Abstract
In this study the residual rate of immunoreactive glycinin in the digestive tracts of pigs of different ages was compared. Results showed that the residual rate of immunoreactivity decreased as the glycinin passed down the gastrointestinal tract in all groups (P<0.05). In the stomach, upper-jejunum or middle-jejunum, the residual rate decreased with increasing age of the pigs. In the ileum, the residual rate of immunoreactivity of glycinin in growing pigs or finishing pigs is significantly higher than in weaning piglets (P<0.05), while no significant difference existed between growing pigs and finishing pigs (P>0.05). In the caecum, the residual rate in finishing pigs is significantly higher than in weaning piglets (P<0.05), however, no significant difference existed between weaning piglets and growing pigs or between growing pigs and finishing pigs (P>0.05). It indicated that the immunoreactivity of glycinin generally decreases as it descends into the gastrointestinal tract and with the age of the pig.
Introduction
Soybeans have been transformedFootnote† into various forms of soy products because of their highly nutritive value (Rajni, Dianne, & Perry, Citation2003). These products are rich in fibre and low in calories compared with animal foods when evaluated on a dry weight or on an equal-protein basis (John & Fordyce, Citation1989). Due to their beneficial effects the usage of soybeans in the world is gradually increasing. However, the presence of glycinin has hampered their wide use seriously as it contributes to an allergic reaction in humans (Sampson, Citation2004) and animals (Lallès, Dreau, Salmon, & Toullec, Citation1996; Li et al., Citation1990). Glycinin has been recognised as one of the major soybean allergens in human and animal diets, which is composed of several kinds of subunits and its molecular mass ranges from 320 to 360 kDa. So far, five major subunits encoded by a small gene family have been identified; A1aB2, A1bB1b, A2B1a, A3B4 and A5A4B3, which can be divided into three groups based on amino acid sequence homology: group I (A1aB2, A1bB1b, A2B1a), group IIa (A5A4B3) and group IIb (A3B4) (Kazuhiro, Toshio, & Miyo, Citation1997; Nielsen, Citation1985). A (acidic polypeptide, MWs≈35,000–43,000 Da) and B (basic polypeptide, MWs≈20,000 Da) are two types of polypeptides with different isoelectric points linked by disulfide bonding (Moreira, Hermodson, Larkins, & Nielsen, Citation1979; Staswick, Hermodson, & Nielsen, Citation1981).
During the past few decades, several papers on glycinin have been published (Astwood, Leach, & Fuchs, Citation1996; Sun, Li, Dong, Qiao, & Ma, Citation2008a; Sun, Li, Li, Dong, & Wang, Citation2008b; Zhao et al., 2008). However, little attention has been paid on the gastrointestinal digestion of glycinin in pigs and humans. For reasons of ethics and efficacy, prospective studies in humans are not possible. Thus, experimentally induced allergic disease in large animals that closely mimic allergic diseases in humans can be a useful model. In this study, pigs in different physiological stages were used as a large-animal model for studying the residual rate of immunoreactive glycinin in vivo due to their physiological and immunological similarity to human beings. The concentration of immunoreactive glycinin in the digesta of each segment (stomach, upper-jejunum, middle-jejunum, ileum and caecum) was detected using inhibition ELISA and the residual rate of immunoreactive glycinin was calculated.
Materials and methods
Characterisation of purified soybean glycinin
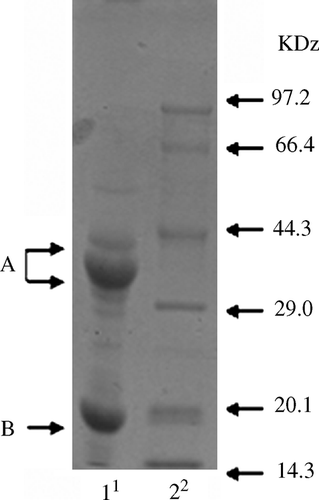
Purified glycinin, donated by Professor Shun-tang Guo from the Food Institute of China Agricultural University (Patent No. 200410029589.4, China), was used in this experiment to eliminate the interference from other components in soybean meal or whole soybean products. The purity glycinin was analysed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) followed by Coomassie Brilliant Blue R-250 staining (Laemmli, Citation1970). The results showed that the gel bands of glycinin are clear and have no hybrid-protein bands which satisfied the needs of our study ().
Diets, animals and management
Diets are formulated to be isocaloric and isonitrogenous and the amino acid content of all diets is balanced. Animals were maintained according to the rules of China Animal Care and Use Committee. Fifteen healthy piglets weaned at 28 days of age, with an average initial body weight of 8.0±0.5 kg, were allocated to three groups (I, II, III), and housed in pens (3×4 m); each pen had five pigs. Diet was fed four times a day in equal quantities at 00:00, 06:00, 12:00 and 18:00 hours. Water was available from drinking nipples all day. All pigs received diets without ingredients originating from soybean products in non-experimental periods; while the pigs received diets containing 4% purified glycininin experimental periods (Sun et al., Citation2008a, Citation2008b). After 3 days of adaptation, the experimental periods of each group started, and lasted for 7 days (30–37, 98–105 and 168–175 days). In experimental periods, Sesquioxide Chromium (0.5%) was added to the diets as the indigestible indicator ().
Table 1. Composition of the diets, as-fed basis (%).
Sample collection
All pigs were anaesthetised with appropriate amount of procaine then slaughtered 2 hours after the last morning meal. The digesta from stomach (between cardiac and pylorus), upper-jejunum (the upper one-third of jejunum), middle-jejunum (the mid-one-third of small jejunum), ileum (from ileocecal ligament to ileocecal valve) and caecum (from ileocecal valve to caecum) were collected separately. Samples (50 g) of each diet were also collected. Globulins were extracted from all samples (Perez, Mills, Lambert, Johnson, & Morgan, Citation2000).
Detection of immunoreactive glycinin
The immunoreactive glycinin in samples were determined by inhibition ELISA (Perez et al., Citation2000). The anti-glycinin polyclonal antibody was made in our laboratory according to the methods described by He, Hu, and Wang (Citation2005).
Indicator technique
According to the indicator technique, based on the concentration of immunoreactive glycinin and indigestible marker Cr2O3, the residual rate of immunoreactive glycinin was calculated using the following formula:
b=Concentration of Cr in diets (mg g−1).
c=Concentration of immunoreactive of glycinin in digesta(mg g−1).
d=Concentration of Cr in digesta (mg g−1).
Statistical analysis
The data were analysed using the GLM procedure (SPSS Inc., Chicago, IL, USA). The results were expressed as mean values±standard error of the mean. P-values ≤ 0.05 were considered statistically significant.
Results and discussion
In this study, all piglets were offered solid feed without ingredients originated from leguminous products three weeks before weaning for two reasons. First, creep feed supplies supplemental nutrients that are required to maintain satisfactory growth rates and achieve heavier weaning weights. Second, the consumption of creep feed is believed to prepare the digestive system of the piglets for digestion of complex carbohydrates and protein that will be supplied as the sole source of nutrients after weaning (Pluske, Dividich, & Verstegen, Citation2003). The feed dropped from the trough of sow was cleared immediately in order to prevent the piglets from eating it.
Results showed that the residual rate of immunoreactive glycinin in digesta is decreasing from stomach to caecum in all groups(P<0.05). The residual rate of immunoreactivity of glycinin in the stomach, upper-jejunum or middle-jejunum was decreased significantly as the piglets aged (P<0.05). In ileum, the residual rate of immunoreactive glycinin in digesta from growing pigs or finishing pigs is significantly higher than weaning piglets (P<0.05), while no significant difference existed between growing pigs and finishing pigs (P>0.05). In the caecum, the residual rate in digesta from finishing pigs is significantly higher than that of weaning piglets (P<0.05), however, no significant difference was found between weaning piglets and growing pigs, or between growing pigs and finishing pigs (P>0.05) ().
Table 2. Analysis of the residual rate of immunoreactive glycinin in digesta.
Although the residual rate of immunoreactivity of glycinin decreased as the digesta descended the gastrointestinal tract, immunoreactive glycinin could still be detected in the digesta collected from the caecum in all pigs. These may be attributed to a number of interacting factors, such as protein structure and enzymatic systems (Lallès et al., Citation1999). The digestion of proteins by proteinases is a complex process. The extent and kinetics of protein hydrolysis is influenced by the protein structure. The richness of glycinin in intra- and inter-molecular disulphide bonds, and the hydrophobicity of basic polypeptides limit its susceptibility to proteolysis (Fukushima, Citation1991). In addition, the gastric pH of piglets soon after weaning does not seem to be adequate for protein digestion compared with the other two groups of pigs. It was high (pH > 4.5) compared with the optimal pH for pepsin action (pH 2–4) (Beynon & Bond, Citation1989). The inefficiency of pepsin hydrolysis may be responsible for the low digestion of soybean proteins in piglets.
It clearly demonstrates that the residual rate of immunoreactive glycinin in digesta from the same digestive segment is reduced with the ageing of pigs as the gastrointestinal tract matures. The significant difference found in the stomach, upper-jejunum and middle-jejunum for the residual rate of immunoreactive glycinin among weaning piglets, growing pigs and finishing pigs may attributed to the great difference in the digestive physiology among these pigs in this experiment. The digestive functionality of piglets is immature in the weaning period. For example, the levels of most of the proteinases did not reach maximum until the pigs were 7 weeks old. Even at the age of 8 weeks, the level of gastric and tryptic proteinases activity apparently had not reached a maximum in the weaned pigs (Hartman et al., Citation1961). In the stomach, the activity of pepsin is obviously hampered by the lack of the hydrochloric acid, which results in a limited inactivation of immunoreactive glycinin. In addition, the stomach emptying time, which has a key role as it determines the rate of food delivery to the small intestine, is about 3–5 hours at 30 days of age (Han, Citation2002), so a lot of immunoreactive glycinin could flow into the small intestine. Lower levels of pancreatic trypsin and chymotrypsin activity were found in weaned pigs (Efird, Armstrong, & Dennis, Citation1982); as a result immunoreactive glycinin cannot be inactivated completely in the small intestine.
Moreover, the weaning period is a period of intense stress for piglets, with profound consequences on growth, physiology and disease outbreaks, as the piglets’ gut adapt to the change in feed type, resulting in severe undernutrition (Lallès, Bosi, Smidt, & Stokes, Citation2007; Lallès, Boudry, & Favier, Citation2004). The rapid changes in the structure and function of the digestive tract undoubtedly result in a transient period of suboptimal digestive competence, which may last from 7 to 14 days and is characterised by low feed intake and little or no weight gain. This in turn affects various aspects of small intestinal architecture and function leading to gut-associated disorders. The extent of the depression in growth depends on how rapidly the piglets can adjust to its new circumstances and regain homeostasis. It often takes piglets 2 or 3 weeks to recover their energy intake and begin to grow at the same rate as they did before weaning (Pluske, Williams, & Aherne, Citation1995; Pluske et al., Citation2003).
All the factors may contribute to the higher residual rate of immunoreactive glycinin in weaning piglets. Compared with weaning piglets, the digestive tract of growing pigs is more mature, which leads to a lower residual rate of immunoreactive glycinin in the stomach, upper-jejunum and middle-jejunum. Finishing pigs had the lowest residual rate of immunoreactive glycinin as those pigs had the strongest digestive capability of these three groups. No significant difference in the residual rate of immunoreactive glycinin was found in the ileum of growing pigs and finishing pigs as they all have a strong digestive capability (Li, Citation2003). The residual rate of immunoreactive glycinin is decreased in the caecum which may be attributed to the actions of microorganisms.
To a certain extent, the variation of immunoreactive glycinin observed in this experiment is similar to the results described by Wang, Qin, Zhao, and Sun (Citation2009); i.e. that the immunoreactive β-conglycinin (another important soybean allergen) tended to decrease with the growth of age of the pig and the distance along the digestive tract at which measurements were made. However, in all groups, the residual rate of immunoreactive glycinin (60.69, 58.49 and 57.09%) is much lower than that of β-conglycinin (66.75, 65.67 and 64.11) in the stomach, while the residual rate of immunoreactive glycinin (9.41, 8.26 and 7.75) is much higher than that of β-conglycinin (3.27, 3.15 and 2.93) in the ileum. These may indicate that differences existed in the digestion between glycinin and β-conglycinin. The digestion of proteins by proteinases is a complex process which may attribute to a number of interacting factors, such as protein structure and enzymatic systems (Lallès et al., Citation1999). The stability to gastric digestion in vitro of β-conglycinin may be ascribed to its higher residence time in the stomach (Astwood et al., Citation1996). The richness of glycinin in intra- and inter-molecular disulphide bonds, and the hydrophobicity of basic polypeptides limit its susceptibility to proteolysis and may explain the higher residual rate of glycinin in the ileum compared to that of β-conglycinin (Fukushima, Citation1991).
Conclusions
This study demonstrated that the residual rates of immunoreactive glycinin in digesta from all groups are gradually decreased from stomach to caecum, whilst it is reduced as the pig ages in any specific digestive sites (except ileum and caecum). This study may give us a better understanding about the residual rates of immunoreactive glycinin in vivo, which may provide some theoretical base for further research on soybean glycinin-related allergic disease.
Acknowledgements
This investigation was financially supported by the National Natural Science Foundation of P.R. China (No. 30430520). The author thanks Xiao-lin Zhang for critical reading of the manuscript.
Notes
†This paper and the paper, Comparative study on the stability of soybean (glycine max) β-conglycinin in vivo, published in Food and Agricultural Immunology, Vol. 20, No. 4, pp. 295–304, form a part of T. Wang's master's thesis (2009)
References
- Astwood , J.D. , Leach , J.N. and Fuchs , R.L. 1996 . Stability of food allergens to digestion in vitro . Nature Biotechnology , 14 : 1269 – 1273 .
- Beynon , R.J. and Bond , J.S. 1989 . Proteolytic enzymes. A practical approach , Oxford : IRL Press .
- Efird , R.C. , Armstrong , W.D. and Dennis , L.H. 1982 . The development of digestive capacity in young pigs: Effects of age and weaning system . Journal of Animal Science , 55 : 1380 – 1387 .
- Fukushima , D. 1991 . Recent progress of soybean protein foods: Chemistry, technology and nutrition . Food Review International , 7 : 323 – 351 .
- Han , Z.K. 2002 . Livestock physiology , (3rd ed.) Beijing, , China : China Agricultural Publisher (in Chinese)
- Hartman , P.A. , Hays , V.W. , Baker , R.O. , Neagle , L.H. and Catron , D.V. 1961 . Digestive enzyme development in the young pig . Journal of Animal Science , 20 : 114 – 123 .
- He , Z.Y. , Hu , G.X. , Wang , C.F. 2005 . Technique of animal immunology . Changchun, , China : Science and Technology Publishing House (in Chinese)
- John , W.E. and Fordyce , E.J. 1989 . Soy products and the human diet . American Journal of Clinical Nutrition , 49 : 725 – 737 .
- Kazuhiro , Y. , Toshio , T. and Miyo , S. 1997 . Biochemical characterization of soybean protein consisting of different subunits of glycinin . Journal of Agricultural and Food Chemistry , 45 : 656 – 660 .
- Laemmli , U.K. 1970 . Cleavage of structural proteins during the assembly of the head of bacteriophage T4 . Nature , 227 : 680 – 685 .
- Lallès , J.P. , Bosi , P. , Smidt , H. and Stokes , C.R. 2007 . Weaning – a challenge to gut physiologists . Livestock Science , 108 : 82 – 93 .
- Lallès , J.P. , Boudry , G. and Favier , C. 2004 . Gut functions and dysfunction in young pigs: Physiology . Animal Research , 53 : 301 – 316 .
- Lallès , J.P. , Dreau , D. , Salmon , H. and Toullec , R. 1996 . Identification of soybean allergens and immune mechanisms of dietary sensitivities in preruminant calves . Research in Veterinary Science , 60 : 111 – 116 .
- Lallès , J.P. , Tukur , H.M. , Salgado , P. , Mills , E.N.C. , Morgan , M.R.A. and Quilien , L. 1999 . Immunochemical studies on gastric and intestinal digestion of soybean glycinin and β-conglycinin in vivo . Journal of Agricultural and Food Chemistry , 47 : 2797 – 2806 .
- Li , D.F. 2003 . Nutrition of swine , (2nd ed.) Beijing, , China : China Agricultural Publisher (in Chinese)
- Li , D.F. , Nelssen , J.L. , Reddy , P.G. , Belcha , F. , Hancock , J.D. and Allee , G.L. 1990 . Transient hypersensitivity to soybean meal in the early-weaned pig . Journal of Animal Science , 68 : 1790 – 1799 .
- Moreira , M.A. , Hermodson , M.A. , Larkins , B.A. and Nielsen , N.C. 1979 . Partial characterization of the acidic and basic polypeptides of glycinin . The Journal of Biological Chemistry , 254 : 9921 – 9926 .
- Nielsen , N.C. 1985 . The structure and complexity of the 11S polypeptide in soybean . Journal of the American Oil Chemists’ Society , 49 : 2733 – 2740 .
- Perez , M.D. , Mills , E.N.C. , Lambert , N. , Johnson , I.T. and Morgan , M.R.A. 2000 . The use of anti-soya globulin antisera in investigating soya digestion in vivo . Journal of Agricultural and Food Chemistry , 80 : 513 – 521 .
- Pluske , J.R. , Dividich , J.L. and Verstegen , M.W.A. 2003 . Weaning the pig: Concepts and consequences , Netherlands : Wageningen Academic Publishers .
- Pluske , J.R. , Williams , I.H. and Aherne , F.X. 1995 . “ Nutrition of the neo-natal pig ” . In The neonatal pig development and survival , Edited by: Varly , M.A. 187 – 235 . Wallingford : CAB International .
- Rajni , M. , Dianne , T.T. and Perry , K.W. 2003 . Characterization of storage proteins in different soybean varieties and their relationship to tofu yield and texture . Food Chemistry , 82 : 265 – 273 .
- Sampson , H.A. 2004 . Update on food allergy . Journal of Allergy and Clinical Immunology , 113 : 805 – 819 .
- Staswick , P.E. , Hermodson , M.A. and Nielsen , N.C. 1981 . Identification of the acidic and basic subunit complexes of glycinin . The Journal of Biological Chemistry , 256 : 8752 – 8755 .
- Sun , P. , Li , D.F. , Dong , B. , Qiao , S.Y. and Ma , X. 2008a . Effects of soybean glycinin on performance and immune function in early weaned pigs . Archives of Animal Nutrition , 62 : 313 – 321 .
- Sun , P. , Li , D.F. , Li , Z.J. , Dong , B. and Wang , F.L. 2008b . Effects of glycinin on IgE-mediated increase of mast cell numbers and histamine release in the small intestine . Journal of Nutrition Biochemistry , 19 : 627 – 633 .
- Wang , T. , Qin , G.X. , Zhao , Y. and Sun , Z.W. 2009 . Comparative study on the stability of soybean (glycine max) β-conglycinin in vivo . Food and Agricultural Immunology , 20 : 295 – 304 .
- Zhao , Y. , Qin , G.X. , Sun , Z.W. , Zhang , X.D. , Bao , N. , Wang , T. , Zhang , B. , Zhang , B.L. , Zhu , D. and Sun , L. 2008 . Disappearance of immunoreactive glycinin and b-conglycinin in the digestive tract of piglets . Archives of Animal Nutrition , 62 : 322 – 330 .