Abstract
Fungal immunomodulatory proteins (FIPs) are a group of novel proteins, purified from medicinal fungi or edible mushrooms that possess immunomodulatory properties. FIP-gts and FIP-fve have been isolated and purified from Ganoderma tsugae and Flammunlina velutipes, respectively. The evaluation of FIP immunomodulatory activity was based on their ability to stimulate human peripheral blood lymphocytes to release interferon-gamma (IFN-γ). We found that FIP-gts exhibited better immunomodulatory activity than FIP-fve. Activities were both greatly reduced with duration of heating. For digestibility, FIP-fve was more resistant than FIP-gts to digestive enzymes in simulated gastric fluid and simulated intestinal fluid. IFN-γ production is only detectable in dimers of FIP-gts as opposed to polymer of FIP-fve. These results suggest that FIP-gts and FIP-fve have activities that are stable and have a strong potential of being applied to food or pharmaceutical products for commercial development.
Introduction
Fungal immunomodulatory proteins (FIP)-gts and FIP-fve have been isolated and purified from Ganoderma tsugae and Flammulina velutipes, respectively (Ko, Hsu, Lin, Kao, & Lin, 1995; Liao et al., Citation2006). A previous study observed a 61.4% (70 invariant amino acid residues) sequence similarity between FIP-gts and FIP-fve. Their structure is similar to that of human immunoglobulin (Ig) heavy chain and exhibits the potential to modulate immune reactions similar to Ling Zhi (LZ)-8, purified from the Oriental medicinal fungus Ganoderma lucidium. FIPs exert immunomodulatory effects of interleukin (IL)-2, interferon-gamma (IFN-γ) and tumour necrosis factor-alpha (TNF-α) production in human peripheral blood lymphocyte (hPBMC; H.C. Hsu, C.I. Hsu, Lin, Kao, & Lin, Citation1997; Ko et al., Citation1995). Earlier demonstrations prove that FIP-fve and FIP-gts are potent T-cell activators and enhance the activation of IFN-γ release in Th1 cells (Hsiao et al., Citation2008; Wang et al., Citation2004). It is well-known that Th1 cells produce mainly IL-2 and IFN-γ, while allergy-associated Th2 cells predominantly produce IL-4 and IL-6. The production of IFN-γ by Th1 cells can augment Th1 development and inhibit the proliferation of Th2 cells, whereas IL-4 produced by Th2 cells shows anti-inflammatory function of Th1 cells. The degree and extent of the production of the initial allergic trigger, IgE, are mainly determined by the co-operative effect between antagonistic IL-4 and IFN-γ functions (Wold, Citation1998). Therefore, modulation of the functional differentiation of allergen-specific Th1 cells may have clinical utility in the treatment of allergy-induced diseases.
In addition, more evidence shows that FIPs may possess anti-cancer activity. For instance, FIP-gts inhibits the growth of A549 lung cancer cells by suppressing telomerase activity in vitro (Liao et al., Citation2007). In a previous study, FIP-gts significantly inhibited lung tumour growth in a mouse model (Liao et al., Citation2008). It has been shown that aqueous extracts of G. tsugae are potent inhibitors of cell proliferation in human breast cancer cell lines MCF-7 and MDA-MB-231 (Yue, Fung, Tse, Leung, & Lau, Citation2006). FIP-gts also reduced cell migration and invasion in cervical cancer cells (Wang et al., Citation2007).
Heating is a common process in food preparation. However, the heating process may result in undesired consequences such as changes to flavour, colour, and most importantly, a decrease in nutritional value. In general, proteins are heat-sensitive compounds. Many food proteins easily lose biological activity after heating. Hence, it is important to predict whether proteins can potentially maintain bioactivity during the heating process. We examined the effects of heating on the immunomodulatory activity of FIP-gts and FIP-fve in hPBMC cells.
The bioactivity of proteins may be denatured or inactivated during extreme physico-chemical operations, such as acid/alkali treatments and diverse storage methods. For efficient storage, proteins and polypeptides are preferably prepared as dry powders by utilising processing methods, such as freeze and spray-drying. However, as the bioactivity of proteins may be lost during processing and storage, protectants are generally required to increase the long-term stability (Liao, Brown, & Martin, 2004). Since the resistance of FIP-gts and FIP-fve to storage and acid/alkali treatments in food processing and pharmaceutical production for the regulation of cellular immune function remained unknown, it is important to understand their remaining bioactivities after processing operations.
Oral administration of FIP-fve may be useful in preventing food allergy responses (Hsieh, Hsu, Lin, Tsai, & Lin, 2003). This suggests that FIP-fve may be resistant to gastrointestinal hydrolysis. However, previous studies have found that there was not a clear relationship between protein digestibility measured in vitro and protein cellular functions (Fu, Abbott, & Hatzos, 2002). Therefore, we have measured and compared the digestive stability of FIP-gts and FIP-fve in simulated gastric fluid (SGF) and simulated intestinal fluid (SIF) digestion.
Some studies have established the immunomodulatory activity of FIP-gts which is in the form of homodimers through N-terminal amphipathic α-helix (Lin, Hung, Hsu, & Lin, 1997). FIP-fve is also predicted to form dimers by 3-D domain swapping of the N-terminal amphipathic α-helix (Paaventhan et al., Citation2003). Hence, the results suggest that FIP-gts or FIP-fve may display immunomodulatory activity via formation of homodimers.
Based on previous studies, the FIP-gts and FIP-fve exhibit a health promotive and therapeutic effect. However, effects of conventional food processing operation on the immunomodulatory activity of FIP-gts and FIP-fve remain unknown. Thus, an investigation was conducted on the effects of heating, pH manipulation and storage variation on the immunomodulatory activity of FIP-gts and FIP-fve. The dimerisation of FIPs and its role in FIPs immunomodulatory activity was investigated. An additional observation on digestibility of FIP-gts and FIP-fve was also conducted.
Materials and methods
Purification of fungal immunomodulatory protein (FIP)-gts and fungal immunomodulatory protein (FIP)-fve
FIP-gts and FIP-fve were isolated and purified as previously described (Ko et al., Citation1995; Liao et al., Citation2006). The fruit bodies were homogenised with 500 ml ice-cold 5% acetic acid in the presence of 0.05M 2-mercaptoethanol, and soluble proteins in the supernatant were precipitated by addition of ammonium sulphate to 95% of saturation. The precipitate was collected and then dialysed against 10 mM sodium acetate (pH 5.2). The dialysate was applied to a carboxymethyl (CM-52) cellulose column that had been equilibrated with 10 mM sodium acetate (pH 5.2). The active fractions were collected and presented as a single peak on absorbance at 280 nm. The active protein was present as a single band on SDS-PAGE at 16 and 13 kDa, respectively. The SDS-PAGE analyses of samples were performed according to Laemmli (Citation1970) in 12% slab gels on a Bio-Rad mini protein II gel apparatus (Hercules, CA, USA). The gels were stained with Coomassie brilliant blue R.
FIP-gts and FIP-fve had less than 30 endotoxin units (EU)/ml (1 EU = 0.1 ng, Limulus Amebocyte Lysate, Cape Cod, MS).
Interferon-gamma (IFN- γ ) production by fungal immunomodulatory protein (FIP)-gts and fungal immunomodulatory protein (FIP)-fve
Different concentrations of FIP-gts and FIP-fve were used as stimuli. For the analysis of IFN-γ stimulatory activity assay, 2×106 cells/ml hPBMCs were cultured in 24-well tissue culture plates and stimulated with different concentration of FIP-gts and FIP-fve for 48 h at 37°C. Supernatants were harvested and assayed as per manufacturer's instructions for the production of IFN-γ using commercially available kits from R&D Systems (Minneapolis, MN, USA).
Heat treatment of fungal immunomodulatory protein (FIP)-gts and fungal immunomodulatory protein (FIP)-fve
FIP-gts and FIP-fve were loaded into polycarbonate microcentrifuge tubes and held in PCR machine for 0, 0.5, 1, 2, 4 and 8 min at 100°C. After the heat treatment, these samples were immediately cooled for 10 min on ice. The heated FIP-gts and FIP-fve were cultured immediately with hPBMCs. After 48 h incubation, the supernatants were harvested for IFN-γ assay by commercial ELISA kits.
Effect of pH on the immunomodulatory activity of fungal immunomodulatory protein (FIP)-gts and fungal immunomodulatory protein (FIP)-fve
The pH stability of FIP-gts and FIP-fve was determined by pre-incubating in buffers at different pH levels in the range of 4.0–10.0 for 4 h at 4°C. The following buffer systems were used: 10 mM acetate buffer for pH 4.0; phosphate buffer for pH 6.0; Tris buffer for 8.0 and sodium carbonate buffer 10.0. After 4 h, the pH values of dialysates were measured using pH indicator paper (pH 3–14, Whatman International, Ltd., Maidstone, UK) to verify their equilibrium. Samples were further dialysed against phosphate-buffered saline (PBS, pH 7.2) for 24 h to investigate the immunomodulatory activity. All dialysed samples were filtered for sterility and stored at −20°C. A 2×106 hPBMC were incubated with the aforementioned dialysed samples. After 48 h incubation, supernatants were harvested to determine their IFN-γ levels.
Deep-freezing and lyophilisation on the immunomodulatory activity of fungal immunomodulatory protein (FIP)-fve
Freshly prepared FIP-fve was divided into five groups: (1) only lyophilised; (2) lyophilised with 30% sucrose; (3) lyophilised with 3.2M ammonium sulphate; (4) deep-frozen; and (5) deep-frozen plus glycerol. All samples were stored at –80°C for two months. After storage, all frozen and lyophilised samples were incubated with hPBMC for 48 h to test IFN-γ levels.
Simulated gastric fluid (SGF) digestion stability assay
SGF digestion stability assay was performed as previously described (Fu et al., Citation2002), with only minor modifications. SGF consisted of 0.032 g/ml pepsin in 30 mM NaCl at pH 1.2. Aliquots (200 µl) of SGF were placed in microcentrifuge tubes and incubated in a water bath at 37°C. FIP-gts and FIP-fve (5 mg/ml in 30 mM NaCl) were added to each of the SGF vials to start the digestion reaction. At intervals of 0, 0.5, 5, 15, 60 and 120 min, 75 µl of 1N NaOH and 70 µl of 5×sample buffer were added to each vial to stop the reaction. The samples were then analysed using SDS-PAGE.
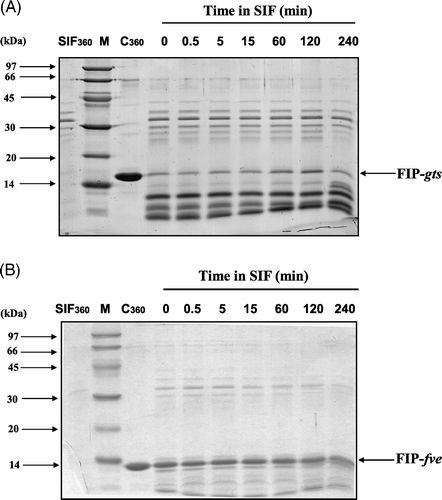
Simulated intestinal fluid (SIF) digestion stability assay
SIF digestion stability assay was performed as previously described (Fu et al., Citation2002), with only minor modifications. SIF consisted of 0.01 g/ml of pancreatin in 50 mM KH2PO4, pH 7.5. Aliquots (64 µl) of SIF were placed in microcentrifuge tubes and incubated at 37°C for 10 min in a water bath. The FIP-gts and FIP-fve samples (10 µl) at concentration of 5 mg/ml (in 50 mM KH2PO4, pH 7.5) were added to each microcentrifuge tube to start the reaction. At intervals of 0, 0.5, 5, 15, 60, 120 and 240 min, 15 µl of 5×sample buffer was added to each tube, and the reaction was immediately stopped by placing the tube in a boiling water bath for 10 min. The samples were then analysed using the SDS-PAGE procedure previously described.
Chemical cross-linking
Chemical cross-linking of FIPs was performed, as previously described (Lin et al., Citation1997) FIP-gts and FIP-fve were separately cross-linked with various concentrations of glutaraldehyde. Both FIP-gts and FIP-fve were dialysed against doubly distilled at 4°C for over 16 h to remove the salt compounds before the measurement of dimerisation. Cross-linking was carried out for 2 h at room temperature. The reaction was stopped with 5 mM Tris/HCl buffer (5 µl), pH 8.0, and samples further incubated for 20 min at room temperature. Portions of the reaction mixtures (10 µl) were analysed by SDS-PAGE (15% polyacrylamide). The remaining mixtures were assayed for the analysis of IFN-γ production.
Preparation of human peripheral blood lymphocyte (hPBMC)
Human PBMCs were obtained from the heparinised peripheral blood of healthy donors by centrifugation over Ficoll-paque gradient medium (Amersham Biosciences, Uppsala, Sweden). For measurement of IFN-γ secretion, 2×106 hPBMC were cultured in a volume of 1 ml (a concentration of 2×106 cells/ml) in RPMI1640 medium (Gibco, Grand Island, NY), supplemented with 10% fetal bovine serum (FBS), 100 µg/ml streptomycin, 100 units/ml penicillin, and 200 mM l-glutamate in 24-well tissue culture plates (Nunc, Roskilde, Denmark) at 37°C in an atmosphere containing 5% CO2.
Interferon-gamma (IFN-s γ) production assay
FIP-gts and FIP-fve, after being treated with the various previously described conditions, were used as a stimulus. For cytokine analysis, 2×106 cells/ml hPBMCs were cultured in 24-well tissue culture plates and stimulated with previously treated FIP-gts and FIP-fve for 48 h at 37°C. Supernatants were harvested and assayed for the production of IFN-γ.
Statistical analysis
All values are given as means±SD. Statistical analysis was performed using ANOVA. In all cases, P values less than 0.05 were considered statistically significant.
Results
Immunomodulatory activities of fungal immunomodulatory protein (FIP)-gts and fungal immunomodulatory protein (FIP)-fve
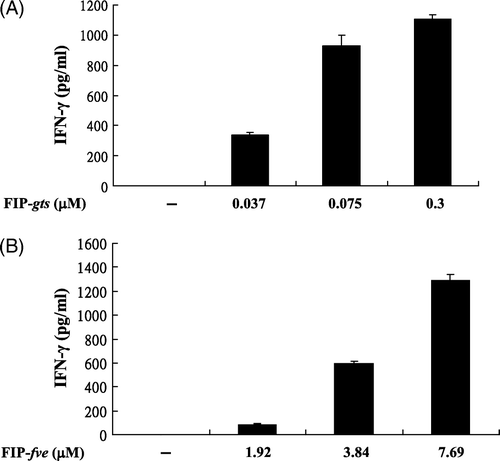
In order to assess the effects of FIP-gts and FIP-fve on the IFN-γ stimulatory activity in hPBMC, cells were treated with various concentrations of FIP-gts (A) and FIP-fve (B) for 48 h. For FIP-gts (A) and FIP-fve (B), untreated PBMCs secreted certain amounts of IFN-γ (<16 pg/ml). After treatment with FIP-gts (0.3 µM) and FIP-fve (7.69 µM), the production of IFN-γ increased significantly in a dose-dependent manner and reached a plateau at 0.3 µM/ml and 7.6 µM/ml, respectively. The results showed that FIP-gts (0.3 µM) and FIP-fve (7.69 µM) had the most IFN-γ stimulatory activity in hPBMCs.
Figure 1. Effects of FIP-gts and FIP-fve on IFN-γ secretion in PBMCs. (A) Effects of FIP-gts on IFN-γ secretion in hPBMCs. Cultured hPBMCs (2×106 cells/ml, 1 ml/well) were treated with the indicated concentrations of FIP-gts in RPMI 1640 supplemented with 10% FBS for 48 h. Conditioned media were subjected to ELISA to measure amounts of secreted IFN-γ (mean value from three independent experiments). (B) Effects of FIP-fve on IFN-γ secretion in hPBMCs. Cultured peripheral T cells (2×106 cells/ml, 1 ml/well) were treated with the indicated concentrations of FIP-fve in RPMI 1640 supplemented with 10% FBS for 48 h. Conditioned media were subjected to ELISA to measure amounts of secreted IFN-γ (mean value from three independent experiments).
Immunomodulatory activities of fungal immunomodulatory protein (FIP)-gts and fungal immunomodulatory protein (FIP)-fve after heat process
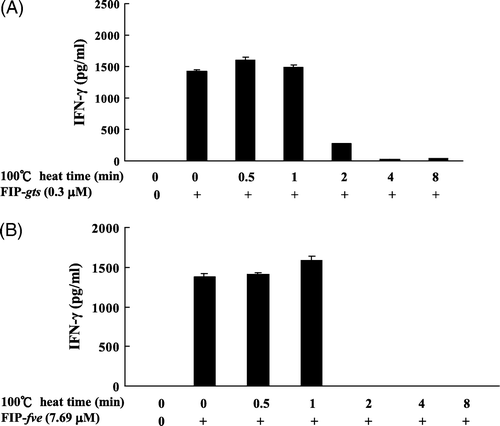
The effects of heating on the immunomodulatory activities of FIP-gts and FIP-fve are shown in A and B. The IFN-γ activity in the FIP-gts and FIP-fve was greatly reduced by the heat treatment at 100°C for 2–8 min. However, the immunomodulatory activities are stable at 100°C for 1 min.
Figure 2. Effect of heating on the stability of FIP-gts and FIP-fve-induced IFN-γ secretion in hPBMCs. The FIP-gts and FIP-fve were pre-incubated at 100°C from 0 to 8 min and residual immunomodulatory activity was determined. Cells cultured without stimulant in the culture supernatants were used as control samples. Human PBMC (2×106 cells/ml) were stimulated by non-heated and heated FIP-gts (0.3 µM) and FIP-fve (7.69 µM) for 48 h. Culture supernatant was collected and assayed for IFN-γ levels by ELISA. The IFN-γ is expressed as pg/ml (mean value from three independent experiments).
Effects of acid and alkali treatments on fungal immunomodulatory protein (FIP)-gts and fungal immunomodulatory protein (FIP)-fve activities
Our study showed that the immunomodulatory activities of FIP-gts and FIP-fve were very stable over a wide pH range (pH 4, pH 6, pH 8, and pH 10, respectively). FIP-gts kept over 90% of its original activity at pH 8.0 and 10. In acid conditions, the IFN-γ production kept 83 and 88%, respectively, of its original levels at pH 4.0 and 6.0. Similarly, FIP-fve kept over 90% of its original activity in all acid/alkali treatments.
Storage stability of deep-frozen and lyophilised fungal immunomodulatory protein (FIP)-fve
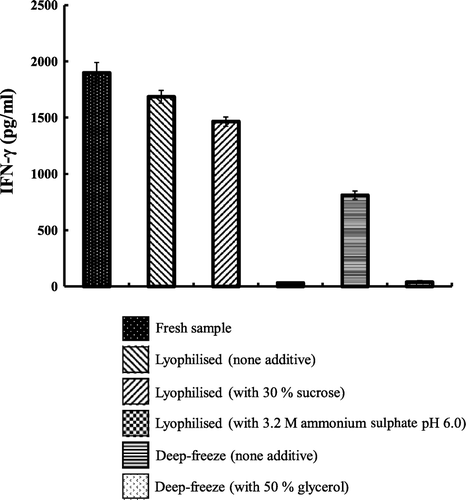
Effects of deep-freeze and lyophilisation on the storage stability of FIP-fve are presented in . The lyophilised method was more beneficial to FIP-fve stability than the deep-freeze method. also shows that the additive-absent lyophilised sample and 30% sucrose lyophilised sample both maintained activities similar to that of fresh sample after storage. The FIP-fve, which was solely deep-freeze, lost about 40% of its original activity. However, the deep-frozen FIP-fve (plus glycerol) and lyophilised FIP-fve containing 3.2M ammonium sulphate were completely inactivated after two months storage.
Figure 3. Stability of the deep-frozen and lyophilised FIP-fve with and without additives during storage. The freshly prepared FIP-fve was divided into five groups: (1) only lyophilised; (2) lyophilised with 30% sucrose; (3) lyophilised with 3.2M ammonium sulphate; (4) deep-frozen; and (5) deep-frozen with glycerol. The production of IFN-γ was measured by ELISA. Three independent experiments were performed. Values are means of three independent experiments.
The digestibilities of fungal immunomodulatory protein (FIP)-gts and fungal immunomodulatory protein (FIP)-fve
The digestibilities of FIP-gts and FIP-fve in SGF are shown in A and B. When FIP-gts is in SGF digestion for 5 min, the protein is nearly completely decomposed (A), but the protein bands of FIP-fve are still prominent in SGF reaction even at 120 min (B). A and B shows the digestion stability of FIPs in SIF. FIP-gts retained some intact proteins, but most were converted to small protein fragments. Although FIP-fve had similar degradation, more intact bands are present.
Figure 4. SDS-PAGE analysis of the degradation of FIP-gts and FIP-fve in SGF. (A) FIP-gts and (B) FIP-fve were treated with SGF. Molecular weight markers (lane M) are indicated on the left-hand side of the gel. SGF and the test protein control were run along. The numbers on top denote the incubation times in minutes. The FIP-gts (A) and FIP-fve (B) in SGF digestion were labelled at the right-hand side of the gel, respectively.
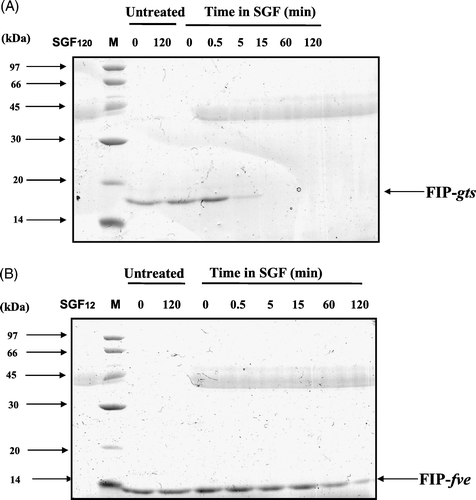
Figure 5. SDS-PAGE analysis of the degradation of FIP-gts and FIP-fve in SIF. (A) FIP-gts and (B) FIP-fve were treated with SIF. Molecular weight markers (lane M) are indicated on the left-hand side of the gel. SIF and the test protein control were run along. The numbers on top denote the incubation times in minutes. The FIP-gts (A) and FIP-fve (B) in SIF digestion were labelled at the right-hand side of the gel.
Immunomodulatory activities for dimerisation of fungal immunomodulatory proteins (FIPs)
The formation of homodimers of FIP-gts is a critical step for binding cell surface receptors to exert immunomodulatory activity (Lin et al., Citation1997). We investigated whether FIP-fve also converts to homodimers. Results in show that when FIP-gts and FIP-fve were incubated only with buffer, only monomeric FIPs were found. When either FIP-gts or FIP-fve reacted with glutaraldehyde (250 µM), both FIP types appeared in the dimeric form, but the dimerisation of FIP-fve was weaker than FIP-gts. also indicates that most FIP-fve remained as monomer (250 µM glutaraldehyde), but FIP-fve has the ability to form a mixture of trimers and tetramers, as well as polymer form. As show in , the formation of FIP-gts dimer maintained its immunomodulatory activity. While FIP-fve produced dimers, trimers or tetramers, these forms lost their immunomodulatory activity.
Figure 6. Chemical cross-linking of FIP-gts and FIP-fve. The dimerisation of FIP-gts and FIP-fve were examined by glutaraldehyde cross-linking reaction. About 12 µg of protein was loaded in each lane, and gels were stained with Coomassie blue.
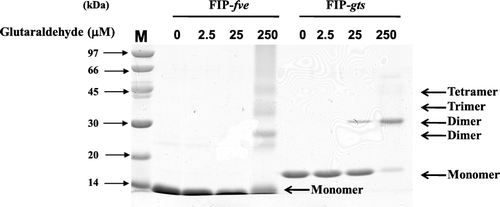
Table 1. The IFN-γ production in dimerisation of FIP-gts and FIP-fve.
Discussion
FIP-gts, a mushroom used for medicinal purposes in China and exhibits the potential to modulate immune responses similar to that of LZ-8 isolates from the Oriental medicinal fungus G. lucidium. Golden needle mushrooms (F. velutipes, FIP-fve) are also a popular edible mushroom in Asia. These medicinal fungi or edible mushroom are all reported to be effective in the promotion of human health (Ko et al., Citation1995; Liao et al., Citation2008). Therefore, it is of interest to compare the effects of conventional food processing operation on the immunomodulatory ability of FIP-gts and FIP-fve. In this study, we first compared the immunomodulatory activity of these two FIPs. As shown in , FIP-gts and FIP-fve had IFN-γ stimulatory activity on hPBMCs and acted in a dose-dependent manner, with FIP-gts more active on a molar basis.
FIP-gts and FIP-fve retained most of their activity after heating treatment at 100°C for 1 min. Our results showed that a systematic short time, high temperature heating process may be appropriate for heat-sensitive biological fluids such as FIPs. It is especially important to preserve bioactive stability of protein in food and pharmaceutical applications. Spray-drying technology is widely used in the food and pharmaceutical industries, producing predominantly amorphous material due to rapid solidification (Gonnissen, Verhoeven, Peeters, Remon, & Vervaet, Citation2008; Partanen et al., Citation2008) from a one-step drying process which causes agglomeration, thus obtaining a homogeneous powder. For example, the short gas residence time, ranging from 3 to 40 seconds, permits spray-drying without thermal degradation, while simultaneously cooling the product through evaporation. This process is suitable for many heat-sensitive products. Perhaps, the spray-drying processing is an appropriate technique for retaining protein bioactivity of FIPs, when preparing a desired protein powder for specific applications.
The immunomodulatory activities of FIP-gts and FIP-fve were very stable over a wide pH range. The results showed that FIP-gts and FIP-fve resisted acid hydrolysis and alkali decomposition in short exposures to various pH conditions. Our results are consistent with those of a previous report (Chang, Chien, Tong, & Sheu, Citation2007), in which the immunomodulatory activities of mushrooms (Agaricus bisporus lectin and Auricularia polytricha) were not influenced by extreme pH levels (2 and 12). Thus, these results show that the immunomodulatory activities obtained from different mushrooms are stable within a wide range of pH values.
Among the additives examined (), the best results were obtained with sucrose. In previous studies (Kawai & Suzuki, Citation2007; Wang, Citation2000), the addition of stabilisers such as polysaccharides enhanced the stability of the protein during lyophilisation. The reasons could be explained by the fact that sucrose acts as cryoprotectants, as protectants for freeze drying, and stabilisers during dehydration. Thus, sucrose makes the protein more stable against both freezing and dehydration during freeze drying (Patist & Zoerb, Citation2005). On the other hand, it was reported that sucrose has stabilising effects on the conformation of different proteins and enzymes in their aqueous solutions and lyophilised forms (Arakawa & Timasheff, Citation1982; Liao et al., Citation2004). Thus, the results suggest that lyophilisation with or without sucrose is effective for preserving the immunomodulatory activity of FIP-fve.
We had previously demonstrated that the edema reaction and systemic anaphylaxis reaction can be prevented by repeat administration of FIP-fve, in vivo (Ko et al., Citation1995). It was previously reported that the oral administration of FIP-fve provides immunoprophylaxis for food allergy and other allergy diseases (Hsieh et al., Citation2003). Our results clearly indicate FIP-fve has higher resistance to SGF and SIF digestion than FIP-gts. In addition, these results also show that FIP-fve was more resistant to SGF than SIF.
It has been reported that the N-terminal α-A-helix of FIP-gts may play a significant role in the formation of homodimers for binding to cell surface receptors to exert its immunomodulatory activity (Lin et al., Citation1997). We studied the possible dimerisation of FIPs and found that FIP-gts forms dimers and exerts immunomodulatory activity. In agreement with data reported previously (Lin et al., Citation1997), most FIP-gts appeared in the dimeric form at a concentration of 250 µM glutaraldehyde. On the contrary, even by the reaction at 250 µM reaction, most FIP-fve seems remained monomer. It has been reported that dimerisation of FIPs occurs through 3D domain swapping of the N-terminal helices and is stabilised predominantly by hydrophobic interactions (Paaventhan et al., Citation2003). Our results demonstrated that while FIP-fve has the ability to form dimers, trimers or tetramers, these forms apparently lose immunomodulatory activity ().
Conclusions
This study shows FIP-gts exhibited better immunomodulatory activity than FIP-fve. Our study also demonstrated the storage/digestion stability, acid/alkali resistance of immune-stimulating activity of FIP-gts and FIP-fve after various treatments. FIPs have attracted a great deal of attention in the field of preventive medicine. The results of this study indicate that FIPs are stable and possesses a high potential for commercial development in food or pharmaceutical products for commercial development.
Acknowledgements
The study was supported by the National Science Council, Taiwan, ROC (NSC 97-2314-B040-014-MY3 and Chung Shan Medical University (CSMU 89-OM-B-017).
References
- Arakawa , T. and Timasheff , S.N. 1982 . Stabilization of protein structure by sugars . Biochemistry , 21 : 6536 – 6544 .
- Chang , H.H. , Chien , P.J. , Tong , M.H. and Sheu , F. 2007 . Mushroom immunomodulatory proteins possess potential thermal/freezing resistance, acid/alkali tolerance and dehydration stability . Food Chemistry , 2 : 597 – 605 .
- Fu , T.J. , Abbott , U.R. and Hatzos , C. 2002 . Digestibility of food allergens and nonallergenic proteins in simulated gastric fluid and simulated intestinal fluid-a comparative study . Journal of Agricultural and Food Chemistry , 50 : 7154 – 7160 .
- Gonnissen , Y. , Verhoeven , E. , Peeters , E. , Remon , J.P. and Vervaet , C. 2008 . Coprocessing via spray drying as a formulation platform to improve the compactability of various drugs . European Journal of Pharmaceutics and Biopharmaceutics , 69 : 320 – 334 .
- Hsiao , Y.M. , Huang , Y.L. , Tang , S.C. , Shieh , G.J. , Lai , J.Y. Wang , P.H. 2008 . Effect of a fungal immunomodulatory protein from Ganoderma tsugae on cell cycle and interferon-gamma production through phosphatidylinostiol 3-kinase signal pathway . Process Biochemistry , 43 : 423 – 430 .
- Hsieh , K.Y. , Hsu , C.I. , Lin , J.Y. , Tsai , C.C. and Lin , R.H. 2003 . Oral administration of an edible-mushroom-derived protein inhibits the development of food-allergic reactions in mice . Clinical and Experimental Allergy , 33 : 1595 – 1602 .
- Hsu , H.C. , Hsu , C.I. , Lin , R.H. , Kao , C.L. and Lin , J.Y. 1997 . Fip-vvo, a new fungal immunomodulatory protein isolated from Volvariella volvacea . The Biochemical Journal , 323 ( Pt 2 ) : 557 – 565 .
- Kawai , K. and Suzuki , T. 2007 . Stabilizing effect of four types of disaccharide on the enzymatic activity of freeze-dried lactate dehydrogenase: Step by step evaluation from freezing to storage . Pharmaceutical Research , 24 : 1883 – 1890 .
- Ko , J.L. , Hsu , C.I. , Lin , R.H. , Kao , C.L. and Lin , J.Y. 1995 . A new fungal immunomodulatory protein, FIP-fve isolated from the edible mushroom, Flammulina velutipes and its complete amino acid sequence . European Journal of Biochemistry , 228 : 244 – 249 .
- Laemmli , U.K. 1970 . Cleavage of structural proteins during the assembly of the head of bacteriophage T4 . Nature , 227 : 680 – 685 .
- Liao , C.H. , Hsiao , Y.M. , Hsu , C.P. , Lin , M.Y. , Wang , J.C. Huang , Y.L. 2006 . Transcriptionally mediated inhibition of telomerase of fungal immunomodulatory protein from Ganoderma tsugae in A549 human lung adenocarcinoma cell line . Molecular Carcinogenesis , 45 : 220 – 229 .
- Liao , C.H. , Hsiao , Y.M. , Lin , C.H. , Yeh , C.S. , Wang , J.C. Ni , C.H. 2008 . Induction of premature senescence in human lung cancer by fungal immunomodulatory protein from Ganoderma tsugae . Food and Chemical Toxicology , 46 : 1851 – 1859 .
- Liao , C.H. , Hsiao , Y.M. , Sheu , G.T. , Chang , J.T. , Wang , P.H. Wu , M.F. 2007 . Nuclear translocation of telomerase reverse transcriptase and calcium signaling in repression of telomerase activity in human lung cancer cells by fungal immunomodulatory protein from Ganoderma tsugae . Biochemical Pharmacology , 74 : 1541 – 1554 .
- Liao , Y.H. , Brown , M.B. and Martin , G.P. 2004 . Investigation of the stabilisation of freeze-dried lysozyme and the physical properties of the formulations . European Journal of Pharmaceutics and Biopharmaceutics , 58 : 15 – 24 .
- Lin , W.H. , Hung , C.H. , Hsu , C.I. and Lin , J.Y. 1997 . Dimerization of the N-terminal amphipathic alpha-helix domain of the fungal immunomodulatory protein from Ganoderma tsugae (Fip-gts) defined by a yeast two-hybrid system and site-directed mutagenesis . Journal of Biological Chemistry , 272 : 20044 – 20048 .
- Paaventhan , P. , Joseph , J.S. , Seow , S.V. , Vaday , S. , Robinson , H. Chua , K.Y. 2003 . A 1.7A structure of Fve, a member of the new fungal immunomodulatory protein family . Journal of Molecular Biology , 332 : 461 – 470 .
- Partanen , R. , Raula , J. , Seppanen , R. , Buchert , J. , Kauppinen , E. and Forssell , P. 2008 . Effect of relative humidity on oxidation of flaxseed oil in spray dried whey protein emulsions . Journal of Agricultural and Food Chemistry , 56 : 5717 – 5722 .
- Patist , A. and Zoerb , H. 2005 . Preservation mechanisms of trehalose in food and biosystems . Colloids and Surfaces. B, Biointerfaces , 40 : 107 – 113 .
- Wang , P.H. , Hsu , C.I. , Tang , S.C. , Huang , Y.L. , Lin , J.Y. and Ko , J.L. 2004 . Fungal immunomodulatory protein from Flammulina velutipes induces interferon-gamma production through p38 mitogen-activated protein kinase signaling pathway . Journal of Agricultural and Food Chemistry , 52 : 2721 – 2725 .
- Wang , P.H. , Yang , S.F. , Chen , G.D. , Han , C.P. , Chen , S.C. Lin , L.Y. 2007 . Human nonmetastatic clone 23 type 1 gene suppresses migration of cervical cancer cells and enhances the migration inhibition of fungal immunomodulatory protein from Ganoderma tsugae . Reproductive Science , 14 : 475 – 485 .
- Wang , W. 2000 . Lyophilization and development of solid protein pharmaceuticals . International Journal of Pharmaceutics , 203 : 1 – 60 .
- Wold , A.E. 1998 . The hygiene hypothesis revised: Is the rising frequency of allergy due to changes in the intestinal flora? . Allergy , 53 ( Suppl. 46 ) : 20 – 25 .
- Yue , G.G. , Fung , K.P. , Tse , G.M. , Leung , P.C. and Lau , C.B. 2006 . Comparative studies of various ganoderma species and their different parts with regard to their antitumor and immunomodulating activities in vitro . Journal of Alternative and Complementary Medicine , 12 : 777 – 789 .