Abstract
A specific monoclonal antibody (mAb) against clenbuterol, with cross-reactivities less than 0.01% for all test compounds except salbutamol (6.4%), was produced with hybridoma technology. The mAb originated from immunogen of clenbuterol–human serum albumin was combined with coating antigen of salbutamol–ovalbumin to develop a heterologous enzyme-linked immunosorbent assay (ELISA). This assay shows very high sensitivity with IC50 of 0.3 ng/ml and LOD of 0.1 ng/ml when it was run in 0.01 M PBS (pH 7.5). Clenbuterol was spiked in chicken and pork samples and after a simple extraction procedure the extracts at appropriate dilution were analysed by ELISA. Satisfactory results were obtained by both intra-assay, with average recoveries of 81–102% and coefficient variations (CVs) of 3–12%, and inter-assay, with average recoveries of 77–95% and CVs of 5–13%. The survey results of ELISA and HPLC for some real world tissue samples were consistent. It suggests that the mAb-based ELISA will be a feasible quantitative/screening method for clenbuterol residue in animal tissues.
Introduction
Clenbuterol–HCl, 4-amino-a-[(tert-butylamino) methyl-3,5-dichlorobenzyl alcohol hydrochloride], is a β2-adrenergic agonist capable of exerting a variety of neurological and cardiovascular effects on animals and humans (Wingert, Mundy, Nelson, Wong, & Curtis, Citation2008). The primary usage of clenbuterol is in veterinary medicine as a bronchodilator (Ventipulmin®). However, because of its growth-promoting properties (Ricks, Dalrymple, Baker, & Ingle, Citation1984), clenbuterol has been used as a feed additive for food-producing animals to induce weight gain and increase muscle mass during their growth. The excessive use of clenbuterol resulted in a high level of residues in animal products (Martinez-Navarro, Citation1990; Pulce et al., Citation1991), which were consumed by humans. Despite concerted efforts by governments to enforce a ban for the abuse of clenbuterol internationally, there have been reports of food poisoning caused by clenbuterol in many countries (Barbosa et al., Citation2005; Salleras et al., Citation1995; Sporano et al., Citation1998; Wang & Zhang, Citation2004).
Currently, various analytical methods have been reported for determination of clenbuterol residues in animal feeds, tissues and body fluids. These include instrumental assays, such as liquid chromatography-mass spectra (LC-MS; Blanca et al., Citation2005; Kootstra et al., Citation2005) and gas chromatography-mass spectra (GC-MS; Gonzalez et al., Citation1997; He, Su, Zeng, Liu, & Huang, Citation2007) and immunoassays with polyclonal or monoclonal antibodies (mAbs; Xu et al., Citation2007; Zhang et al., Citation2006). Compared to instrumental assay, immunoassay is more rapid, cost effective and high throughput, and thus widely employed as a screening tool to detect the presence of an analyte in divers matrices. In recent years, concerns about potential misuses and abuse of clenbuterol in livestock industry have increased. There are many clenbuterol enzyme-linked immunosorbent assay (ELISA) kits commercially available in China. However, the most of them show various cross-reactivities with some structurally related agonists. We herein developed a sensitive and specific mAb-based ELISA for the detection of clenbuterol. To our knowledge, the heterologous ELISA based on the combination of mAb from clenbuterol–human serum albumin (clenbuterol–HSA) and coating antigen salbutamol–ovalbumin (salbutamol–OVA) is firstly reported here, which highly increases the assay sensitivity for clenbuterol. This ELISA is expected to be a suitable screening tool for clenbuterol in laboratory prior to instrumental assay. Furthermore, it is possible to achieve field-portable analysis, and to analyse numerous samples simultaneously.
Materials and methods
Reagents and materials
All reagents were of analytical grade unless specified otherwise. Clenbuterol–HCl, salbutamol and other compounds for cross-reaction were purchased from National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Chemicals purchased from Sigma (St. Louis, MO, USA) were goat anti-mouse IgG-horseradish peroxidase (IgG-HRP), HSA, OVA, complete and incomplete Freund's adjuvant, dimethyl sulphone (DMSO), polyethylene glycol 4000 (PEG 4000), 3,3'5,5’-tetramethylbenzidine (TMB), penicillin and streptomycin. Other reagents were purchased from Beijing Reagent Corporation (Beijing, China).
Balb/c mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Murine myeloma cell line Sp2/0Ag14 was purchased from the American Type Culture Collection (Manassas, VA, USA; ATCC Catalogue No. CRL-1581). Hypoxanthine-thymidine (HT), hypoxanthine aminopterin thymidine (HAT) and RPMI 1640 supplements were bought from Life Technologies (Grand Island, NY, USA). Foetal bovine serum from Hyclone (Logan, UT, USA) was heat inactivated at 56°C for 30 min prior to use. Hybridoma cloning factor (ORIGEN) was obtained from Fisher Scientific (Pittsburgh, PA, USA). Cell culture plastic wares were obtained from Costar (Cambridge, MA, USA). The ELISAs were carried out in 96-well polystyrene microplates (JET Bio-chemicals International, Inc., Toronto, Canada). All water used was purified twice successively by deionisation and distillation.
Preparation of conjugates
Clenbuterol–protein conjugates
Clenbuterol was diazotised and coupled to carrier proteins, HSA and OVA, according to the method of Degand et al. (Kim & Kim, Citation1997) with little modification. Clenbuterol (10 mg) was dissolved in 1.33 ml of distilled water (7.5 mg/ml), and the pH was adjusted to 2.5 by adding 1 N HCl. To this clenbuterol solution, NaNO2 (0.67 ml, 15 mg/ml) solution was added dropwise in the dark at 4°C with constant stirring followed by incubation for 30 min at 4°C to diazotise clenbuterol. To remove unreacted nitrous acid, ammonium sulphamate (50 mg/ml) was added until no more nitrogen bubbles were given off. Diazotisation of clenbuterol was confirmed by the formation of a deep yellow colour after reaction of an aliquot of the above solution with N,N-dimethylaniline. The diazo-clenbuterol solution was added to carrier proteins (3.33 ml; 55 mg/ml in 0.1 M PBS, pH 7.5), then the final pH was adjusted to 7.5 with NaOH (1 N) followed by overnight incubation at 4°C. Clenbuterol–carrier protein conjugate was dialysed against 0.01 M PBS at 4°C for three days in the dark with frequent changes of the dialysis solution.
Salbutamol–protein conjugates
Salbutamol–OVA conjugate was prepared according to the method described by Shelver and Smith (Citation2000). Briefly, 576 mg of salbutamol–H2SO4 (1 mmol) and 120 mg (1 mmol) of succinic anhydride were mixed in 1 ml anhydrous DMF. The solution was stirred at room temperature overnight and salbutamol hemisuccinic was formed. A 100 µl aliquot of salbutamol hemisuccinic solution and 26.2 µl (0.11 mmol) of tributylamine was mixed and stirred on ice for 10 min, followed by adding isobutylchloroformate (0.11 mmol). The reaction was brought to room temperature and stirred for another 1 h. The mixture was dropped into an ice-cold protein solution (100 mg OVA dissolved in 0.1 M sodium borate, pH 8.5). The resulting solution was brought to room temperature and allowed to react overnight. The final solution was dialysed against PBS as above. All the conjugates were lyophilised and stored at 4°C until use.
Production of monoclonal antibody (mAb)
Immunisation
Balb/c female mice (8–10 weeks old) were immunised with clenbuterol–HSA conjugate. First dose consisting of around 100 µg of the conjugate was intra-peritoneally injected as an emulsion of sterilised physiological saline and complete Freund's adjuvant. Booster injections were given two, four and six weeks after the initial dose, with the same amount of immunogen emulsified in incomplete Freund's adjuvant. One week after the last injection, mice were tail-bled and titres of antisera were determined by indirect ELISA. Mice with the highest titre antisera were selected to be spleen donors for hybridoma production and received a final soluble intra-peritoneal injection of 100 µg of conjugate in physiological saline (without adjuvant), three days prior to cell fusion.
Cell fusion
The mice were sacrificed and their spleen cells were removed. The spleen cells were fused with mouse Sp2/0 myeloma cells at the ratio of 10:1 in the presence of 50% PEG 4000 which was used as fusion agent. The fused cells (hybridomas) were distributed in 96-well culture plates supplemented with HAT medium containing 20% foetal calf serum with peritoneal macrophages as feeder cells from young Balb/c mice. The growth of hybridomas in the plates was incubated at 37°C with 5% CO2.
Hybridoma selection and cloning
About 12–14 days after cell fusion, culture supernatants were screened with both non-competitive and competitive indirect ELISAs, coated with conjugate of clenbuterol–OVA, for the presence of antibodies that recognised clenbuterol. Selected hybridomas were subcloned by limiting dilution. Stable antibody-producing clones were expanded and stored in liquid nitrogen.
Monoclonal antibody (mAb) production and characterisation
Parts of stable subclones were expanded and then injected into female Balb/c mice intra-peritoneally which were preinjected with 0.5 ml of liquid paraffin one week later. Ten days after the injection, the ascites were collected and subjected to purification by ammonium sulphate precipitation. Class and subclass determination was performed using Pierce ImmunoPure Monoclonal Antibody Isotyping Kit (Rockford, IL, USA). The purified mAb was lyophilised and stored at –20°C until use.
Indirect competitive ELISA
The optimal mAb and coating antigen concentrations were initially screened with checkerboard titration. The indirect competitive ELISAs for clenbuterol were carried out as follows. Microplate wells were coated overnight at 4°C with coating antigens (5 ng of clenbuterol–OVA or salbutamol–OVA in 100 µl per well of 0.05 M carbonate–bicarbonate buffer, pH 9.6). The next day, the plate was washed four times with PBS containing 0.05% Tween-20 (PBST) and then blocked with 1% BSA in PBS (150 µl/well) by incubation for 1 h at ambient temperature. The plate was washed again four times and a solution of 50 µl per well of analytes or standards and 50 µl per well of mAb (2 µg/ml) were added and incubated at 37°C for 30 min. Peroxidase-labelled goat anti-mouse IgG (1:5000 in PBST; 100 µl/well) was then added, and the plate was incubated 30 min at 37°C. The plate was washed as above and then incubated with substrate solution (100 µl/well) containing Part A (1.0 g of carbamide peroxide, 35.8 g of Na2HPO4·12H2O, 10.3 g of citric acid monohydrate and 100 µl Tween-20 dissolved in 1 l of distilled water, pH 5.0) and Part B (10.3 g of citric acid monohydrate and 40 ml dimethyl sulphoxide containing 700 mg of TMB added into 960 ml of distilled water, pH 2.4), which were mixed at a ratio of 1:1 just prior to use. The reaction was stopped with 2 M of sulphuric acid solution after an incubation of 10–15 min at ambient temperature. The optical density (OD) was read at 450 nm. The data were fitted with the four-parameter logistic equation using SigmaPlot 2000 (Version 6.0).
Effects of buffer pH, salt concentrations and solvents on ELISA
The effects of the chemicals on ELISA were examined by running standard curves in buffer with various pH values, percentages of salt concentrations and organic solvents. The variables of ODmax (the OD value in the absence of clenbuterol) and half-maximum inhibition concentration (IC50, the value comes from the parameter determined by the least-squares fit of the four-parameter equation) were evaluated. To determine effects of pH, clenbuterol was dissolved in 0.01 M PBS with pH values of 5.0, 6.0, 7.0, 7.5, 8.0 and 9.0. To determine effects of salt concentrations, clenbuterol was dissolved in PBS (pH 7.5) with NaCl concentrations of 0, 0.01, 0.02, 0.05, 0.1 and 1.0 M. To evaluate solvent effects, methanol was diluted in PBS to yield final solvent compositions of 0, 5, 10, 20 and 30% (v/v).
HPLC analysis
The HPLC system used was an ESA chromatographic system (ESA, Chelmsford, MA, USA) equipped with two 582 pumps, an organiser chamber, a PEEK pulse damper, a manual injector fitted with a 20 µl loop (Rheodyne 7725i, CA, USA) and a 5600A 16 channels CoulArray detector. Clenbuterol was analysed by reversed-phase HPLC analysis according to the method reported by Zhang, Gan, and Zhao (Citation2003). Briefly, analysis of clenbuterol was carried out on an ODS Hypersil (150×4 mm i.d. and 5 µm particle size, Hewlett-Packard, USA) column. The column temperature was 30°C. The mobile phase component A was 50 mM phosphoric acid–30 mM triethylamine and the pH was adjusted to 4.0 with 2 M sodium hydroxide solution. The mobile phase component B was acetonitrile and methanol in the proportion of 45:30 (v/v). A mixture of mobile phase components A and B (80:20, v/v) was used in the method and the flow rate was kept constantly at 0.8 ml/min. Mobile phase was always freshly prepared, filtered through a 0.22 µm membrane and sonicated before use. The injection volume was 20 µl.
Sample preparation
The recovery and precision of ELISA were evaluated by analysing clenbuterol spiked in chicken and pork tissues at five levels (0.1, 0.9, 2.7, 5.0 and 10 ng/g). Animal tissue (5.0 g) was mixed with 20 ml hydrochloric acid (0.1 M) and homogenised at maximum speed for 30 s using an Ultraturrax homogeniser. The mixture was centrifuged at 5000 rpm for 10 min and the supernatant was collected.
For ELISA, the supernatant was adjusted to pH 11–12 with sodium hydroxide (1 N) and then transferred to a separatory funnel followed by partitioning with ethyl ether (20 ml×3). The ether layer was collected and dried under a gentle nitrogen stream. The residue was dissolved in 0.5 ml of water/methanol (1:1, v/v) and then diluted at least 10-fold for ELISA. Intra-assay variation was measured on the basis of four replicates within a day, and inter-assay variation was determined on the basis of three replicates performed sequentially on separated days.
For HPLC, 15 ml ethyl ether was mixed with the collected supernatant. The mixture was vortex mixed for 5 min and centrifuged for 10 min at 5000 rpm. The organic layer was discarded. Another 15 ml ethyl ether was added to the aqueous layer and the process was repeated. The pH value of the remaining aqueous layer was adjusted to 11–12 by adding 1 N sodium hydroxide solution. Ten millilitres ethyl ether was added and vortex mixed vigorously for 5 min, and then the sample was centrifuged for 10 min at 5000 rpm. The ethyl ether layer was collected. To the remaining aqueous phase, another 10 ml ethyl ether was added and the extraction process was repeated. The twice collected ethyl ether was combined and evaporated to dryness under a continuous flow of nitrogen at 60°C. The residue was redissolved in 1 ml mobile phase and passed through a 0.22 µm filter before the HPLC analysis.
Results and discussion
Monoclonal antibody (mAb) production and characterisation
Two cell fusion experiments were performed. Growing hybridomas, which were observed in 95% of the wells, were screened for clenbuterol antibodies. The culture supernatants were tested by indirect non-competitive ELISA against clenbuterol–OVA, OVA and HSA. Amongst the growing hybridomas wells, 25 of them with hybridomas produced antibodies that recognised only the conjugate but not HSA or OVA. Hybridomas from these 25 wells were cloned by limiting dilution. Competitive ELISA was performed to evaluate the ability of these 25 mAbs to recognise clenbuterol. Ten mAbs showed that ability and five of them showed a significant competition in the presence of clenbuterol. Isotyping, performed with a commercial kit, showed that all five clones were IgG1κ. Hybridomas (3F7) were injected intra-peritoneally into paraffin-primed mice to produce ascites, which was purified and selected for further study.
Specificity of mAb to clenbuterol was evaluated through the cross-reactivity of the homologous ELISA for some clenbuterol analogues (). Cross-reactivity values were calculated as percentages of the IC50 of the standard clenbuterol relative to the IC50 of the test compounds. This mAb has shown high specificity to clenbuterol, as the cross-reactivity values achieved were very low (<0.01%) for all the test compounds except salbutamol (6.4%; ).
Table 1. Cross-reactivity of clenbuterol related compounds.
Competitive ELISA
Competitive ELISA formats can be divided into homologous and heterologous formats (Schneider & Hammock, Citation1992). In homologous format the same hapten is used for immunisation and assay purposes, whereas in heterologous format the immunising hapten and the ELISA coating hapten differ in their molecular structures. Hapten heterology is commonly used to eliminate problems associated with the high affinity of the antibodies to the spacer arm that leads to no or poor inhibition by the target compound (Jülicher, Mussenbrock, Renneberg, & Cammann, Citation1995). Heterologous assay, especially heterology in structure of hapten would largely improve the sensitivity of ELISA assay (Kim, Cho, Lee, & Lee, Citation2003; Wang et al., Citation2009[]). Thus, on an initial step we explored the influence of heterologous haptens on the sensitivity to optimise the ELISA.
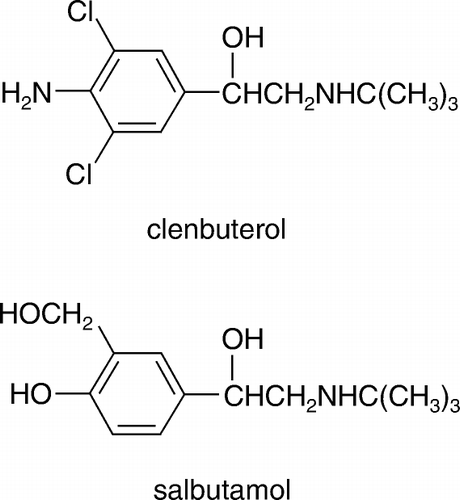
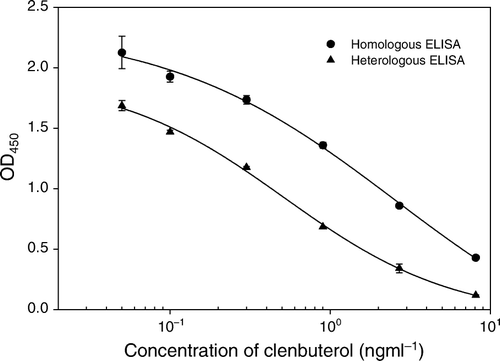
Salbutamol is another β-adrenergic agonist with the similar chemical structure to clenbuterol except for the substituents in the benzene ring (). Cross-reactivity between these two compounds has been observed. Thus, salbutamol was coupled to OVA through the phenyl hydroxy group to develop a heterologous ELISA. shows the inhibition curves of clenbuterol in both homologous and heterologous ELISAs. At the optimal concentrations of the coating antigens (0.05 µg/ml for both clenbuterol–OVA and salbutamol–OVA) and mAb (2 µg/ml), the IC50 of heterologous ELISA and homologous was 0.3 and 2.5 ng/ml, respectively. The limits of detect (LOD, calculated as IC10 values) were 0.1 and 0.7 ng/ml, respectively. It is obvious that the heterologous ELISA shows higher sensitivity than the homologous one in spite of the decrease of ODmax values. The heterologous ELISA was herein employed in the rest of the study.
Chemical effects on ELISA performance
Immunoassay performance is commonly affected by chemical parameters such as organic solvents, ionic strength, pH and other substances in the sample matrix. The effects of these parameters were estimated by comparing inhibition curves and IC50 values obtained under various conditions with that of a control.
Assay pH values varying from seven to nine caused little fluctuation in the IC50 and ODmax values of ELISA (A). However, this assay is correspondingly sensitive to lower pH (≤6.0). High or low pH generally change the ionic interactions within the antibody and finally chaotropic ions disrupt the water structure around the antibody, all thereby changing the antibody structure and allowing the release of an antigen (Crabbe, Haasnoot, Kohen, Salden, & Van Peteghem, Citation1999). In most immunoassay systems, neutral assay buffer provides the best conditions for the binding of antibody and antigen. At pH 7.5, this assay shows good shape of inhibition curve with an IC50 around 0.3 ng/ml.
Figure 3. Effects of pH values (A), ionic strength (B) and solvent content (C) of assay buffer on ELISA. Each value represents the mean of four replicates.
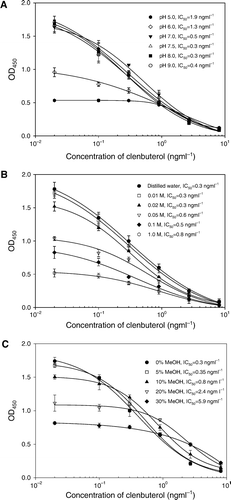
The ELISA sensitivity was altered gradually with the increase of NaCl concentration (B). When NaCl concentrations moved up from 0 to 0.02 M, the IC50 kept stable at the concentration of approximate 0.3 ng/ml. The decline of ODmax values in the presence of weak ionic strength may be due to the dispersion and weakening of the non-specific binding derived from antibodies. Concentration of NaCl higher than 0.02 M caused the drop of sensitivity (B), because the much stronger ionic strength may weaken the binding of antibody and antigen. Therefore, the ELISA was carried out in the assay buffer containing 0.01 M NaCl. In practice, ionic strength of real samples can be adjusted by simple dilution with water or concentrated buffer.
In addition, the effect of methanol on ELISA was studied because it is water miscible and would be used in sample preparation. C shows normalised dose response curves at different methanol compositions. The IC50 values were slightly affected by methanol up to 5% (v/v). If higher solvent concentrations are necessary in some assays, up to 10% of methanol could be used because the sensitivity decrease is acceptable at this concentration. When the methanol content achieved 20%, IC50 and ODmax had obviously been decreased.
Determination of clenbuterol in tissue samples
In order to evaluate and correct the interference caused by a variety of food matrices, extracts from blank animal tissues were initially determinated with ELISA. To minimise the effects of physiochemical factors on the ELISA, the final extracts need to be diluted at least 10 folds with PBS prior to the assay. The LOD of ELISA was consequently moved up to 1.0 ng/ml in the animal tissue extracts. The ELISA was applied to detect low, medium and high concentrations clenbuterol spiked in chicken and pork. The average recoveries in intra-assay ranged from 81 to 102% with coefficient variations (CVs) between 3 and 12%, and in inter-assay ranged from 77 to 95% with CVs between 5 and 13% (). In the analysis of HPLC, the average recoveries and CVs were in a range of 96–113% and 4–10%, respectively. It is noticed that all the recoveries of clenbuterol by ELISA are less than those by HPLC (). However, both methods are comparable. In principle, the average recoveries and CVs of ELISA obtained in this study are acceptable for the detection of clenbuterol in animal tissues; thus this ELISA was applied to survey clenbuterol residues in 20 chicken and 30 pork tissues collected from the local markets. Meanwhile, six pork samples and four chicken samples were selected randomly from the above samples and analysed with HPLC. Clenbuterol has not been determined in any of the muscle tissue samples from the markets surveyed in the present study, by either ELISA or HPLC. The results of the ELISA and HPLC for real world samples are consistent.
Table 2. Recoveries and precision of clenbuterol in spiked samples by ELISA and HPLC.
Conclusion
We have developed a sensitive heterologous ELISA for clenbuterol based on a specific mAb (3F7) produced from clenbuterol–HSA and a coating antigen salbutamol–OVA. At optimal experimental conditions, the IC50 and LOD of this assay were 0.3 and 0.1 ng/ml, respectively. No significant variations were observed when the assay was run in PBS with 5% methanol. This ELISA was finally applied to analyse clenbuterol residue in chicken and pork samples. The acceptable recoveries of both intra-assay (81–102%) and inter-assay (77–95%) from spiked samples and the consistent results between the ELISA and the HPLC suggested that this ELISA will be a feasible quantitative/screening method for clenbuterol residue in animal tissues.
References
- Barbosa , J. , Cruz , J. , Martins , J. , Silva , J.M. , Neves , C. Alves , C. 2005 . Food poisoning by clenbuterol in Portugal . Food Additives and Contaminations , 22 : 563 – 566 .
- Blanca , J. , Munoz , P. , Morgado , M. , Mendez , N. , Aranda , A. Reuvers , T. 2005 . Determination of clenbuterol, ractopamine and zilpaterol in liver and urine by liquid chromatography tandem mass spectrometry . Analytica Chimica Acta , 529 : 199 – 205 .
- Crabbe , P. , Haasnoot , W. , Kohen , F. , Salden , M. and Van Peteghem , C. 1999 . Production and characterization of polyclonal antibodies to sulfamethazine and their potential use in immunoaffinity chromatography for urine sample pre-treatment . Analyst , 124 : 1569 – 1575 .
- Gonzalez , P. , Fente , C.A. , Franco , C. , Vazquez , B. , Quinto , E. and Cepeda , A. 1997 . Determination of residues of the β-agonist clenbuterol in liver of medicated farm animals by gas chromatography-mass spectrometry using diphasic dialysis as an extraction procedure . Journal of Chromatography B , 693 : 321 – 326 .
- He , L.M. , Su , Y.J. , Zeng , Z.L. , Liu , Y.H. and Huang , X.H. 2007 . Determination of ractopamine and clenbuterol in feeds by gas chromatography-mass spectrometry . Animal Feed Science and Technology , 132 : 316 – 323 .
- Jülicher , P. , Mussenbrock , E. , Renneberg , R. and Cammann , K. 1995 . Broadening the antibody specificity by hapten design for an enzyme-linked immunoassay as an improved screening method for the determination of nitroaromatic residues in soils . Analytica Chimica Acta , 315 : 279 – 287 .
- Kim , Y.H. and Kim , Y.S. 1997 . Effects of active immunization against clenbuterol on the growth-promoting effect of clenbuterol in rats . Journal of Animal Science , 75 : 446 – 453 .
- Kim , Y.J. , Cho , Y.A. , Lee , H.S. and Lee , Y.T. 2003 . Investigation of the effect of hapten heterology on immunoassay sensitivity and development of an enzyme-linked immunosorbent assay for the organophosphorus insecticide fenthion . Analytica Chimica Acta , 494 : 29 – 40 .
- Kootstra , P.R. , Kuijpers , C.J.P.F. , Wubs , K.L. , van Doorn , D. , Sterk , S.S. van Ginkel , L.A. 2005 . The analysis of beta-agonists in bovine muscle using molecular imprinted polymers with ion trap LC/MS screening . Analytica Chimica Acta , 529 : 75 – 81 .
- Martinez-Navarro , J.F. 1990 . Food poisoning related to consumption of illicit beta-agonist in liver . Lancet , 336 : 1311
- Pulce , C. , Lamaison , D. , Keck , G. , Bostvironnois , C. , Nicolas , J. and Descotes , J. 1991 . Collective human food poisonings by clenbuterol residues in veal liver . Veterinary and Human Toxicology , 33 : 480 – 481 .
- Ricks , C.A. , Dalrymple , R.H. , Baker , P.K. and Ingle , D.L. 1984 . Use of a β-agonist to alter fat and muscle deposition in steers . Journal of Animal Science , 59 : 1247 – 1255 .
- Salleras , L. , Domínguez , A. , Mata , E. , Taberner , J.L. , Moro , I. and Salvà , P. 1995 . Epidemiologic study of an outbreak of clenbuterol poisoning in Catalonia, Spain . Public Health Reports , 110 : 338 – 342 .
- Schneider , P. and Hammock , B.D. 1992 . Influence of the ELISA format and the hapten-enzyme conjugate on the sensitivity of an immunoassay for s-triazine herbicides using monoclonal antibodies . Journal of Agricultural and Food Chemistry , 40 : 525 – 530 .
- Shelver , W.L. and Smith , D.J. 2000 . Development of an immunoassay for the β-adrenergic agonist ractopamine . Journal of Immunoassay , 21 : 1 – 23 .
- Sporano , V. , Grasso , L. , Esposito , M. , Oliviero , G. , Brambilla , G. and Loizzo , A. 1998 . Clenbuterol residues in non-liver containing meat as a cause of collective food poisoning . Veterinary and Human Toxicology , 40 : 141 – 143 .
- Wang , C.M. , Liu , Y.H. , Guo , Y.R. , Liang , C.Z. , Li , X.B. and Zhu , G.N. 2009 . Development of a McAb-based immunoassay for parathion and influence of the competitor structure . Food Chemistry , 115 : 365 – 370 .
- Wang , X.N. , & Zhang , G.P. 2004 . Illegal use of clenbuterol hydrochloride in China and its food safety analysis . Chinese Journal of Animal Science , 40 , 52 54 . In Chinese
- Wingert , W.E. , Mundy , L.A. , Nelson , L. , Wong , S.C. and Curtis , J. 2008 . Detection of clenbuterol in heroin users in twelve postmortem cases at the Philadelphia medical examiner's office . Journal of Analytical Toxicology , 32 : 522 – 528 .
- Xu , T. , Wang , B.M. , Sheng , W. , Li , Q.X. , Shao , X.L. and Li , J. 2007 . Application of an enzyme-linked immunosorbent assay for the detection of clenbuterol residues in swine urine and feeds . Journal of Environmental Science and Health B , 42 : 173 – 177 .
- Zhang , G.P. , Wang , X.N. , Yang , J.F. , Yang , Y.Y. , Xing , G.X. Li , Q.M. 2006 . Development of an immunochromatographic lateral flow test strip for detection of β-adrenergic agonist clenbuterol residues . Journal of Immunological Methods , 312 : 27 – 33 .
- Zhang , X.Z. , Gan , Y.R. and Zhao , F.N. 2003 . Determination of clenbuterol in pig liver by high-performance liquid chromatography with a coulometric electrode array system . Analytica Chimica Acta , 489 : 95 – 101 .