzodiazepines such as nitrazepam (100%), clonazepam (80%), 7-aminonitrazepam (130%), 7-aminoclonazepam (90%), delorazepam (80%) and nordazepam (55%). For the hapten b-BSA system, the ratio CR of nitrazepam was 90%, clonazepam 100%, 7-aminonitrazepam 80%, 7-aminoclonazepam 105% and delorazepam 95%. Little or no CR was measured for other ataractics such as oxazepam, diazepam, triazolam, promethazine hydrochloride. The primary aim was to develop a class selective immunoassay with high CR to the benzodiazepines, with little or no CR to other substances. The hapten a-BSA system was selected for further experiments. The standard curve obtained using the hapten a-BSA system for the detection of benzodiazepines is shown in .
Table 3. Cross-reactivities of benzodiazepines and similar substances.
By comparing the affinities of the antibody for various benzodiazepines with the structures of the drugs (, ), it can be concluded that: (a) the antibody binds with the highest affinities to benzodiazepines that have a hydrogen in positions 1 and 3. However, the benzodiazepines with a methyl or a bulkier group in the position 1 or 3 have lower affinities for the antibody; (b) addition of an electron-withdrawing substituent in position 7 such as NO2 or Cl does not affect the binding of the benzodiazepines to the antibody; and (c) elimination of the carbon ring in position 5 has a dramatic negative effect on the affinities of the antibody.
Precision and accuracy
To assess the precision and accuracy of the assay, urine samples spiked with nitrazepam at three concentrations, corresponding to 5.0, 10.0 and 20.0 µg/ml were studied, as shown in . Coefficient of variation (CV) was below 14% for all the samples, demonstrating an acceptable level of precision.
Table 4. Determination of nitrazepam in spiked urine samples by IC-ELISA.
Recovery
To estimate reliability, a recovery test was performed using urine samples. Known amounts of standard to urine samples were analysed and the value was measured by ELISA compared with the true value. shows the recoveries determined at three different concentration levels. It can be observed that values range from 75 to 103%, indicating the method has a good accuracy.
Conclusions
The broad specificity antibody obtained during the study can recognise six benzodiazepines, i.e, nitrazepam, clonazepam, 7-aminonitrazepam, 7-aminoclonazepam, delorazepam and nordazepam. The pre-treatment of samples is quite suitable for urine, and the recoveries were between 82 and 103%, with the coefficients of variation lower than 14%. Thus, this assay is useful for screening benzodiazepines and the use of such a general assay has several advantages. The method was relative easy to perform and has potential for automation. Only a small sample is needed for it. Most important, it can be applied to pre-screen out samples containing benzodiazepines residues, enhancing efficiency of further instrumental analysis such as GC-MS. Furthermore, the detection limits of ELISA can be comparable with those obtained by use of instrumental methods.
References
- Ashton , H. 1994 . ELISA detection of clonazepam and 7-aminoclonazepam in whole blood and urine . Drugs , 48 : 25 – 40 .
- Bailey , L. , Ward , M. and Musa , M.N. 1994 . Clinical pharmacokinetics of benzodiazipines . Journal of Clinical Pharmacology , 34 : 804 – 811 .
- Barrett , A.M. , Walshe , K. , Kavanagh , P.V. , McNamara , S.M. , Moran , C. Burdett , J. 1999 . A comparison of five commercial immunoassays for the detection of flunitrazepam and other benzodiazepines in urine . Addiction Biology , 4 : 81 – 87 .
- Borrey , D. , Meyer , E. , Ducheteau , L. , Lambert , W. , Van Peteghem , C. and De Leenheer , A. 2002 . Enzymatic hydrolysis improves the sensitivity of emit screening for urinary benzodiazepines . Clinical Chemistry , 48 : 2047 – 2049 .
- Chen , W. , Peng , C.F. , Jin , Z.Y. , Qiao , R.R. , Wang , W.K. Zhu , S.F. 2009 . Ultrasensitive immunoassay of 7-aminoclonazepam in human urine based on CdTe nanoparticle bioconjugations by fabricated microfluidic chip . Biosensors and Bioelectronics , 24 : 2051 – 2056 .
- Dixon , R. 1984 . Radioimmunoassay of benzodiazepines . Methods in Enzymology , 84 : 490 – 515 .
- Elian , A.A. 2003 . ELISA detection of clonazepam and 7-aminoclonazepam in whole blood and urine . Forensic Science International , 134 : 54 – 56 .
- Gambart , D. , Cardenas , S. , Gallego , M. and Valcarcel , M. 1998 . An automated screening system for benzodiazepines in human urine . Analytical Chimica Acta , 366 : 93 – 103 .
- Hollister , L.E. , Muller-Oerlinghausen , B. , Rickerls , K. and Shader , R.I. 1993 . Clinical uses of benzodiazepines . Journal of Clinical Psychopharmacology , 13 : 1S – 169 .
- Huang , W. and Moody , D.E. 1995 . Immunoassay detection of benzodiazepines and benzodiazepine metabolites in blood . Journal of Analytical Toxicology , 19 : 333 – 342 .
- Jain , R. 1993 . Simultaneous determination of diazepam and its metabolites in urine by thin-layer chromatography and direct densitometry . Journal of Chromatography , 615 : 365 – 368 .
- Kemp , P. , Sneed , G. , Kupiec , T. and Spiehler , V. 2002 . Validation of a microtiter plate ELISA for screening of postmortem blood for opiates and benzodiazepines . Journal of Analytical Toxicology , 26 : 504 – 512 .
- Kuang , H. , Li , Q.S. , Shen , C.Y. , Xu , J.Z. , Yuan , Y. Xu , C.L. 2009 . A highly sensitive method for the determination of 7-aminonitrazepam, a metabolite of nitrazepam, in human urine using high performance electrospray liquid chromatography tandem mass spectrometry . Biomedical Chromatography , 23 : 740 – 744 .
- Lehmann , B. and Boulieu , R. 1995 . Determination of midazolam and its unconjugated 1-hydroxy metabolite in human plasma by high-performance liquid chromatography . Journal of Chromatography B , 674 : 138 – 142 .
- Li , Y.L. , Hao , X.L. , Ji , B.Q. , Xu , C.L. , Chen , W. Shen , C.Y. 2009a . Rapid determination of 19 quinolone residues in spiked fish and pig muscle by HPLC tandem mass spectrometry . Food Additives and Contaminants , 26 ( 3 ) : 306 – 313 .
- Li , Q.S. , Liu , L.Q. , Chen , W. , Peng , C.F. , Wang , L.B. and Xu , C.L. 2009b . Gold nanoparticle-based immunochromatographic assay for the detection of 7-aminoclonazepam in urine . International Journal of Evironmental Analytical Chemistry , 89 : 261 – 268 .
- Li , Y.L. , Ji , B.Q. , Chen , W. , Liu , L.Q. , Xu , C.L. Peng , C.F. 2008 . Production of new class-specific polyclonal antibody for determination of fluoroquinolones antibiotics by indirect competitive ELISA . Food and Agricultural Immunology , 19 : 251 – 264 .
- Liu , L.Q. and Xu , C.L. 2007 . Development and evaluation of a rapid lateral flow immunochromatographic strip assay for screening 19-nortestosterone . Biomedical Chromatography , 21 : 861 – 866 .
- Meatherall , R. and Fraser , A.D. 1998 . Comparison of four immunoassays for the detection of lorazepam in urine . Therapeutic Drug Monitoring , 20 : 673 – 675 .
- Miller , E.I. , Wylie , F.M. and Oliver , J.S. 2006 . Detection of benzodiazepines in hair using ELISA and LC–ESI-MS–MS . Journal of Analytical Toxicology , 30 : 441 – 448 .
- Miller , N.S. , Gold , M.S. and Subst , J. 1991 . Introduction-benzodiazepines: A major problem . Journal of Substance Abuse Treatment , 8 : 3 – 7 .
- Peng , C.F. , Chen , Y.W. , Chen , W. , Xu , C.L. , Kim , J.M. and Jin , Z.Y. 2008a . Development of a sensitive heterologous ELISA method for analysis of acetylgestagen residues in animal fat . Food Chemistry , 109 : 647 – 653 .
- Peng , C.F. , Shen , C.Y. , An , K.J. Xu , C.L. 2008b . Analysis of acetylgestagens residues in animal fat by liquid chromatography tandem mass spectrometry . Chinese Journal of Analytical Chemistry , 36 : 1117 – 1120 .
- Peng , C.F. , Li , Z.K. , Zhu , Y.Y. , Chen , W. , Yuan , Y. Liu , L.Q. 2009 . Simultaneous and sensitive determination of multiplex chemical residues based on multi-color quantum dot probes . Biosensors and Bioelectronics , 24 : 3657 – 3662 .
- Pirnay , S. , Ricordel , I. , Libong , D. and Bouchonnet , S. 2002 . Sensitive method for the detection of 22 benzodiazepines by gas chromatography-ion trap tandem mass spectrometry . Journal of Chromatography A , 954 : 235 – 245 .
- Rasanen , I. , Ojanpera , I. and Vuori , E. 2000 . Quantitative screening for benzodiazepines in blood by dual column gas chromatography and comparison of the results with urine immunoassay . Journal of Analytical Toxicology , 24 : 46 – 53 .
- Schafroth , M. , Thormann , W. and Allemann , D. 1994 . Comparison of four immunoassays for the detection of lorazepam in urine . Electrophoresis , 15 : 72 – 78 .
- Tomita , M. , Okuyama , T. , Sato , S. and Ishizu , H. 1993 . Simultaneous determination of nitrazepam and its metabolites in urine by micellar electokinetic capillary chromatography . Journal of Chromatography , 621 : 249 – 255 .
- Valentour , J.C. , Monforte , J.R. , Lorenzo , B. and Sunshine , I. 1975 . Fluorometric screening method for detecting benzodiazepines in blood and urine . Clinical Chemistry , 21 : 1976 – 1979 .
- Wang , L.Y. , Peng , C.F. , Chen , W. and Xu , C.L. 2008 . A direct enzyme-linked immunosorbent assay for hexoestrol residues . Food and Agricultural Immunology , 19 : 61 – 75 .
- Wang , L.Y. , Xu , C.L. and Peng , C.F. 2006 . Development and optimization of an indirect enzyme-linked immunosorbent assay for the determination of Hexoestrol . Food and Agricultural Immunology , 17 : 157 – 171 .
- Wernly , P. and Thormann , W. 1992 . Drug of abuse confirmation in human urine using stepwise solid-phase extraction and micellar electrokinetic capillary chromatography . Analytical Chemistry , 64 : 2155 – 2159 .
- Wolff , K. , Garretty , D. and Hay , A.W.H. 1997 . Micro-extraction of commonly abused benzodiazepines for urinary screening by liquid chromatography . Annals of Clinical Biochemistry , 34 : 61 – 67 .
- Xie , H.L. , Liu , L.Q. and Xu , C.L. 2009 . Development and validation of an immunochromatographic assay for rapid multi-residues detection of cephems in milk . Analytica Chimica Acta , 634 : 129 – 133 .
- Xu , C.L. , Chu , X.G. , Peng , C.F. , Jin , Z.Y. and Wang , L.Y. 2006a . Development of a faster determination of 10 anabolic steroids residues in animal muscle tissues by liquid chromatography tandem mass spectrometry . Journal of Pharmaceutical and Biomedical Analysis , 41 : 616 – 621 .
- Xu , C.L. , Chu , X.G. , Peng , C.F. , Liu , L.Q. , Wang , L.Y. and Jin , Z.Y. 2006b . Comparison of enzyme-linked immunosorbent assay with liquid chromatography–tandem mass spectrometry for the determination of diethylstilbesterol residues in chicken and liver tissues . Biomedical Chromatography , 20 : 1056 – 1064 .
- Xu , C.L. , Peng , C.F. , Hao , K. , Jin , Z.Y. and Chu , X.G. 2006c . Simultaneous determination of 9 anabolic steroids residues in animal muscle tissues by gas chromatography-mass spectrometry . Analytical Letters , 39 : 555 – 568 .
- Xu , C.L. , Peng , C.F. , Wang , L.Y. , Hao , K. and Jin , Z.Y. 2006d . Separation and identification of synthetic antigens of hexoestrol residue in animal derived food by HPLC-MS . Food and Agricultural Immunology , 17 : 21 – 27 .
- Yuan , Y. , Xu , C.L. and Peng , C.F. 2008 . Analytical methods for the detection of corticosteroids-residues in animal-derived foodstuffs . Critical Reviews in Analytical Chemistry , 38 : 227 – 241 .