Abstract
The purpose of this study was to determine the effect of active or passive immunisation against cholecystokinin (CCK) peptide on feed intake and body weight gain in pigs. For active immunisation, recombinant CCK-33 concatenate polypeptide was employed to immunise pigs, while passive immunisation was achieved by egg powders containing CCK-specific antibodies from laying hens that were immunised with recombinant CCK-33 peptide. Enzyme-linked immunosorbent assay showed that the active immunised pigs had significantly higher levels of CCK antibodies in serum than the control ones (p<0.05). Actively or passively immunised pigs had significantly higher body weight, average daily gain and average daily feed intake than that of the control ones (p<0.05). The results suggest that active or passive immunisation against CCK-33 peptide has stimulatory effects on growth of pigs. Thus, immunisation against CCK-33 may be used as an alternative way to enhance pig growth.
Introduction
A growing body of evidence suggests that signals from the gastrointestinal tract is directly involved in the control of feed intake of animals (Ritter, Citation2004a; Strader & Woods, Citation2005). The gastrointestinal signals are collectively called ‘satiety signal’, including dozens of gastrointestinal hormones that are known to be secreted from the endocrine cells lining the gastrointestinal tract in response to the physicochemical properties of ingested feed passing along the lumen (Gomez et al., Citation1996; le Roux & Bloom, Citation2005; Liddle, Citation1997; Lloyd, Citation1994; McIntosh, Citation1985; Srivastava, Kamath, Barry, & Dayal, Citation2004; Woods, Citation2004). The gastrointestinal hormones may signal the central nervous system in either endocrine or paracrine manner (Harrold, Citation2004). Subsequently, the central nervous system interprets these signals and sends signals to numerous organs in the periphery to modulate visceral functions and feeding behaviour (Figlewicz, Lacour, Sipols, Porte, & Woods, Citation1987; Moran, Ladenheim, & Schwartz, Citation2001).
Cholecystokinin (CCK) is one of the gastrointestinal hormones, secreted primarily in two forms, CCK-33 and CCK-8 by the I-cells within the duodenal and jejunal mucosa (Polak et al., Citation1975). Previous studies showed that nutrient-stimulated release of CCK by enteroendocrine cells triggers several downstream events, including pancreatic enzyme secretion, excretion of bile into the intestine, slowing of gastric emptying and inhibition of feed intake (Liddle, Citation1997; Matson & Ritter, Citation1999; Ritter, Covasa, & Matson, 1999). Additionally, CCK was shown to function as a trophic hormone in supporting the growth of stomach, pancreas and intestine (Barrowman, Citation1975; Enochs & Johnson, Citation1977; Kuntz, Pinget, & Damge, Citation2004). Vagus nerves appear to be necessary for CCK-mediated inhibition of feed intake, since destruction of these neuron fibres attenuates the reduction of feed intake by CCK. It has been suggested that CCK may act on its target tissues via two G-protein coupled receptors, CCK-A and CCK-B, both of which are expressed by the vagus nerves (Ritter, Citation2004a, Citationb; Sun & Ferguson, Citation1997).
Neutralisation of plasma CCK (active immunisation with CCK-8-hsG conjugate) or blockage of CCK receptors was shown to stimulate animal feed intake and daily body weight gain in several animal species including growing swine (25.6–77 kg, Yorkshine and Large white) and gilts (Pekas, Citation1991; Pekas & Trout, Citation1990, Citation1993), but decrease feed intake in lambs (Spencer, Citation1992). In the present study, we examined whether active or passive immunisation against plasma CCK affects feed intake and daily body weight gain of growing pigs (43–80 kg, Du×(chang×da)).
Materials and methods
DNA constructs
The open reading frame of chicken CCK-33 was amplified by PCR using the specific primers pair CCKup (5′-CAT GGA TCC GGT TCT ACT GGC CGC T-3′)/CCKdn (5′-CAT GAG CTC CCA AAA TCC ATC CAG CC-3′; the homology of chicken CCK-33 to pig CCK-33 was as high as 95%). The PCR product was digested with Bam HI and Sac I and cloned into plasmid pRSET-A (Invitrogen, Karlsruhe, Germany) to generate recombinant plasmid pRSET-1CCK. The insert of pRSET-1CCK was then excised out by two double restriction digestions of Bam HI/Hind III and Bgl II/Hind III and joined together into pRSET to generate pRSET-2CCK. Following a similar procedure, pRSET-4CCK was generated. The final product pRSET-4CCK was verified by PCR, enzyme digestive identification and sequencing.
Expression and purification of concatenated CCK-33 peptide
To express concatenate CCK peptide, the plasmid pRSET-4CCK was transformed into BL-21 Escherichia coli cells. A single ampicillin-positive clone was picked and grown in LB broth. When OD600 reached approximately 0.5, IPTG was added to the bacterial culture to induce expression of recombinant CCK protein. Samples of the bacterial culture were removed at 2, 3 and 4 hours after addition of IPTG, lysed and analysed by Western blot.
IPTG-induced bacterial cells were collected by centrifugation, resuspended in 40 ml/l buffer B (8 M urea, 0.1 M NaP, pH 8.0), and lysed by sonication. After centrifugation for 20 min at 10,000 rpm in a 50 ml Oakridge tube, the supernatant was collected and added to a column loaded with 50% Ni-NTA resin. The column was washed with 25–30 ml buffer B and 40 ml of buffer C (8 M urea, 0.1 M NaP, pH 6.3) to remove bacterial proteins. The bound protein was eluted with buffer F (8 M urea, 5% glacial acetic acid) and analysed by SDS-PAGE and Western blotting.
Preparation of oil-emulsion vaccine
The vaccine was prepared with purified concatenated CCK-33 peptide of 0.5 mg/ml in oil-emulsion adjuvant.
Preparation of the CCK-33-specific antibody
Sixty-four healthy laying hens were housed in the experimental area of the Guangdong Wen's Food Co., Ltd. (Guangdong, China) and allotted randomly to two groups, Group 1 was used for control and Group 2 for immunisation with recombinant CCK-33 peptide. Each group consisted of eight replicates with four hens in each replicate. The hens in Group 2 received the vaccine at 1 ml/bird. Control hens were injected at the same ages with physiological saline prepared in oil emulsion. The hens were inoculated at 20 d intervals for three times. For detection of immune responses, blood samples and eggs were collected from the immunised and control birds at 28, 48, 68 and 94 testing days and analysed by enzyme-linked immunosorbent assay (ELISA). Eggs containing CCK-33-specific antibody were collected for preparation of egg powder.
Immunisation of pigs actively by vaccine and passively by CCK-33-specific antibody
A total of 96 triple hybrid pigs of 75 d were housed in the experimental area of the Guangdong Wen's Food Co., Ltd. and allotted randomly to four groups and each treatment consisted of three replicates with eight pigs in each replicate. Their diets and nutrition level were listed in . Pre-trial feeding was done for 7 d, and then the pigs were immunised at 82 d. For active immunisation, each pig was injected intraperitoneally with 2 ml of the oil-emulsion vaccine at 82 and 110 d of age, respectively. Control pigs were injected at the same ages with physiological saline prepared in oil-emulsion adjuvant. Blood samples were collected from the immunised and control animals at 128 d and analysed by ELISA. For passive immunisation, egg powder containing CCK-33-specific antibody was added to the feed of pigs at 100 g/t, and pigs of another groups were fed egg powder at 100 g/t without CCK-33-specific antibody. All the experiments were ended at 128 d.
Table 1. Basal diet and nutrition level of pigs.
Measurements of average daily gain, average daily feed intake and feed to gain ratio
At the beginning and termination of the experiment each pig was weighted at 9 am after food was withheld for one night. The amount of feed consumed was recorded during the experiment to determine average daily gain, average daily feed intake and feed to gain ratio.
Statistical analyses
Statistical analyses were performed using one-way ANOVA procedure of SPSS 11.0 software. Differences between individual means were determined by Duncan's new multiple range test. Probability value (p) < 0.05 was considered significant. Data are expressed as the mean±SE.
Results
Verification of pRSET-4CCK
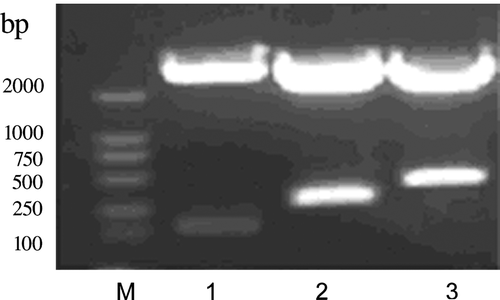
shows that the recombinant plasmid of pRSET-1CCK, pRSET-2CCK and pRSET-4CCK was digested with Bam HI and Sac I and yielded about 120, 230 and 450 bp bands in size, respectively, which were 1CCK, 2CCK and 4CCK. The recombinant pRSET-4CCK was sequenced (Shanghai Boya Ltd.) and conformed to our original design essentially (data not shown).
Expression of 4CCK protein
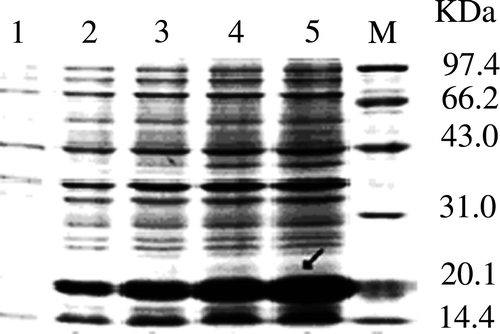
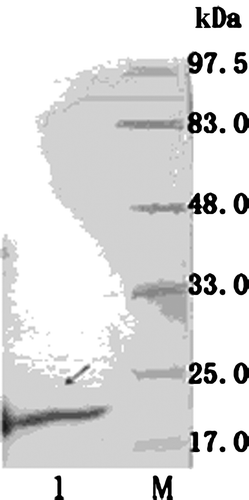
SDS-PAGE analysis of the 4CCK fusing protein was displayed in . As expected, the expression of CCK protein was induced by 1 mmol/L IPTG and its molecular was about 20 kDa. Further testing of the expressed protein with Western blot using rabbit anti-CCK hyper-immune serum (Sigma, USA) revealed a specific protein band of about 20 kDa in size (), which demonstrated that the fusion protein could react with CCK-33-specific antibody.
Detection of anti-CCK antibody in hen serum and yolk
and show the serum and yolk antibody levels against CCK-33. It can be seen that the CCK-specific antibody in serum and yolk in treated group was much higher than that of control except that anti-CCK antibody in yolk at 94 d was relatively low. As CCK was an endogenous protein, it is plausible that a longer period of immunisation raised tolerance that made anti-CCK antibody in yolk at 94 d was relatively low.
Table 2. Detection of hens anti-CCK antibody level in serum (n=32).
Table 3. Detection of hens anti-CCK antibody level in yolk (n=32).
Detection of anti-CCK antibody in pig serum
The immune responses of pigs were determined by ELISA (). At 128 d, significant levels of antibody against CCK were detected in the plasma of pigs immunised with recombinant CCK-33 peptide. The results indicated that pigs immunised with 0.5 mg/ml of concatenated recombinant CCK-33 peptide produced significantly high levels of anti-CCK antibody in their blood stream.
Table 4. Comparison of antibody levels between control and immunised groups at 128 d (n=24).
Analysis of the body weight and feed intake of pigs
We compared the body weight, average daily gain, average daily feed intake and feed to gain ratio of the actively or passively immunised pigs to that of the control animals (). It shows that the final weight, average daily gain and average daily feed intake of the actively or passively immunised pigs were significantly higher than that of the control pigs (p<0.05). There was no difference between the two control groups (p>0.05), and there were also no difference between the actively immunised pigs and passively immunised pigs (p>0.05). There was no significant difference in feed to gain ratio among the four groups (p>0.05).
Table 5. Comparison of body weights, feed intakes and feed efficiencies between control and treated groups (n=24).
Discussion
CCK is an endogenous protein and anti-CCK antibody was produced in the serum of hens. According to the results of hen antibody against CCK, a longer period of immunisation raised the negative feedback of CCK antibody reaction with endogenous CCK, which stimulated animals to produce more endogenous CCK, thus it may caused the concentration of CCK antibody relatively low. In addition, CCK and gastrin are highly similar peptides, CCK antibodies may also bind and neutralise gastrin hormones (Colucci et al., Citation2005; Stubbs et al., Citation2002). While gastrin stimulates gastric acid release and is not known to directly control food intake, it is possible that anti-gastrin activity could influence acidity and thereby influence overall intake and weight gain, which indicated that alternative mechanisms for CCK antibodies increased feeding weight gain may exist.
Feed intake in animals is thought to be controlled by a complex network of endocrine signals. Stimulation of feed intake has been explored to maximise the growth potential of animals. Our findings suggest that neutralisation of endogenous CCK-33 hormone through active immunisation increases feed intake and stimulates body growth in pigs, which is in line with the evidence generated in growing swine (26.5–77 kg), barrows and gilts (Pekas, Citation1991; Pekas & Trout, Citation1990, Citation1993), and supports the hypothesis that endogenous CCK functions as a negative appetite regulator (Ritter et al., Citation1999). Given that no apparent pathological condition was observed in the immunised animals, we propose that immunisation against plasma CCK may be used as a means to stimulate feed intake and growth in pigs.
We found that passive immunisation against CCK-33 for improving feed intake and growth of pigs were equally effective to active immunisation but was relatively more expensive. Passive immunisation has potential advantages over vaccination in that higher doses of antibody can be administered in feed, its apparent pathological effect such as the stress and ache on animal was less and the antibody dose can be controlled so that individual variability in serum antibody levels might be minimised, and the onset of effect is immediate. In addition, the usefulness of egg yolk immunoglobulin Y (IgY) has been assessed for therapeutic application by passive immunisation through oral ingestion of IgY because of its broad stability towards heat, acid and pepsin (Shin et al., Citation2002). Although the principle and procedure of IgY absorption in gastrointernal tract is still unknown, the phenomenon has been observed and utilised in prevention or control of many infections (Kuroki et al., Citation1997; Marquardt et al., Citation1999; Sunwoo, Nakano, Dixon, & Sim, Citation1996). For these reasons, passive immunisation with egg yolk-derived antibodies is of interest as a possible therapeutic alternative to vaccination. Our reports suggest that passive immunisation with CCK antibodies is a feasible strategy for improvement of feed intake and growth of pigs.
Acknowledgements
This study was supported by grant from Natural Science Group Foundation (04205804) and Science Technology Strategic Plan (2006A20403002) of Guangdong, People's Republic of China.
References
- Barrowman , J.A. 1975 . The tropic action of gastro-intestinal hormones . Digestion , 12 : 92 – 104 .
- Colucci , R. , Blandizzi , C. , Tanini , M. , Vassalle , C. , Breschi , M.C. and Del Tacca , M. 2005 . Gastrin promotes human colon cancer cell growth via CCK-2 receptor-mediated cyclooxygenase-2 induction and prostaglandin E2 production . British Journal of Pharmacology , 144 : 338 – 348 .
- Enochs , M.R. and Johnson , L.R. 1977 . Trophic effects of gastrointestinal hormones: Physiological implications . Federation Proceedings , 36 : 1942 – 1947 .
- Figlewicz , D.P. , Lacour , F. , Sipols , A. , Porte , D. Jr , & Woods , S.C. 1987 . Gastroenteropancreatic peptides and the central nervous system . Annual Review of Physiology , 49 , 383 395 .
- Gomez , G. , Udupi , V. , Qi , X. , Lluis , F. , Rajaraman , S. Thompson , J.C. 1996 . Growth hormone upregulates gastrin and peptide yy gene expression . American Journal of Physiology , 271 : E582 – E586 .
- Harrold , J.A. 2004 . Hypothalamic control of energy balance . Current Drug Targets , 5 : 207 – 219 .
- Kuntz , E. , Pinget , M. and Damge , P. 2004 . Cholecystokinin octapeptide: A potential growth factor for pancreatic beta cells in diabetic rats . Journal of Pancreas , 5 : 464 – 475 .
- Kuroki , M. , Ohta , M. , Ikemori , Y. , Icatlo , F.C. , Kobayashi , C. Jr , Yokoyama , H. , et al. 1997 . Field evaluation of chicken egg yolk immunoglobulins specific for bovine rotavirus in neonatal calves . Archives of Virology , 142 , 843 851 .
- le Roux , C.W. and Bloom , S.R. 2005 . Peptide yy, appetite and food intake . Proceedings of the Nutrition Society , 64 : 213 – 216 .
- Liddle , R.A. 1997 . Cholecystokinin cells . Annual Review of Physiology , 59 : 221 – 242 .
- Lloyd , K.C. 1994 . Gut hormones in gastric function . Baillieres Clinical Endocrinology and Metabolism , 8 : 111 – 136 .
- Marquardt , R.R. , Jin , L.Z. , Kim , J.W. , Fang , L. , Frohlich , A.A. and Baidoo , S.K. 1999 . Passive protective effect of egg-yolk antibodies against enterotoxigenic Escherichia coli K88_ infection in neonatal and early-weaned piglets . FEMS Immunology and Medical Microbiology , 23 : 283 – 288 .
- Matson , C.A. and Ritter , R.C. 1999 . Long-term CCK-leptin synergy suggests a role for CCK in the regulation of body weight . American Journal of Physiology , 276 : R1038 – R1045 .
- McIntosh , C.H. 1985 . Gastrointestinal somatostatin: Distribution, secretion and physiological significance . Life Sciences , 37 : 2043 – 2058 .
- Moran , T.H. , Ladenheim , E.E. and Schwartz , G.J. 2001 . Within-meal gut feedback signaling . International Journal of Obesity and Related Metabolic Disorders , 25 ( Suppl. 5 ) : S39 – S41 .
- Pekas , J.C. 1991 . Effect of cholecystokinin immunization, enhanced food intake and growth of swine on lean yield and carcass composition . Journal of Nutrition , 121 : 563 – 567 .
- Pekas , J.C. and Trout , W.E. 1990 . Stimulation of food intake and growth of swine by cholecystokinin immunization . Growth Development and Aging , 54 : 51 – 56 .
- Pekas , J.C. and Trout , W.E. 1993 . Cholecystokinin octapeptide immunization: Effect on growth of barrows and gilts . Journal of Animal Science , 71 : 2499 – 2505 .
- Polak , J.M. , Bloom , S.R. , Rayford , P.L. , Pearse , A.G. , Buchan , A.M. and Thompson , J.C. 1975 . Identification of cholecystokinin-secreting cells . Lancet , 2 : 1016 – 1018 .
- Ritter , R.C. 2004a . Gastrointestinal mechanisms of satiation for food . Physiology and Behavior , 81 : 249 – 273 .
- Ritter , R.C. 2004b . Increased food intake and CCK receptor antagonists: Beyond abdominal vagal afferents . American Journal of Physiology Regulatory Integrative Comparative Physiology , 286 : R991 – R993 .
- Ritter , R.C. , Covasa , M. and Matson , C.A. 1999 . Cholecystokinin: Proofs and prospects for involvement in control of food intake and body weight . Neuropeptides , 33 : 387 – 399 .
- Shin , J.H. , Yang , M. , Nam , S.W. , Kim , J.T. , Myung , N.H. Bang , W.G. 2002 . Use of egg yolk-derived immunoglobulin as an alternative to antibiotic treatment for control of Helicobacter pylori infection . Clinical and Diagnostic Laboratory Immunology , 9 : 1061 – 1066 .
- Spencer , G.S. 1992 . Immunization against cholecystokinin decreases appetite in lambs . Journal of Animal Science , 70 : 3820 – 3824 .
- Srivastava , A. , Kamath , A. , Barry , S.A. and Dayal , Y. 2004 . Ghrelin expression in hyperplastic and neoplastic proliferations of the enterochromaffin-like (ecl) cells . Endocrine Pathology , 15 : 47 – 54 .
- Strader , A.D. and Woods , S. 2005 . Gastrointestinal hormones and food intake . Gastroenterology , 128 : 175 – 191 .
- Stubbs , M. , Khan , K. , Watson , S.A. , Savage , K. , Dhillon , A.P. and Caplin , M.E. 2002 . Endocytosis of anti-CCK-B/gastrin receptor antibody and effect on hepatoma cell lines . Journal of Histochemistry and Cytochemistry , 50 : 1213 – 1217 .
- Sun , K. and Ferguson , A.V. 1997 . Cholecystokinin activates area postrema neurons in rat brain slices . American Journal of Physiology , 272 : R1625 – R1630 .
- Sunwoo , H.H. , Nakano , T. , Dixon , W.T. and Sim , J.S. 1996 . Immune responses in chickens against lipopolysaccharide of Escherichia coli and Salmonella typhimurium . Poultry Science , 75 : 342 – 345 .
- Woods , S.C. 2004 . Gastrointestinal satiety signals i. An overview of gastrointestinal signals that influence food intake . American Journal of Physiology , 286 : G7 – G13 .