Abstract
The immunoreactivity and structural variation of β-conglycinin in digesta from digestive tracts of pigs were measured by inhibition ELISA and sodium dodecyl sulphate polyacrylamide gel electrophoresis, respectively. Results showed that the immunoreactivity disappearance proportion of β-conglycinin significantly increased from stomach to the caecum in all groups (P<0.05). In the stomach, upper-jejunum and middle-jejunum, the immunoreactivity disappearance proportion of β-conglycinin significantly increased among these three groups (P<0.05), while it has no significant difference in ileum and caecum (P>0.05). The α′, α subunits of β-conglycinin were easier to digest than the β subunit. It indicated that immunoreactivity disappearance proportion of β-conglycinin tended to increase with the growth of age and the descending down of digestive tract, while the β subunits of β-conglycinin are more stable to digestion than α′, α subunits.
Introduction
Soybean (Glycine max) is one of the most important plant protein sources and it is widely used in human and animal diets due to its beneficial effects on human and animal health. However, it was also considered to be an important allergenic food, which induced allergic symptoms, may range from skin, gastrointestinal or respiratory reactions to anaphylaxis (Sampson, Citation2004; Scott & Hugh, Citation2006). β-conglycinin, one of the major soybean storage proteins, which account for about 30% of the total seed proteins, is considered to be an important soybean allergen (Thomas et al., Citation2008; You et al., Citation2008). The presence of β-conglycinin in soybean (Glycine max) has limited its wide use in human and animal diets. β-conglycinin is made up of α′, α and β subunits, and their molecular weights (MWs) are about 76 kDa, 72 kDa and 53 kDa, respectively (Koshiyama & Fukushima, Citation1976; Nobuyuki et al., Citation2003). Researche into β-conglycinin allergy have been described primarily in children and young animals (Scott & Hugh, Citation2006), but little data on the prevalence of β-conglycinin allergy in adults and matured animals has been published. To increase our knowledge on soybean allergy, a study on the stability of soybean β-conglycinin in vivo was made. In this study, pigs in different physiological stages were used; purified β-conglycinin was fed to the pigs in order to eliminate interference from other components in soybean products. The immunoreactivity disappearance proportion of β-conglycinin was calculated after the detection of immunoreactivity for β-conglycinin in the digesta of each segment using inhibition ELISA and the structural variation of β-conglycinin were analysed by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). This may provide further information on the β-conglycinin allergy in vivo, which may have important implications for preventing and treating soybean-related allergic disease in human beings and animals.
Materials and methods
Animals and experimental design
Animals were maintained according to the rules of China Animal Care and Use Committee. Fifteen healthy pigs with an average initial body weight of 8.0±0.5 kg, weaned at 28 days of age, were allocated to three groups randomly, each group with five replicates. All pigs received diets without ingredients originating from leguminous products in non-experimental periods; while they received diets containing 4% purified β-conglycinin (donated by Professor Shun-tang Guo from the Food Institute of China Agricultural University; Patent No. 200410029589.4) in experimental periods. The purity of β-conglycinin was analysed by SDS-PAGE. The experimental periods of each group started after 3 days of adaptation, and lasted for 7 days, Group A are from 30 to 37 days of age (weaning piglets), Group B are from 98 to 105 days of age (growing pigs) and Group C are from 168 to 175 days of age (finished pigs). An indigestible indicator chromium sesquioxide (0.5%) was added to the diets in experimental periods. Pigs were fed supplements without ingredients originating from leguminous products during the suckling period in order to adapt to this routine, and all diets were formulated on an isoenergetic and isonitrogenous basis ().
Table 1. Composition of the diets, as-fed basis (%).
Sample collection and preparation
The digesta from stomach, upper-jejunum, middle-jejunum, ileum and caecum were collected immediately after the pigs were killed 2 hours after the morning meal. The diets (50 g) of feed to each pig were also collected. β-conglycinin was extracted using 10 volumes of 0.025M Tris-HCl (pH 8.6) for the diets and digesta from each segment, by shaking for 30 min at room temperature using a wrist-action shaker (Perez, Mills, Lambert, Johnson, & Morgan, Citation2000). Extracts were clarified by centrifuging at 10,000 r/min for 15 min at 4°C and were stored at −80°C until required.
Inhibition ELISA
Following the methods described by Perez, Mills, Lambert, Johnson, and Morgan (Citation2000), the immunoreactivity of β-conglycinin was determined with some modifications.
Microtitre plates were coated with β-conglycinin by adding 0.3 ml/well of a 1 µg ml−1 solution of each protein dissolved in 50 mM sodium carbonate–bicarbonate buffer (pH 9.6) and incubating for 3 h at 37°C. The plates were blocked with 1% bovine serum albumin (0.1 ml/well), followed by three washes with phosphate-buffered saline containing 0.5 ml L−1 Tween 20 (PBST) for 1 h at 37°C. After washing three times with PBST, the samples diluted with appropriate PBS were added in triplicate to the corresponding globulin-coated plates (0.1 ml/well) together with 0.1 ml/well of anti-β-conglycinin antibody (prepared according to He, Hu, & Wang (Citation2005) diluted 1:1000 (v v−1) in PBST. After incubating for 2 h at 37°C, the plates were washed three times with PBST before adding 0.1 ml/well of goat anti-rabbit IgG labelled with horseradish peroxidase (Sigma, St. Louis, MO, USA) diluted 1:5000 (v v−1) with PBST. Following a further incubation for 2 h at 37°C, the plates were washed three times with PBST prior to adding 0.1 ml/well of substrate solution (0.7 mg ml−1) based on o-phenylenediamine (Sigma, St. Louis, MO, USA). The reaction was terminated by 50 µl 2M H2SO4 after 10 min incubation, and the optical density value at 490 nm was determined using the Immuno-Microplate Autoreader (Bio-rad Model 680, USA). Then the immunoreactivity of β-conglycinin was calculated based on the principle of inhibition ELISA.
The immunoreactivity disappearance proportion of β-conglycinin
The immunoreactivity disappearance proportion of β-conglycinin was calculated according the indicator technique based on the value measured by inhibition ELISA using the formula:
a = Immunoreactivity of globulins in diets (mg g−1).
b = Concentration of Cr in diets (mg g−1).
c = Immunoreactivity of globulins in digesta (mg g−1).
d = Concentration of Cr in digesta (mg g−1).
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE)
The structural variations of β-conglycinin in digesta were analysed by SDS-PAGE using BIO-RAD Mini-PROTEAN® Tetra Cell (BIO-RAD Laboratories, Inc., USA) followed by Laemmli (Citation1970). It was carried out in the presence of Tris-Glycine buffer (25 mM Tris, 192 mM Glycine, pH 8.3) with a 12% acrylamide separating and a 5% acrylamide stacking gel. The volume of globulin extracts loaded on the gels was 20 µl per well. The protein MW marker (low) was loaded in a separate well for 10 µl. The electrophoresis was performed for 15 min at 100 V for the acrylamide stacking gel and 45 min at 200 V for the acrylamide separating gel. Gels were stained for protein using Coomassie blue R250.
Statistical analysis
The data were analysed using the General linear model (GLM) procedure (SPSS Inc., Chicago, USA). The model used was:
where Yij=observation, Î1/4 = population mean, Ti=segment effect (i=1–5), Pj=physiological stage effect (j=1–3), TiPj=interaction between segment effectÃ×physiological stages effect and εij=residual error. The results were expressed as mean values±standard error of the mean. Significant differences were accepted if P≤0.05.
Results
The purity of β-conglycinin
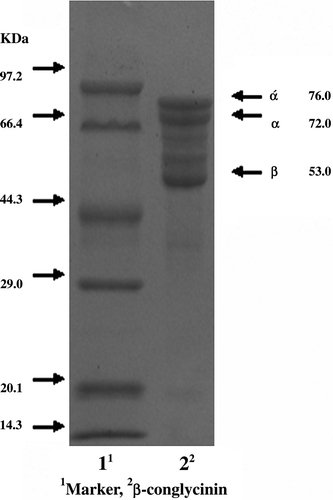
From the SDS-PAGE gels, we can see that the protein bands of β-conglycinin are made of α′, α and β subunits and their MWs are about 76 kDa, 72 kDa and 53 kDa, respectively. These protein bands are clear and they satisfied the needs of our study ().
The immunoreactivity disappearance proportion of β-conglycinin in digesta
Interaction between segments and physiological stages (segments×physiological stages) was found. The results showed that the immunoreactivity disappearance proportion of β-conglycinin significantly increased from stomach to the caecum in all groups (P<0.05). In the stomach, upper-jejunum and middle-jejunum, the immunoreactivity disappearance proportion of β-conglycinin significantly increased among these three groups (P<0.05), while the immunoreactivity disappearance proportion of β-conglycinin in ileum and caecum has no significant difference (P≥0.05). In the stomach, only 33% immunoreactivity of β-conglycinin disappeared, while near-66% immunoreactivity of β-conglycinin disappeared in the small intestine which may indicate that there was a difference between gastric and intestinal digestion of β-conglycinin. However, the immunoreactivity was quite high along the gastrointestinal tract, even in the caecum (≈99%) the immunoreactivity disappearance proportion of β-conglycinin was not up to 100% ().
Table 2. Analysis of the immunoreactivity disappearance proportion for β-conglycinin in digesta.
The structure variation of β-conglycinin in digesta
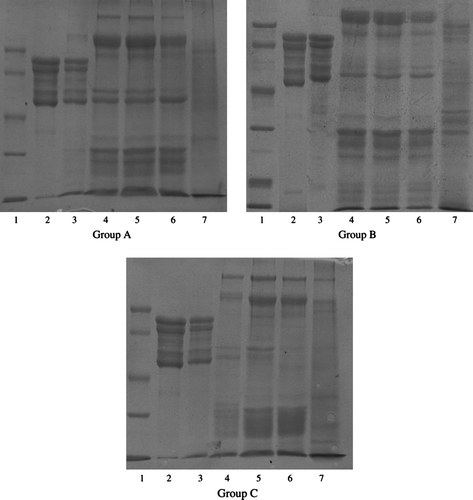
The SDS-PAGE gels stained for proteins showed that protein bands of the α′, α and β subunits of β-conglycinin were detected in gastric digesta of all groups. From the upper-jejunum, the protein bands of the α′, α subunits of β-conglycinin disappeared, while the β subunit is quite stable along the gastrointestinal tract as it is still could be detected in upper and middle-jejunum of these three groups. In ileum, the β subunit still could be detected in Groups A and B, while it nearly could not be detected in Group C. No clear protein bands can be detected in the digesta from caecum ().
Figure 2. SDS-PAGE gels of β-conglycinin in the digesta. Lane 1: the protein molecular weight marker (low; KDa): phosphorylase b 97.200; bovine serum albumin, 66.400; ovalbumin, 44.300; carbonic anhydrase, 29.000; soybean inhibitor, 201.00; α-lactalbumin, 14.300. Lane 2: purified β-conglycinin (added to diets). Lane 3–7: β-conglycinin extracted from the digesta of stomach, upper-jejunum, middle-jejunum, ileum and caecum.
Discussion
The purity of glycinin and β-conglycinin
Many studies have been made on the soybean allergens, but the reliability coefficients of these researches were limited as they often used heated soybean flour, ethanol-treated soybean protein concentrate or soybean isolates (Lalles et al., Citation1999; Perez et al., Citation2000). These soybean products may contain more than one kind of antinutritional factors. Purified β-conglycinin was used in this study in order to eliminate the interference from the other antinutritional factors in soybean. The SDS-PAGE gels showed that the bands of β-conglycinin are clear and have no hybrid-protein bands. It satisfied in the use of this study.
Creep feed
From one week of age, piglets were offered solid feed without ingredients originated from leguminous products in order to satisfy the nutrients required for the fast growth rates. Moreover, creep feed is thought to be a way that can improve the digestive capability of pigs after weaning (Pluske, Dividich, & Verstegen, Citation2003; Pluske, Williams, & Aherne, Citation1995).
Comparison of the immunoreactivity disappearance proportion of β -conglycinin in digesta
On the basis of immunological investigations, an interaction between physiological stages and segments was found in this study. Results showed that the immunoreactivity disappearance proportion of β-conglycinin tended to increase as it descended down the gastrointestinal tract, which is in agreement with the results in calves (Sissions & Thurston, Citation1984; Tukur, Lalles, Mathis, Caugant, & Toullec, Citation1993). Moreover, it is also tended to increase with the growth of the age of pigs as their digestive tracts became more and more matured. Only 33% immunoreactivity of β-conglycinin disappeared in the stomach which may attribute to the stability of β-conglycinin to pepsin (Astwood, Leach, & Fuchs, 1996). The immunoreactivity disappearance proportion of β-conglycinin continually increased as β-conglycinin continued to get digested in the small intestine. However, the immunoreactivity of β-conglycinin did not disappear completely, as it still could be detected in the digesta from caecum in all groups, and this may increase the possibility of the occurrence of allergic reaction in pigs (Baratt, Strachan, & Poter, Citation1978; Li et al., Citation1990).
Significant difference of the immunoreactivity disappearance proportion of β-conglycinin in stomach, upper and middle-jejunum among weaning pigs, growing pigs and finishing pigs have been found in this study, which may attribute to the great difference in digestive physiology of these pigs. In the weaning period, the digestive function of piglets is immature as the activity of most proteinases did not show their best until the time the pigs were 8 weeks of age (Efird, Armstrong, & Dennis, Citation1982; Han, Citation2002; Hartma, Hays, Baker, Neagle, & Catron, Citation1961). Moreover, there were profound changes in piglets’ digestive physiologies following weaning as the diets changed from liquid to solid. Villus atrophy and digestive enzyme activity depression have been documented (Funderburke & Seerley, Citation1990; Lalles, Bosi, Smidt, & Stokes, Citation2007; Lalles, Boudry, & Favier, Citation2004). At this time the pigs showed a suboptimal digestive competence as the rapid changes in the structure and function of the digestive tract occurred. This may lead to the low immunoreactivity disappearance proportion of β-conglycinin in weaning piglets compared with growing and finished pigs. This study also found that the immunoreactivity disappearance proportion of β-conglycinin among weaning piglets, growing pigs and finished pigs are not significantly different in ileum and caecum. This may be attributed to two reasons: first, the ileum is the last part of small intestine and its shorter length may not play a very important role in the digestion of proteins (Li, Citation2003); second, the microorganisms in the caecum interfere with the digestion of proteins too. From the data we achieved in this study, we could find that growing pigs had a higher immunoreactivity disappearance proportion of β-conglycinin than Group A weaning piglets, which may be attributed to the much more mature digestive tract and stronger digestive capability. Finished pigs had the highest immunoreactivity disappearance proportion of β-conglycinin as the pigs had the strongest digestive capability in the three groups.
Comparison of the structure variation of β-conglycinin in digesta
The digestion of β-conglycinin in the stomach is limited as the protein bands of the α′, α and β subunits of β-conglycinin were detected in gastric digesta of all groups which is coincident with the results from Astwood et al. (Citation1996) that β-conglycinin are stable to pepsin in vitro. However, from the upper—jejunum, the protein bands of the α′, α subunits of β-conglycinin disappeared, while the β subunit still could be detected in upper—and middle-jejunum of the three groups. In ileum, the β subunit still could be detected in Groups A and B, while it could not be detected in Group C which may be attributed to the high maturity of the digestive tract of pigs in Group C. The results indicated that β subunit is more stable along the small intestine than the other two subunits. This may be attributed to two reasons. First, α and α′ subunits are rich in arginine, while β subunit had a higher content of hydrophobic amino acids (Hirano, Chikafusa, & Kyuya, Citation1984; Thanh & Shibasaki, Citation1977). Second, enzymes show their specificity when they recognise substrates. Trypsin acts on arginine and lysine residues, while chymotrypsin acts on large hydrophobic residues such as tryptophan, tyrosine and phenylalanine (Beynon & Bond, Citation1989; Thomas, Citation2006). All of these may contribute to the results that α and α′ subunits are much more susceptible to trypsin and chymotrypsin than β subunit which we achieved in this study. In the caecum, no clear protein bands can be detected which may be attributed to the actions of microorganisms (Li, Citation2003). Recent investigations have made it clear that the entering of many nutrients into the caecum could increase the susceptibility to gut disorders and diarrhoea, which is confirmed with the observations in this study.
Conclusions
In conclusion, the immunoreactivity disappearance proportion of β-conglycinin tend to increase with the growth of age and the descending down of the digestive tract; and the β subunit of β-conglycinin are more stable to digestion than α′, α subunits.
Acknowledgements
This study was completed at the College of Animal Science and Technology (Jilin Agricultural University, Changchun, China) and the Jilin University Swine Breeding Center (Jilin University, Changchun, China). Financial support for this study was provided by the National Natural Science Foundation of P. R. China (No. 30430520). The author thanks X.L. Zhang for critical reading of the manuscript.
References
- Astwood , J.D. , Leach , J.N. and Fuchs , R.L. 1996 . Stability of food allergens to digestion in vitro . Nature Biotechnology , 14 : 1269 – 1273 .
- Baratt , M.E.J. , Strachan , P.J. and Poter , P. 1978 . Antibody mechanisms implicated in digestive disturbance following ingestion of soya protein in calves and piglets . Clinical and Experimental Immunology , 31 : 305 – 312 .
- Beynon , R.J. and Bond , J.S. 1989 . Proteolytic enzymes. A practical approach , Oxford : IRL Press .
- Efird , R.C. , Armstrong , W.D. and Dennis , L.H. 1982 . The development of digestive capacity in young pigs: Effects of age and weaning system . Journal of Animal Science , 55 : 1380 – 1387 .
- Funderburke , D.W. and Seerley , R.W. 1990 . The effects of postweaning stressors on pig weight change, blood, liver and digestive tract characteristics . Journal of Animal Science , 68 : 155 – 162 .
- Han , Z.K. 2002 . Livestock physiology , 3rd ed , Beijing : China Agricultural Publisher .
- Hartma , P.A. , Hays , V.W. , Baker , R.O. , Neagle , L.H. and Catron , D.V. 1961 . Digestive enzyme development in the young pig . Journal of Animal Science , 20 : 114 – 123 .
- He , Z.Y. , Hu , G.X. and Wang , C.F. 2005 . Technique of animal immunology , Changchun : Science and Technology Publishing House .
- Hirano , H. , Chikafusa , F. and Kyuya , H. 1984 . The complete amino acid sequence of the A subunit of the glycinin seed storage protein of the soybean (Glycine max (L.)) Merrill . Journal of Biological Chemistry , 259 : 14371 – 14377 .
- Koshiyama , I. and Fukushima , D. 1976 . Identification of the 7S globulin with β-conglycinin in soybean seeds . Phytochemistry , 15 : 157 – 159 .
- Laemmli , U.K. 1970 . Cleavage of structural proteins during the assembly of the head of bacteriophage T 4 . Nature , 227 : 680 – 685 .
- Lalles , J.P. , Bosi , P. , Smidt , H. and Stokes , C.R. 2007 . Weaning – a challenge to gut physiologists . Livestock Science , 108 : 82 – 93 .
- Lalles , J.P. , Boudry , G. and Favier , C. 2004 . Gut functions and dysfunction in young pigs: Physiology . Animal Research , 53 : 301 – 316 .
- Lalles , J.P. , Tukur , H.M. , Salgado , P. , Mills , E.N.C. , Morgan , M.R.A. and Quilien , L. 1999 . Immunochemical studies on gastric and intestinal digestion of soybean glycinin and β-conglycinin in vivo . Journal of Agricultural and Food Chemistry , 47 : 2797 – 2806 .
- Li , D.F. 2003 . Nutrition of swine , 2nd ed , Beijing : China Agricultural Publisher .
- Li , D.F. , Nelssen , J.L. , Reddy , P.G. , Belcha , F. , Hancock , J.D. Allee , G.L. 1990 . Transient hypersensitivity to soybean meal in the early-weaned pig . Journal of Animal Science , 68 : 1790 – 1799 .
- Nobuyuki , M. , Takako , F. , Shiori , S. , Nauko , I. , Junko , K. Mayumi , M. 2003 . Molecular and structural analysis of electrophoretic variants of soybean seed storage proteins . Phytochemistry , 64 : 701 – 708 .
- Perez , M.D. , Mills , E.N.C. , Lambert , N. , Johnson , I.T. and Morgan , M.R.A. 2000 . The use of anti-soya globulin antisera in investigating soya digestion in vivo . Journal of the Science of Food and Agriculture , 80 : 513 – 521 .
- Pluske , J.R. , Dividich , J.L. and Verstegen , M.W.A. 2003 . Weaning the pig: Concepts and consequences , Wageningen, , The Netherlands : Wageningen Academic Publishers .
- Pluske , J.R. , Williams , I.H. and Aherne , F.X. 1995 . “ Nutrition of the piglet ” . In The neonatal pig development and survival , Edited by: Varly , M.A. 187 – 235 . Wallingford, , UK : CAB International .
- Sampson , H.A. 2004 . Update on food allergy . Journal of Allergy and Clinical Immunology , 113 : 805 – 819 .
- Scott , H.S. and Hugh , A.S. 2006 . Food allergy . Journal of Allergy and Clinical Immunology , 117 : 470 – 475 .
- Sissions , J.W. and Thurston , S.M. 1984 . Survival of dietary antigens in the digestive tract of calves intolerant to soybean products . Research in Veterinary Science , 37 : 242 – 246 .
- Thanh , V.H. and Shibasaki , K. 1977 . Beta-conglycinin from soybean proteins. Isolation and immunological and physicochemical properties of the monomeric forms . Biochimica et Biophysica Acta , 490 : 370 – 384 .
- Thomas , A. 2006 . Digestive functions . Anesthesia and Intensive Care Medicine , 7 ( 2 ) : 59 – 60 .
- Thomas , H. , Olga , W. , Barbara , K.B.W. , Carsten , B.J. , Joseph , S. Lorenza , P.G. 2008 . Soybean (Glycine max) allergy in Europe: Gly m 5 (b-conglycinin) and Gly m 6 (glycinin) are potential diagnostic markers for severe allergic reactions to soy . Journal of Allergy and Clinical Immunology , 123 : 452 – 458 .
- Tukur , H.M. , Lalles , J.P. , Mathis , C. , Caugant , I. and Toullec , R. 1993 . Digestion of soybean globulins, glycinin, α-conglycinin and β-conglycinin in the preruminant and ruminant calf . Canadian Journal of Animal Science , 73 : 891 – 905 .
- You , J.M. , Li , D.F. , Qiao , S.Y. , Wang , Z.R. , He , P.L. Ou , D.Y. 2008 . Development of a monoclonal antibody-based competitive ELISA for detection of β-conglycinin, an allergen from soybean . Food Chemistry , 106 : 352 – 360 .