Abstract
Glycinin and β-conglycinin have been identified as major food/feed allergens. But effects of glycinin and β-conglycinin on enterocyte migration in piglets are scare. Fifteen weanling (7.06±0.18 kg) General No. 1 barrows, weaned at 28 days, were used. The piglets were randomly allotted to three (A, B and C) treatments with five replicates. The piglets in the A group (control group) were fed diets without ingredients originating from leguminous products, while the piglets in the B or C groups were fed the diets containing purified glycinin or β-conglycinin, which replaced protein in Group A by 4%. All the experimental periods were followed for 7 days. Five-micrometre thick sections of small intestinal tissue were stained with the TUNEL method to assess apoptotic activity, and with Ki-67 immunohistochemistry to assess cellular proliferation. The results indicated that glycinin or β-conglycinin increased proliferative index and apoptotic index in duodenum for piglets (P<0.05).
Introduction
Soybean meal is the most common source of supplementary protein used in swine production, attributing to several factors including its widespread availability, high protein, lysine and energy content as well as its palatability (Qiao et al., Citation2003). Unfortunately, glycinin and β-conglycinin, the main storage globulin in soybean proteins, has long been recognised as a source of dietary allergens of pigs (Dunsford, Knabe, & Hanesly, Citation1989; Li et al., Citation1991; Miller et al., Citation1984). To date, studies have focused on the negative effects of soybean allergens (Miller et al., Citation1984), the techniques for inactivation processing (Qin, ter Elst, Bosch, & van der Poel, Citation1996) and the mechanism of hypersensitivity (Guo, Piao, Cao, Ou, & Li, Citation2008; Sun, Li, Dong, Qiao, & Ma, Citation2008a; Sun, Li, Li, Dong, & Wang, Citation2008a, 2008b) have been conducted. Previously, piglets fed soybean protein presents reduced villus height and increased crypt depth owing to soybean-induced allergy (Dunsford et al., Citation1989; Li et al., Citation1990; Qin et al., Citation2002). But the effects of glycinin and β-conglycinin on mucosal cell turnover in pigs are scarce. Qiao et al. (Citation2003) report that moist extruded full-fat soybeans in weanling pig diets can slow the migration rate and turnover time of epithelial cells of the small intestine compared with soybean meal. However, whether piglets fed purified glycinin or β-conglycinin suffer bad enterocyte apoptosis, proliferation and migration have not been identified.
The objective of the present study was to investigate the effects of glycinin or β-conglycinin on gut morphology and enterocyte migration of piglets. Piglets were fed purified glycinin or β-conglycinin to eliminate the interference from other components in soybean meal or whole soybean products. In this study, enterocyte migration distance (EMD), enterocyte apoptosis and proliferation were measured, and relative enterocyte migration rate (REMR) was calculated. This study was developed to provide the further information on gut morphology and enterocyte migration of pigs fed with glycinin or β-conglycinin, which are essential criteria to explore the soybean-induced hypersensitivity.
Materials and methods
Purification of glycinin and β-conglycinin
Purified glycinin and β-conglycinin suspension were kindly donated by Professor Shuntang Guo at the Food Institute of China Agricultural University (Patent number: 200410029589.4, Beijing, China). The globulins sample contained over 85% glycinin or β-conglycinin determined by the Kjeldahl method and SDS-PAGE analysis (Guo, Piao, Ou, Li, & Hao, Citation2007; Laemmli, Citation1970).
Experimental design
Experiments with piglets from the same herd were conducted at Jilin University Experimental pig farm (Changchun, China). In order to complement the innutrition and have piglets eating solid feed, all the piglets were fed from 7 days of age with dry whole milk. Moreover, the diets devoid of soybean protein were used before experiment to avoid the experimental effects from the oral tolerance of soybean. All of the piglets were weaned on the 28th day after birth. Fifteen weaned piglets (7.06±0.18 kg) General No. 1 barrows were selected. After 3 days of adaptation, the pigs were randomly allotted to three (A, B and C) treatments. Each treatment had five replicates.
The piglets in the A group (control group) were fed diets without ingredients originating from leguminous products, while the piglets in the B or C groups were fed the diets containing purified glycinin or β-conglycinin, which replaced protein in Group A by 4%. All the experimental periods were followed for 7 days. The composition and nutrient content of the diets are shown in . The diets were formulated to meet NRC (1998) requirements. The indicator Cr2O3 (0.5%) was added to the diets.
Table 1. Ingredient composition and nutrient levels of the diets (as-fed basis).
Initial and final live weight, and feed consumption data recorded at the beginning and end of the study were used to calculate the growth performance results including average daily gain, average daily feed intake and feed efficiency.
Sample collection and chemical analysis
After the experimental periods, all the piglets were anesthetised with excess procaine and slaughtered 1 hour after the morning meal. Samples of small intestinal tissue were taken at the middle of the duodenum, jejunum and ileum. After being washed in saline, the samples were fixed in formalin, embedded in paraffin. Five-micrometre thick sections were stained by the TUNEL method and by Ki-67 immunohistochemistry.
TUNEL procedure and assessment of apoptotic cells
To detect the enterocyte apoptosis, we applied the TUNEL method using a commercially available kit (Cat: KGA7042, Kengen, Nanjing, China). Paraffin sections were dewaxed with xylene, dehydrated with graded dilutions of ethanol and then washed with phosphate-buffered saline (PBS). Subsequently, the tissue specimens were digested with 20 µg/mL proteinase K at room temperature for 15 minutes. Following the application of an equilibrium buffer, sections were incubated in working strength terminal deoxynucleotidyl transferase (TdT) containing biotin-11-dUTP at 37°C for 60 minutes. After being washed in PBS, sections were treated with streptavidin-horseradish peroxides (HRP) for 30 minutes at 37°C. Colour was developed in a 0.05% diaminobenzidine (DAB) substrate solution. Sections were counterstained with methyl green. Negative control sections were produced by omission of the TdT. Positive control sections were produced by DNAse I digestion of the DNA, giving rise to a strong nuclear staining of all cells. Cells were defined as apoptotic when the whole nuclear area of the cell labelled positively or when they contained small positively labelled globular bodies (apoptotic bodies).
Immunostaining with Ki-67
Sections were placed on positively charged glass slides and deparaffinised. They were then exposed to microwave pretreatment (in 10 mmol/L citrate buffer, pH 6 at 850 W for 20 minutes). To detect the enterocyte proliferation, binding of Ki-67 (monoclonal; Cat: RM-9106; Thermo, Fremont, CA, USA) was achieved using a labelled streptavidin–peroxidase complex methods (Ultrasensitive Kit, KIT9706, Maixin, Fuzhou, China). Sections were then counterstained with hematoxylin, dehydrated, cleared and permanently mounted. Negative control sections were performed without the primary antibody.
Enterocyte turnover
In each biopsy, TUNEL or Ki-67 staining were quantitated by counting the number of positive epithelial cells in at least 20 wells per sample oriented half-crypt or half-villus units and dividing this number by the total number of crypt cells to obtain the apoptotic index (AI) and the proliferative index (PI). The EMD is the distance from crypt basin to villus apex along which the enterocyte migrates. According to Miller and Slade (Citation2003), combination of EMD and PI data enables calculation of proportional estimates of REMR:
Statistical analysis
Data of enterocyte apoptosis and proliferation, EMD and REMR coefficients were analysed using 3×3 factorial arrangement: three diets (non-leguminous meal, glycinin meal and β-conglycinin) and three parts of digestive tract types (duodenum, mid-jejunum and ileum) using the general linear model procedure of SPSS 11.5. The results were expressed as mean values±standard deviation (SD). Differences among means were tested using Duncan's multiple range tests. Statements of statistical significance were based upon P≤0.05.
Results
The growth performance data are presented in . Generally, when piglets were fed diets with glycinin or β-conglycinin, average daily gain, average daily feed intake and feed conversion ration during 4–5 weeks of age reduced (P=0.002, P<0.001 and P=0.03, respectively). However, there was no difference between glycinin treatment and β-conglycinin treatment.
Table 2. Coefficients of diet type on performance in pigs of different growth periods.
Apoptotic cells in the villus and Ki-67 positive cells in the crypt of mid-jejunum in piglets fed with soybean antigens are presented in and . Data for enterocyte apoptosis, proliferation and migration affected by diet type and part of digestive tract type in piglets are shown in . Compared with the control, AI and PI were significantly increased in duodenum for piglets fed with glycinin or β-conglycinin (P<0.001). Glycinin or β-conglycinin had no effects on other part of digestive tract (P>0.05). The AI and PI had the same variation for piglets (P<0.001), the highest in the mid-jejunum and the lowest in the ileum. Diets have no significant effects on EMD and REMR in piglets (P>0.05). Parts of digestive tract have significant effects on EMD in piglets (P<0.001), the highest in duodenum and the lowest in ileum. REMR of mid-jejunum and ileum decreased in piglets compared to duodenum (P<0.001).
Figure 1. Apoptotic cells (arrows) in the villus of mid-jejunum in piglets fed with soybean antigens (TUNEL, original magnification×800).
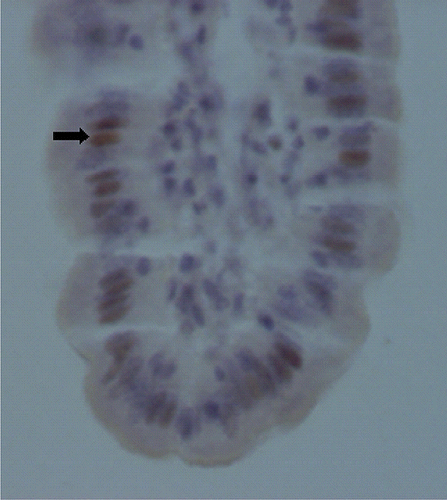
Figure 2. Ki-67 positive cells (arrows) in the crypt of mid-jejunum in piglets fed with soybean antigens (Ki-67 immunostaining, original magnification×800).
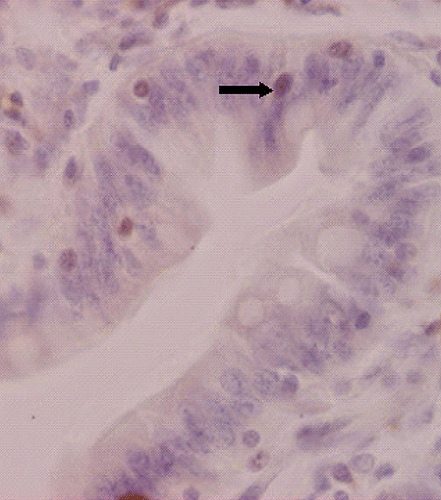
Table 3. Effects of diet type and part of digestive tract type on enterocyte apoptosis, proliferation and migration in piglets, growers and finishers.
Discussion
Many studies have been conducted in young pigs to investigate the allergenicity of soybean proteins that often cause disorders of immune function and reduced performance (Li et al., Citation1990; Zhao et al., Citation2008). This study evaluated the effects of glycinin or β-conglycinin on gut morphology and enterocyte migration of piglets. The results may provide new information for exploring the soybean-induced hypersensitivity.
Many studies have been developed to investigate the performance of piglets fed soybean products. These studies typically observe greater average daily gain and feed conversion ration in pigs fed diets based on milk product compared to those fed diets based on soybean meal (Chae et al., Citation1999; Leibholz, Citation1982; Sohn et al., Citation1994; Wilson & Leibholz, Citation1981). It is hypothesised that residues of antigenic materials cause piglet gastrointestinal hypersensitivity and lower performance after weaned (Li et al., Citation1991). Recently, glycinin or β-conglycinin purification was used for feeding trial to confirm this hypothesis (Sun et al., Citation2008a, Citation2008b; Zhao et al., Citation2008). Consistent with these reports, reduced performance was also observed in glycinin- or β-conglycinin-sensitised piglets in this study.
Previous studies have shown that transient hypersensitivity to soybean antigens may be responsible for these morphological changes, such as villus atrophy, crypt hypertrophy (Dunsford et al., Citation1989; Kenworthy, Citation1976; Li et al., Citation1990; Qin et al., Citation2002), and increased the EMD in piglets fed with soybean meal diet, inferred from the results of increased crypt depth and constant villus height (Salgado et al., Citation2002). Partly consistent with Qiao et al. (Citation2003), dietary glycinin or β-conglycinin had no significant effects on EMD of piglets in this study. Values of EMD determined in this experiment were within the range of previous reports (Qiao et al., Citation2003; Salgado et al., Citation2002), which varied in different parts of digestive tract similar to the results of Qiao et al. (Citation2003).
An important finding of current study is that increased PI, AI and REMR only in duodenum of piglets fed with glycinin or β-conglycinin, not in other parts of digestive tract of piglets. This is similar to the results that moist extruded full-fat soybeans slow the epithelial migration rate in piglets compared soybean meal since soybean antigens are the major different ingredient between moist extruded full-fat soybeans and soybean meal from the techniques for inaction processing (Qiao et al., Citation2003; Zhao et al., Citation2007).
Under normal conditions, the mucosa of the small intestine is one of the most dynamic tissues of the body. In the mouse, about 1200 new epithelial cells migrate onto a villus per day (Hall, Coates, Ansari, & Hopwood, Citation1994), equal to roughly 1 g of tissue (109 cells) for every 5 days (Potten, Citation1992). To maintain homeostasis, this high rate of proliferation must balance an equivalent rate of cell loss. This was consistent with the results in this study that increased apoptosis was matched by increased proliferation. We found that the enterocyte proliferation and apoptosis accelerated in duodenum of piglets fed with soybean antigens. This can be borne out by the fact of villus atrophy and crypt hypertrophy (Dunsford et al., Citation1989; Kenworthy, Citation1976; Li et al., Citation1990; Qin et al., Citation2002). However, the mechanism of proliferation induced by glycinin or β-conglycinin is non-existent to the best of our knowledge due to the relative scarcity of reports.
Different intestinal sites have different PI, AI and REMR. The data in this study indicated that the upper intestine presented higher PI, AI and REMR. It may be related with the fact that the upper intestine is the main part of digestion and absorption of nutritional materials, where massive native diets passed and incidence of mechanical damnification was high.
Taken together, the current study demonstrates glycinin or β-conglycinin increased PI, AI and REMR in duodenum for piglets, although there is no difference between glycinin and β-conglycinin group. Different intestinal sites have different PI, AI, EMD and REMR. Therefore, we can draw the conclusion that glycinin or β-conglycinin accelerates enterocyte proliferation, apoptosis and migration for piglets.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of P.R. China (GrantNo. 30430520).
References
- Chae , B.J. , Han , I.K. , Kim , J.H. , Yang , C.J. , Hancock , J.D. , Kim , I.H. and Anderson , D.A. 1999 . Effects of dietary protein sources on ileal digestibility and growth performance for early-weaned pigs . Livestock Production Science , 58 : 45 – 54 .
- Dunsford , B.R. , Knabe , D.A. and Hanesly , W.E. 1989 . Effect of dietary soybean meal on the microscopic anatomy of the small intestine in the early weaned pigs . Journal of Animal Science , 67 : 1855 – 1863 .
- Guo , P.F. , Piao , X.S. , Cao , Y.H. , Ou , D.Y. and Li , D.F. 2008 . Recombinant soybean protein β-conglycinin α′-subunit expression and induced hypersensitivity reaction in rats . International Archives of Allergy Immunology , 145 : 102 – 110 .
- Guo , P.F. , Piao , X.S. , Ou , D.Y. , Li , D.F. and Hao , Y. 2007 . Characterization of the antigenic specificity of soybean protein β-conglycinin and its effects on growth and immune function in rats . Archives of Animal Nutrition , 61 : 189 – 200 .
- Hall , P.A. , Coates , P.J. , Ansari , B. and Hopwood , D. 1994 . Regulation of cell number in the mammalian gastrointestinal tract: The importance of apoptosis . Journal of Cell Science , 107 : 3569 – 3577 .
- Kenworthy , R. 1976 . Observations on the effects of weaning in the young pigs: Clinical and histopathological studies of intestinal function and morphology . Research in Veterinary Science , 21 : 69 – 75 .
- Laemmli , U.K. 1970 . Cleavage of structural proteins during the asssembly of the head of bacteriophage T4 . Nature , 227 : 680 – 685 .
- Leibholz , J. 1982 . Utilization of casein, fishmeal and soybean proteins in dry diets for pigs between 7 and 28 days of age . Animal Production , 34 : 9 – 15 .
- Li , D. , Nelssen , J.L. , Reddy , P.G. , Belcha , F. , Hancock , J.D. , Allee , G.L. , Goodband , R.D. and Klemm , R.D. 1990 . Transient hypersensitivity to soybean meal in the early-weaned pig . Journal of Animal Science , 68 : 1790 – 1799 .
- Li , D. , Nelssen , J.L. , Reddy , P.G. , Belcha , F. , Klemm , R.D. , Giesting , D.W. , Hancock , J.D. , Allee , G.L. and Goodband , R.D. 1991 . Measuring suitability of soybean products for early-weaned pigs with immunological criteria . Journal of Animal Science , 69 : 3299 – 3307 .
- Miller , B.G. , Newby , T.J. , Stokes , C.R. , Hampson , D.J. , Brown , P.J. and Bourne , F.J. 1984 . The importance of dietary antigen in the cause of post weaning diarrhea in pigs . American Journal of Veterinary Research , 45 : 1730 – 1733 .
- Miller , H.M. and Slade , R.D. 2003 . “ Digestive physiology of the weaned pig ” . In Weaning the pig concepts and consequences , Edited by: Pluske , J.R. , Dividich , J.L. and Verstegen , M.W.A. 117 – 144 . Wageningen, , The Netherlands : Wageningen Academic Publishers .
- Potten , C.S. 1992 . The significance of spontaneous and induced apoptosis in the gastrointestinal tract of mice . Cancer and Metastasis Reviews , 11 : 179 – 195 .
- Qiao , S. , Li , D. , Jiang , J. , Zhou , H. , Li , J. and Thacker , P. 2003 . Effects of moist extruded full-fat soybeans on gut morphology and mucosal cell turnover time of weanling pigs . Asian-Australian Journal of Animal Science , 16 : 63 – 69 .
- Qin , G. , ter Elst , E.R. , Bosch , M.W. and van der Poel , A.F.B. 1996 . Thermal processing of whole soya beans: Studies on the inaction of antinutritional factors and effects on ileal digestibility in piglets . Animal Feed Science and Technology , 57 : 313 – 324 .
- Qin , G.X. , Xu , L.M. , Jiang , H.L. , van der Poel , A.F.B. , Bosch , M.W. and Verstegen , M.W.A. 2002 . The effect of Chinese and Argentine soybeans on nutrient digestibility and organ morphology in Landrace and Chinese Min pigs . Asian-Australian Journal of Animal Science , 15 : 555 – 564 .
- Salgado , P. , Freire , J.P.B. , Mourato , M. , Cabral , F. , Toullec , R. and Lallès , J.P. 2002 . Comparative effects of different legume protein sources in weaned piglets: Nutrient digestibility, intestinal morphology and digestive enzymes . Livestock Production Science , 74 : 191 – 202 .
- Sohn , K.S. , Maxwell , C.V. , Buchanan , D.S. and Southern , L.L. 1994 . Improved soybean protein sources for early-weaned pigs: I. Effects on performance and total track amino acid digestibility . Journal of Animal Science , 72 : 622 – 630 .
- Sun , P. , Li , D. , Dong , B. , Qiao , S. and Ma , X. 2008a . Effects of soybean glycinin on performance and immune function in early weaned pigs . Archives of Animal Nutrition , 62 : 313 – 321 .
- Sun , P. , Li , D. , Li , Z. , Dong , B. and Wang , F. 2008b . Effects of glycinin on IgE-mediated increase of mast cell numbers and histamine release in the small intestine . Journal of Nutrition of Biochemistry , 19 : 627 – 633 .
- Wilson , R.H. and Leibholz , J. 1981 . Digestion in the pig between 7 and 35 d of age. I. The performance of pigs given milk and soya-bean proteins . British Journal of Nutrition , 115 : 301 – 319 .
- Zhao , Y. , Qin , G. , Sun , Z. , Zhang , X. , Bao , N. , Wang , T. , Zhang , B. , Zhang , B. , Zhu , D. and Sun , L. 2008 . Disappearance of immunoreactive glycinin and β-conglycinin in the digestive tract of piglets . Achieves of Animal Nutrition , 62 : 322 – 330 .
- Zhao , Y. , Qin , G. , Wang , T. , Zhang , B. and Zhu , D. 2007 . Comparisons of immunocompetence and inhibit competence of main antinutritional factors in different processed soybean products . Soybean Science , 26 : 930 – 934 .