Abstract
Stroke induces cerebral lesions through the combined action of multiple mechanisms, the inflammatory response on the damage of cerebral tissues after ischemia has become a focus. The Euphoria longan (Lour.) Steud is a fruit and traditional Chinese medicine, with the function of anti-inflammatory and immune regulation. In the present experiments, we investigate the mechanisms underlying the inhibitory effects of polysaccharides of the E. longan (Lour.) Steud on inflammatory responses induced by middle cerebral artery occlusion (MCAO) in rats. Compared with model group, polysaccharides of the E. longan (Lour.) Steud could obviously reduce the neurological function score, the brain water content, the infract volume, the myeloperoxidase (MPO) activity, the tumor necrosis factor-α(TNF-α) and interleukin-1β (IL-1β) level. Polysaccharides of the E. longan (Lour.) Steud have a protective effect on cerebral ischemia/reperfusion injury, which may be related to decreasing inflammatory response mediated by MPO, TNF-α and IL-1β in the brain.
Stroke, frequently encountered following vascular disorders or clinical practices, is a leading cause of death and permanent disability in developed countries, for which there is currently no effective treatment. Brain injury can develop with cerebral ischemia/reperfusion (I/R) due to stroke and other cardiovascular diseases. Stroke induces cerebral lesions through the combined action of multiple mechanisms, such as energy metabolism dysfunction (Eng, Turgay, & Michael, Citation2003) of brain tissues following cerebral ischemic injury, massive free radicals producing (Warner, Sheng, & Batinic-Haberle, Citation2004), phospholipase activation (Chan, Citation2004), energy failure, and programmed cell death (Chen & Huang, Citation2009; Lambeau & Gelb, Citation2008; Mehta, Manhas, & Raghubir, Citation2007), etc. Over the past few years, the inflammatory response on the damage of cerebral tissues after ischemia has become a focus (Allan & Rothwell, Citation2001; Iadecola & Alexander, Citation2001; Li et al., Citation2007; Nobuya, Olga, Kenneth, & Mirochnitchenko, Citation2002; Wang & Smythe, Citation2003).
The Euphoria longan (Lour.) Steud is Sapindaceae (also known as the soapberry family) plant. The E. longan (Lour.) Steud is a fruit and traditional Chinese medicine, which contains polysaccharide, saponins, flavonoid glycosides category, sugars, amino acids and trace elements. Polysaccharide is the main component of the E. longan (Lour.) Steud with the function of antioxidant, immune regulation, promoting intelligence, which was commonly used in the prevention and treatment of cerebrovascular diseases, immune function disorders and other diseases (Okuyama, Citation1999). The molecular weight of polysaccharides of the E. longan (Lour.) Steud is 1.1×105. Pyranose is the major component of polysaccharides of the E. longan (Lour.) Steud, which is composed of glucose, with two glycoside configurations, α-hemiacetal hydroxyl and β-hemiacetal hydroxyl coexisting. Polysaccharides of the E. longan (Lour.) Steud is capable of inhibiting peroxidation in vitro and suppressing excessive production of free radicals in vivo (Huang & Liu, Citation2008; Li, Long, & Xie, Citation2004). The aim of this study was to elucidate the beneficial effects of polysaccharides of the E. longan (Lour.) Steud on the cerebral injury. Based on the results of experiments, nimodipine has showed a beneficial effect on active treatment so it was used as a control in this study.
Materials and methods
Drug and animals
Polysaccharides of the E. longan (Lour.) Steud (purity > 95%, made in Guilin Medical University, Guangxi Province, China) was dissolved in normal saline (NS). One hundred and eighty Sprague-Dawley rats (♂, 220–280 g, Grade II, No. 20090102) were supplied by the Experimental Animal Center of Guilin Medical University. All the experimental procedures were performed in accordance with the guidelines of the Experimental Research Institute of Guilin Medical University.
Focal cerebral ischemia/reperfusion model
Rats were randomly divided into I/R group, sham-operated group, and high-, medium- and low-dose of polysaccharides of the E. longan (Lour.) Steud groups (0.2, 0.1 and 0.05 g kg−1 d−1, respectively), and nimodipine (0.02 g kg−1 d−1) group. The polysaccharides of the E. longan (Lour.) Steud groups were intragastrically administered with drugs for 14 days. One hour after the last administration, all rats were anesthetized with 10% chloralhydrate. A nylon monofilament with a round tip was inserted into the external carotid artery and advanced into the internal carotid until a slight resistance was felt. Such resistance indicated that the filament had reached the circle of Willis (Longa, Weinstein, Carlson, & Cummins, Citation1989). The rectal temperature was maintained at 37–38°C with a heating lamp and heating pad during the operation. The room temperature was controlled in the range of 25–27°C throughout the experimental procedure. After 2 h ischemia, the suture was removed and the animals were allowed to recover.
Neurological deficit evaluation and infarct volume measurement
Neurological deficits were evaluated after 24 h reperfusion using a five-point scoring system according to the method of Longa et al. (1989). Then the 60 rats were anesthetized with 10% chloralhydrate (350 mg kg−1) ip. The brains were carefully removed after rats were sacrificed at different times and sliced into to 2 mm slices. The slices were stained with 2, 3, 5-triphenyltetrazolium chloride (TTC) for 30 min at 37°C, they were photographed on line, as previously described in detail (Li et al., 2007). Image analysis software (NIH Image, National Institutes of Health, Bethesda, Version 1.63) was used for the measurement of the infarcted area.
Brain edema
After 24 h reperfusion, 60 rats were decapitated. The two hemisphere were weighed separately, and then were heated to dry at 160°C using heater. The degree of brain edema was represented by water content, which was calculated by following equation: water content=(wet weight − dry weight)/wet weight×100%.
MPO activity, tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) level
After 24 h reperfusion, 60 rats were decapitated. Brain samples of the ischemic hemisphere were taken from the cortex of the middle cerebral artery (MCA) area and the caudate putamen on ice and stored at −80°C for later analysis. The spectrophotometer methods and the enzyme-linked immunosorbent assay (ELISA) method were used to assay myeloperoxidase (MPO) activity, tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) level according to the description of the kits (Boster, Wuhan, China), respectively.
Statistics
The data were expressed as mean±standard deviation. The Statistical Package for Social Sciences version 13.0 (SPSS Inc, Chicago, IL, USA) was used for standard statistical analysis including one-way ANOVA and Student's t-test. A value of P<0.05 was considered statistically significant.
Results
Infarct tissue was visualized as an area of unstained part in vehicle group, in contrast to viable tissue, which stained red; while there was no infract part in sham-operated group. As shown in and , after treatment with polysaccharides of the E. longan (Lour.) Steud, the infarcted volume and the brain water content were significantly reduced, and the neurological deficit was ameliorated (P<0.01 or P<0.05). As shown in and Figures , the MPO activity, the TNF-α and IL-1β level were significantly higher in model group than that of sham-operation group; treatments of polysaccharides of the E. longan (Lour.) Steud were alleviated the increase in MPO activity, TNF-α and IL-1β level as compared with model group (P<0.01 or P<0.05).
Figure 1. Effect of polysaccharides of the Euphoria longan (Lour.) Steud on the infracted volume in cerebral ischemia/reperfusion rats. (A) sham-operated group; (B) I/R group; (C) polysaccharides of the Euphoria longan (Lour.) Steud 0.2 g kg−1 group.
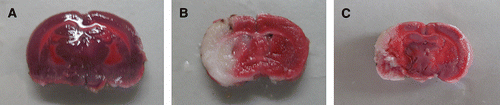
Figure 2. Effect of polysaccharides of the Euphoria longan (Lour.) Steud on IL-1β level in ischemia/reperfusion rats vs ischemia/reperfusion group: *P<0.05; **P<0.01.
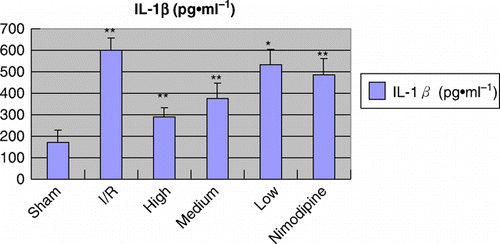
Figure 3. Effect of polysaccharides of the Euphoria longan (Lour.) Steud on TNF-α level in cerebral ischemia/reperfusion rats vs ischemia/reperfusion group: *P<0.05; **P<0.01.
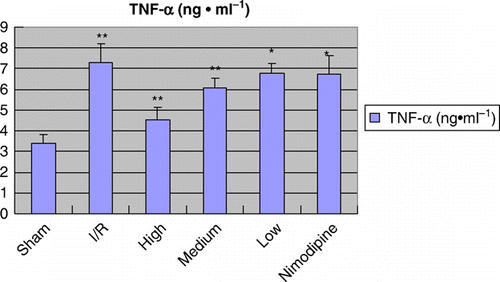
Figure 4. Effect of polysaccharides of the Euphoria longan (Lour.) Steud on MPO activity in cerebral ischemia/reperfusion rats vs ischemia/reperfusion group: *P<0.05; **P<0.01.
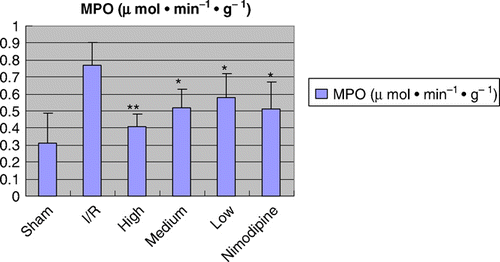
Table 1. Effect of polysaccharides of the Euphorbia longan (Lour.) Steud on brain function, the brain water content and the infract volume in cerebral ischemia/reperfusion rats 
Table 2. Effect of polysaccharides of the Euphoria longan (Lour.) Steud on MPO activity, the contents of IL-1β and TNF-α in cerebral ischemia/reperfusion rats 
Discussion
In this study, we have shown that polysaccharides of the E. longan (Lour.) Steud have positive effects on experimental cerebral ischemia in rats. The mean infarct size, water content and neurological score of rats treated with polysaccharides of the E. longan (Lour.) Steud were significantly lower than that of the control group.
Brain inflammation has been implicated in the development of brain edema and secondary brain damage in I/R (Allan & Rothwell, Citation2001; Feuerstein, Wang, & Barone, Citation1998). Many factors have been implicated in the mechanisms of the secondary injury, including inflammatory reaction induced by release of proinflammatory cytokines and adhesion molecules. Some studies have also shown that the inflammation in cerebral I/R is developed as an outcome of the two sequential: (1) the mobilization and infiltration of peripheral inflammatory cells into the brain and (2) the activation of microglia and resident perivascular/parenchymal macrophages (Giulian, Citation1990; Stanimirovic & Satoh, Citation2000; Storini, Rossi, & Marrella, Citation2005). Brain microvasular endothelium which performs the function of blood – brain barrier is an important responsive and regulatory component in cerebral inflammatory process. Over-expression and over-secretion of proinflammatory cytokines and chemotactic factors, such as TNF-α, IL-1, IL-6, IL-8, etc. have led to microvascular vasoparalysis, neutrophils infiltration and additional clotting of microcirculation, thus exacerbating initial ischemic damage. MPO is an enzyme localized in neutrophils. MPO activity reflected neutrophils infiltration. By measuring its activity, the degree of neutrophils accumulation and infiltration after stroke can be quantified (Matsuo et al., Citation1994). So the anti-inflammatory drugs may play a protective effect on the second injury by decreasing neutrophils infiltration or proinflammatory cytokines after transient ischemic insult. In the present experiments, we demonstrate that polysaccharides of the E. longan (Lour.) Steud significantly reduce MPO activity, concentrations of TNF-α and IL-1β in the brain tissue.
A major limitation of the present study is that the polysaccharides of the E. longan (Lour.) Steud were administered 14 days prior to middle cerebral artery occlusion (MCAO). From a therapeutic standpoint, such a treatment regimen would be of some clinical value in human stroke patients. At the later experiments, we will perform to test whether polysaccharides of the E. longan (Lour.) Steud are capable of providing benefit when given after reperfusion.
Our results suggest polysaccharides of the E. longan (Lour.) Steud have significant protection against cerebral I/R injury, which may be related to decreasing inflammatory response mediated by TNF-α and IL-1β in the brain.
Acknowledgements
We thank Mr Yu Ye, Guangxi Medical University, for his excellent technical assistance. The study was supported by the Natural Science Foundation of Guilin Medical University, China under Grant 2009JC005.
References
- Allan , S.M. and Rothwell , N.J. 2001 . Cytokines and acute neurodegeneration . Nature Reviews Neuroscience , 2 : 734 – 744 .
- Chan , P.H. 2004 . Reactive oxygen radicals in signaling and damage in the ischemic brain . Journal of Cerebral Blood Flow and Metabolism , 21 : 2 – 14 .
- Chen , J. and Huang , R-B. 2009 . Protective effect of Yulangsan polysaccharide on focal cerebral ischemia/reperfusion injury in rats and its underlying mechanism . Neurosciences , 14 ( 4 ) : 7 – 12 .
- Eng , H.L. , Turgay , D. and Michael , A.M. 2003 . Mechanisms, challenges and opportunities in stroke . Nature Reviews Neuroscience , 43 : 99 – 415 .
- Feuerstein , G.Z. , Wang , X. and Barone , F.C. 1998 . The role of cytokines in the neuropathology of stroke and neurotrauma . Neuroimmunomodulation , 5 : 143 – 159 .
- Giulian , D. 1990 . Microglia, cytokines, and cytotoxins: Modulators of cellular responses after injury to the central nervous systems . Journal of Immunology & Immunopharmacology , 10 : 15 – 21 .
- Huang , R-Q. and Liu , X-M. 2008 . Study on the extract of Longan seeds to improve oxidation resistance funtion of mouses . Journal of South China Normal University , 1 : 108 – 112 .
- Iadecola , C. and Alexander , M. 2001 . Cerebral ischemia and inflammation . Current Opinion in Neurology , 14 : 89 – 94 .
- Lambeau , G. and Gelb , M.H. 2008 . Biochemistry and physiology of mammalian secreted phospholipases A2 . Annual Review of Biochemistry , 77 : 495 – 520 .
- Li , T-J. , Yan , Q. , Mao , J-Q. , Yang , P-Y. , Rui , Y-C. and Chen , W-S. 2007 . Protective effects of Guizhi-Fuling-capsules on rat brain ischemia/reperfusion injury . Journal of Pharmacological Sciences , 105 : 34 – 40 .
- Li , X. , Long , S. and Xie , Y. 2004 . The extraction of Euphoria longan (Lour.) Steud and Litchichinensis sonn polysaccharides and its effect of cleaning oxygen radical . Journal of Guang Xi Medical University , 21 : 342 – 344 .
- Longa , E.Z. , Weinstein , P.R. , Carlson , S. and Cummins , R. 1989 . Reversible middle cerebral artery occlusion without craniectomy in rats . Stroke , 20 : 84 – 91 .
- Matsuo , Y. , Onodera , H. , Shiga , Y. , Nakamura , M. , Ninomiya , M. and Kihara , T. 1994 . Correlation between myeloperoxidase-quantified neutrophil accumulation and ischemic brain injury in the rat. Effect of neutrophil depletion . Stroke , 25 : 1469 – 1475 .
- Mehta , S.L. , Manhas , N. and Raghubir , R. 2007 . Molecular targets in cerebral ischemia for developing novel therapeutics . Brain Research Reviews , 54 : 34 – 66 .
- Nobuya , I. , Olga , P. , Kenneth , R. and Mirochnitchenko , O. 2002 . Inflammatory response and glutathione peroxidase in a model of stroke . The Journal of Immunology , 168 : 1926 – 1933 .
- Okuyama , E. 1999 . Adenosine, the antiolytic-like principle of the Arillus of Euphoria longana . Planta Medica , 65 : 115 – 119 .
- Stanimirovic , D. and Satoh , K. 2000 . Inflammatory mediators of cerebral endothelium: A role in ischemic brain inflammation . Brain Pathology , 10 : 113 – 126 .
- Storini , C. , Rossi , E. and Marrella , V. 2005 . C1-inhibitor protects against brain ischemia-reperfusion injury via inhibition of cell recruitment and inflammation . Neurobiology of Disease , 19 : 10 – 17 .
- Wang , X. and Smythe , G.A. 2003 . Assessment of hydroxyl radical generation and radical scavenging activity of Chinese medicinal herbs using GC-MS . Rodox Report: Communications in Free Radical Research , 8 : 223 – 228 .
- Warner , D.S. , Sheng , H. and Batinic-Haberle , I. 2004 . Oxidants, antioxidants and the ischemic brain . Journal of Experimental Biology , 207 : 3221 – 3231 .