Abstract
The interaction between oxidised low-density lipoprotein (LDL) and macrophages is known to be important in the development of arteriosclerosis. Macrophages take up oxidised LDL and then become foam cells, which contribute to the thickening of the blood vessel wall. In our previous paper, some flavonoids found in vegetables and fruits were shown to have a protective effect against arteriosclerosis. In this communication, to elucidate the effect of fucose-related compounds on oxidised LDL uptake in macrophages, the inhibitory activity of various fucose-related compounds on the 1,1-dioctadecyl-3,3,3',3'-tetramethylindo-carbocyanine perchlorate-acetylated-LDL (DiI-ac-LDL) uptake reaction in mouse macrophage cell line J774.1 was measured. Fucoidan, 4-deoxy-fucose and fucose-galactose-N-acetyl-glucose significantly inhibited uptake of DiI-ac-LDL into macrophages. The IC50 of 4-deoxy-fucose was 108.4 µM, the highest value of the fucose-related compounds used in the experiments. The protective effects of the fucose-related compounds on atherosclerosis can be used as new health foods.
Introduction
It is well known that major risk factors for atherosclerosis include high plasma low-density lipoprotein (LDL) concentrations and LDL modifications. Oxidative modification of LDL is thought to play a key role during early atherogenesis (Boullier et al., Citation2001; Glass & Witztum, Citation2001; Greaves & Gordon, Citation2005). Macrophages in blood infiltrate the vessel wall and phagocytose foreign material. Macrophages recognise the oxidised LDL as foreign material. Oxidised LDL is taken up by macrophages at an enhanced rate via their scavenger receptors (Parthasarathy, Printz, Boyd, Joy, & Steinberg, Citation1986), leading to the formation of lipid-laden foam cells, a hallmark of the early atherosclerotic changes (Berliner et al., Citation1995). Because macrophages cannot digest the oxidised LDL, they accumulate the lipids and become foam cells. As smooth muscle cells proliferate, the vessel wall gradually thickens. These changes lead to vessel wall rigidity and to increased blood pressure and subsequently to arteriosclerosis and risk of infarction. Thus, the uptake of oxidised LDL by macrophages appears to be an important point of intervention in this disease process.
We reported that a macrophage cell line has a very useful of the phenomenon, which is the cell line uptake only oxidised LDL; some flavonoids have inhibitory effects on the intake of oxidised LDL (Yamaki, Goto, & Takano-Ishikawa, 2007). In general, macrophages have some radical scavenger receptors for ingestion of some bacteria and foreign objects. Some scavenger receptors are mannose-type, and recognise sugar structures found in the cell wall. Some external objects have fucose structures on the cell wall that are important targets for macrophages. Some fucose compounds are expected as anti-atherosclerosis components. Type A scavenger receptors have fucose-related side chain components that are expected to inhibit macrophage scavenging effects.
In this communication, we conducted experiments to determine whether or not some fucose-related compounds inhibit the intake of acetyl LDL (as oxidised LDL) by macrophages. We previously reported that fucoidan inhibits leukocyte rolling in local area recruitment during inflammation (Yamaki et al., Citation1998). Selectins are important factors in the rolling step of molecules that adhere to vessel walls. Their structure contains intracellular, transmembrane and extracellular domains. The extracellular domain has many fucose side chains which bind to other molecules such as P-selectin glycoprotein legand-1 (PSGL-1). Macrophages have some receptors against bacteria and foreign materials. These receptors recognise surface molecules, especially sugar structures. These sugar structures are comprised mainly of fucose, sialic acid and mannose. Therefore, fucose-related compounds are expected to inhibit binding to the LDL receptor. In this communication, we describe experiments conducted to measure the inhibitory effect of fucose-related compounds on the uptake of LDL by macrophage cell line J774.1.
Materials and methods
Materials
Acetylated LDL (Ac-LDL) labelled with 1,1-dioctadecyl-3,3,3',3'-tetramethylindo-carbocyanine perchlorate (DiI) were purchased from Biomedical Technologies Inc. (Stoughton, MA, USA). Fucoidan was obtained from Sigma Chemical Co. (St. Louis, MO, USA). Fucose and other related compounds were obtained from Calbiochem (La Jolla, CA). All the other chemicals used were reagent grade.
Cells and culture
The J774.1 mouse macrophage cell line was provided by the Japanese Collection of Research Bioresources. The J774.1 cells were maintained in 10% FCS in Dulbecco's modified eagle medium (DMEM) supplemented with 100 U/ml penicillin and 100 µg/ml streptomycin. The cells were maintained at 37°C in an atmosphere of 5% CO2 and 95% air.
Preparation of test sample solution
Fucoidan, fucose and the related compounds were solubilised in Hanks’ balanced salt solution (HBSS). The final alcohol concentration was below 0.1% in the culture plate. Under these conditions, none of the solubilisation solvents altered DiI-LDL and 1,1-dioctadecyl-3,3,3',3'-tetramethylindo-carbocyanine perchlorate-acetylated-LDL (DiI-ac-LDL) uptake activity.
1,1-dioctadecyl-3,3,3',3'-tetramethylindo-carbocyanine perchlorate-acetylated-low-density lipoprotein (DiI-ac-LDL) uptake measurement
Inhibition experiments of test compounds
The subcultured cells were incubated overnight with HBSS. After the overnight incubation, the test compounds were added to the wells of the plate. After 10 min incubation, DiI-ac-LDL was added to each well. The plates were incubated for 3 h in a CO2 incubator. As a control, HBSS was added to the wells instead of samples. After the incubation, each well was washed with HBSS to stop the reaction and remove remain DiI-ac-LDL. After cell lysis using Triton X-100 (10%), the DiI probe fluorescent intensity of each well was measured over time.
Statistical analysis
The values were compared using the unpaired Student's t-test using a significance level of P<0.05. For multiple group comparisons were performed using one-way ANOVA with a Bonferroni correction. Differences among means were considered significant at P<0.05. Other significant differences between control and test values were tested using a two-tailed multiple t-test with a Bonferroni correction. Asterisks indicate P<0.05.
Results and discussion
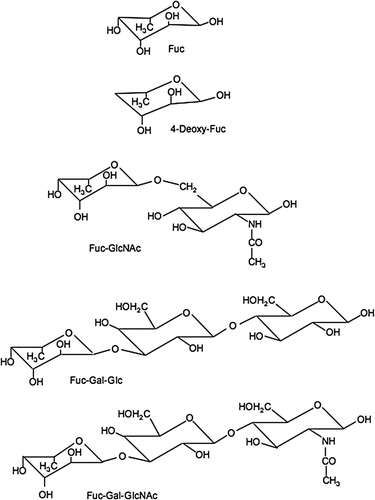
shows the compounds used in these experiments on Ac-LDL uptake inhibition in the J774.1 mouse macrophage cell line. Previously, we reported a method of measuring DiI-ac-LDL uptake by the J774.1 cell line as a model of oxidised LDL uptake by macrophages in the vessel wall.
Figure 1. Chemical structures of fucose and the related compounds used in the experiments. Note: These compounds were used in the inhibitory experiments on Ac-LDL uptake in the J774.1 mouse macrophage cell line. These are chemical structures of the compounds Fuc, fucose (6-deoxy-galactose); Gal, Galactose; GlcNAc, N-acetyl-Glucose; Glc, Glucose.
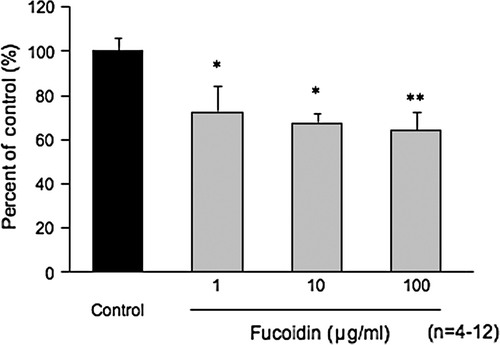
Macrophages have scavenger receptors such as Type A and B, and LOX. We also reported that isoflavones were effective inhibitors of LDL uptake by macrophages. Because isoflavones have not only an antioxidant effect, but also an anti-uptake effect, we speculate isoflavones may have a health benefit. shows the effects of fucoidan, fucose polysaccharide from seaweed, on the uptake reaction of DiI-ac-LDL by macrophage J774.1 cells. The fucoidan significantly inhibited DiI-ac-LDL uptake at concentrations ranging from 1 to 100 µg/ml. Fucoidan is used in inhibitory experiments as a control due to the presence of its sugar side chains. Some contact reactions of leukocytes (polymorphonuclear, monocytes and macrophages, and plasma cells and T/B cells), such as leukocyte rolling and adhesion in vessels, transport to local inflammation and homing to lymph nodes, etc. are mediated by fucose side chains expressed on cell surfaces.
Figure 2. Inhibitory effects of fucoidan on Ac-LDL uptake by the macrophage cell line J774.1. Note: Fucoidan was added to each well of the plates. Dil-ac-LDL was added to the wells and the plates were incubated for 3 h. After incubation, incorporated fluorescence in the cells was measured. Data are expressed as means and standard errors of means. *p<0.05; **p<0.01 vs. control group.
We tried to do inhibitory experiments using some fucose-related compounds. We used original focuses (D and L stereoisomers), 4-deoxy fucose, and galactose and NAc-glucose connected 2–3 oligosugars. Influence of these sugar compounds on the macrophage viability were not confirmed in the maximum concentration of 100 µM by microscope viewing. Of the fucose-related compounds, 4-deoxy fucose was most effective on the LDL uptake reaction of the macrophage cell line. 4-deoxy-fucose significantly inhibited update by 40% in 100 µM in a dose-dependent manner, from 1 to 100 µM (A). Fuc-Gal-GlcNAc had also inhibitory effect on the uptake reaction significantly (B). Thus, inhibition of LDL uptake by Fuc-Gal-GlcNAc was lower than that of 4-deoxy-fuc, which inhibited uptake by 30% in 10–100 µM. Estimated IC50 values of these compounds are shown in . Estimated IC50 values of 4-deoxy-fuc was the highest value of the compounds used in the experiment at 108.4 µM. The estimated IC50 of Fuc-Gal-GlcNAc was 625.3 µM. Other compounds had very low activity, in the order of D and L-Fuc > Fuc-Gal-Glc > >Fuc-GlcNac.
Figure 3. Inhibitory effects of fucoses and related fucose compounds on Ac-LDL uptake by the macrophage cell line J774.1. (A) D-Fucose, L-fucose and 4-deoxy-Fuc were added to the wells of the macrophage culture plates. (B) Fuc-Gal-Glc, Fuc-Gal-GlcNAc and Fuc-GlcNAc were added to the well. Dil-ac-LDL was added to the well and the plates were incubated for 3 h. After incubation, incorporated fluorescence in the cells was measured. Data are expressed as means and standard errors of means. *p<0.05; **p<0.01 vs. control group.
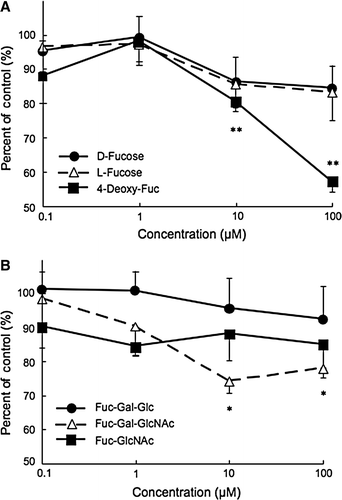
Table 1. Estimated inhibitory concentration of 50% (IC50) on LDL uptake by the macrophage cell line J774.1. IC50 (µM) were estimated from regression curves of concentration vs. percentage of inhibition.
These results suggest that the active moiety of the compounds are the 4-deoxy site of fucose and the N-acetyl site of glucose components. It is necessary to do more precise experiments using synthetic compounds.
Some of the fucose-related compounds were recognised as antigens on membranes of mammalian cells. The 4-deoxy-fuc is a known inhibitor of fucosyltransferase, an enzyme involved in the recognition of external or internal antigens (Allahverdian, Wojcik, & Dorscheid, Citation2006; Becker & Lowe, Citation2003; Du & Hindsgaul, Citation1996). Oxidised LDL and fucoidan bind to a scavenger receptor on the membrane of macrophages. The signal triggered by LDL and fucoidan binding is transduced to cytosol components, and microfilaments act to take up the oxidised LDL by phagocytosis. It was reported that fucoidan mediated two signalling pathways: c-Jun NH2-terminal protein kinase (JNK) and p38 mitogen-activated protein kinase pathway via p21-activated kinase (PAK), playing critical roles in proIL-1/IL-1 regulation (Hsu, Chiu, Wen, Chen, & Hua, Citation2001).
There are some oligosugars in human milk. Fuc-Gal-GlcNAc also is contained in human milk, and probably takes part in some self-defence systems (Ruiz-Palacios, Cervantes, Ramos, Chavez-Munguia, & Newburg, 2003).
We reported that macrophage J774.1 takes the oxidised LDL into the cell body. Type A scavenger receptors are involved in the uptake reaction and some isoflavones and resveratrol inhibit this uptake (Yamaki et al., Citation2007). When inflammatory leukocytes, which are polymorphonuclear leukocytes and mononuclear leukocytes, recruit to a local inflammatory area, adhesion molecules such as selectin and integlins play very important roles in adherence to and penetration of the vascular wall. Leukocyte rolling is a prerequisite for infiltration of endothelial cells, and the adhesion molecule selectin is involved in the reaction. It is well known that fucoidan, a seaweed polysaccharide, strongly inhibits the rolling reaction (Yamaki et al., Citation1998). Leukocyte adhesion and uptake of external substances are thought to similar reactions, and it supposed that recognition of surface antigen(s) by macrophages is common to both. Thus, it seems clear that fucoidan inhibits the uptake of LDL. Furthermore, it is reported that the relation between LDL receptor-related protein-1 activity and fucosylation is important for scavenging activity (Lee et al., Citation2006).
In addition, Fuc-Gal-GlcNAc is an antigen involved in the Campylobacter, a bacterium known to cause infant-related diarrhoea and combination with small intestine coating as an O-antigen (Ruiz-Palacios et al., Citation2003). Fucose oligosaccharide is plentiful in mother's milk, and these oligosaccharides might have a protective effect against infant diarrhoea (Morrow et al., Citation2004).
From these results, including protection against infection by fucose oligosaccharide and inhibition of scavenger receptor activity, these fucoidan-related compounds may be used in foods for improved health and to protect against infection and arteriosclerosis.
Acknowledgements
This study was supported in part by a grant from a Ministry of Education, Culture, Sports, Science and Technology of Japan research project ‘Lifestyle-related diseases overcoming and health enhancement by using traditional medicine’.
References
- Allahverdian , S. , Wojcik , K.R. and Dorscheid , D.R. 2006 . Airway epithelial wound repair: Role of carbohydrate sialyl Lewis X . American Journal of Physiology Lung Cell Molecular Physiology , 291 : L828 – L836 .
- Becker , D.J. and Lowe , J.B. 2003 . Fucose: Biosynthesis and biological function in mammals . Glycobiology , 13 ( 7 ) : 41R – 53R .
- Berliner , J.A. , Navab , M. , Fogelman , A.M. , Frank , J.S. , Demer , L.L. Edward , P.A. 1995 . Atherosclerosis: Basic mechanisms. Oxidation, inflammation, and genetics . Circulation , 91 : 2488 – 2496 .
- Boullier , A. , Bird , D.A. , Chang , M.K. , Dennis , E.A. , Friedman , P. Gillotre-Taylor , K. 2001 . Scavenger receptors, oxidized LDL, and atherosclerosis . Annals of the New York Academy of Sciences , 947 : 214 – 222 .
- Du , M. and Hindsgaul , O. 1996 . Recognition of beta-D-Gal p-(1– > 3)-beta-D-Glc pNAc-OR acceptor analogues by the Lewis alpha-(1– > 3/4)-fucosyltransferase from human milk . Carbohydrate Research , 286 : 87 – 105 .
- Glass , C.K. and Witztum , J.L. 2001 . Atherosclerosis: The road ahead . Cell , 104 : 503 – 516 .
- Greaves , D.R. and Gordon , S. 2005 . Thematic review series: The immune system and atherogenesis. Recent insights into the biology of macrophage scavenger receptors . Journal of Lipid Research , 46 : 11 – 20 .
- Hsu , H.Y. , Chiu , S.L. , Wen , M.H. , Chen , K.Y. and Hua , K.F. 2001 . Ligands of macrophage scavenger receptor induce cytokine expression via differential modulation of protein kinase signaling pathways . Journal of Biological Chemistry , 276 : 28719 – 28730 .
- Lee , S.H. , Takahashi , M. , Honke , K. , Miyoshi , E. , Osumi , D. Sakiyama , H. 2006 . Loss of core fucosylation of low-density lipoprotein receptor-related protein-1 impairs its function, leading to the upregulation of serum levels of insulin-like growth factor-binding protein 3 in Fut8-/-mice . Journal of Biochemistry , 139 : 391 – 398 .
- Morrow , A.L. , Ruiz-Palacios , G.M. , Altaye , M. , Jiang , X. , Guerrero , M.L. Meinzen-Derr , J.K. 2004 . Human milk oligosaccharides are associated with protection against diarrhea in breast-fed infants . Journal of Pediatrics , 145 : 297 – 303 .
- Parthasarathy , S. , Printz , D.J. , Boyd , D. , Joy , L. and Steinberg , D. 1986 . Macrophage oxidation of low-density lipoprotein generates a modified form recognized by the scavenger receptor . Arteriosclerosis , 6 : 505 – 510 .
- Ruiz-Palacios , G.M. , Cervantes , L.E. , Ramos , P. , Chavez-Munguia , B. and Newburg , D.S. 2003 . Campylobacter jejuni binds intestinal H(O) antigen (Fuc1, 2Gal1, 4GlcNAc), and fucosyloligosaccharides of human milk inhibit its binding and infection . Journal of Biological Chemistry , 278 : 14112 – 14120 .
- Yamaki , K. , Goto , M. and Takano-Ishikawa , Y. 2007 . The inhibitory effects of isoflavonoids and resveratrol on oxidized low-density lipoprotein uptake in macrophage cell line J774.1 . Food and Agricultural Immunology , 18 : 67 – 74 .
- Yamaki , K. , Thorlacius , H. , Xie , X. , Lindbom , L. , Hedqvist , P. and Raud , J. 1998 . Characteristics of histamine-induced leukocyte rolling in the undisturbed microcirculation of the rat mesentery . British Journal of Pharmacology , 123 : 390 – 399 .